ANTIAGREGANTS IN ACUTE CORONARY SYNDROME Karlis TRUSINSKIS Interventional






- Slides: 42

ANTIAGREGANTS IN ACUTE CORONARY SYNDROME Karlis TRUSINSKIS Interventional Cardiologist Pauls Stradins Clinical University Hospital Riga, LATVIA

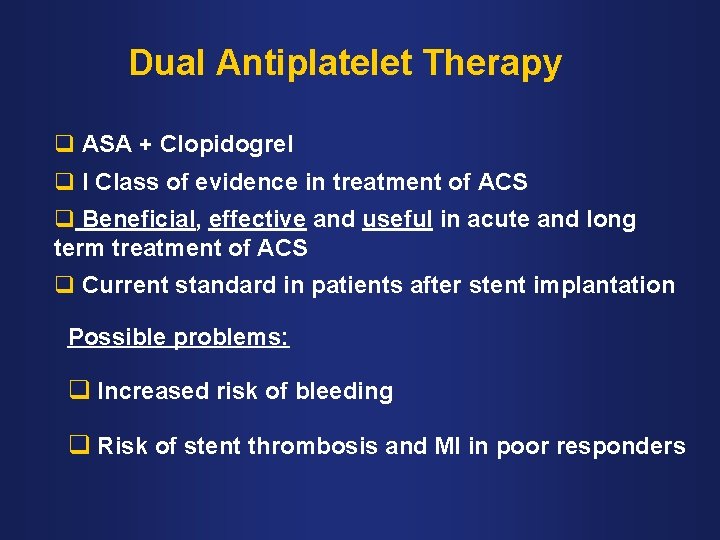
Dual Antiplatelet Therapy q ASA + Clopidogrel q I Class of evidence in treatment of ACS q Beneficial, effective and useful in acute and long term treatment of ACS q Current standard in patients after stent implantation Possible problems: q Increased risk of bleeding q Risk of stent thrombosis and MI in poor responders

Stent thrombosis of LAD bifurcation

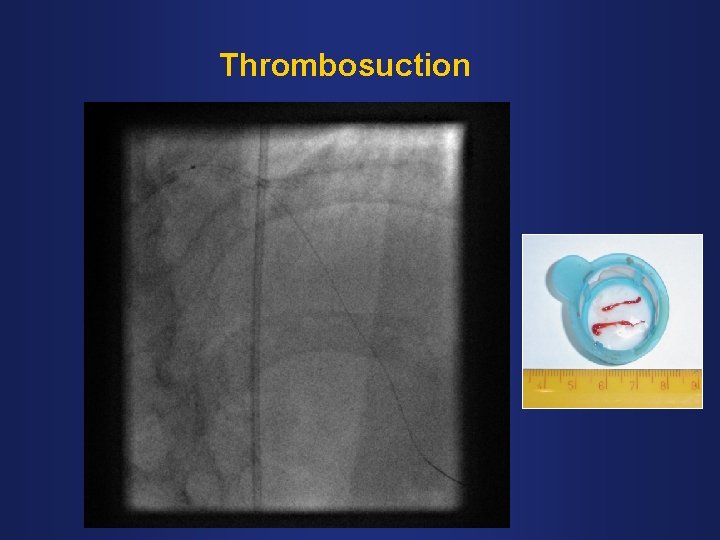
Thrombosuction

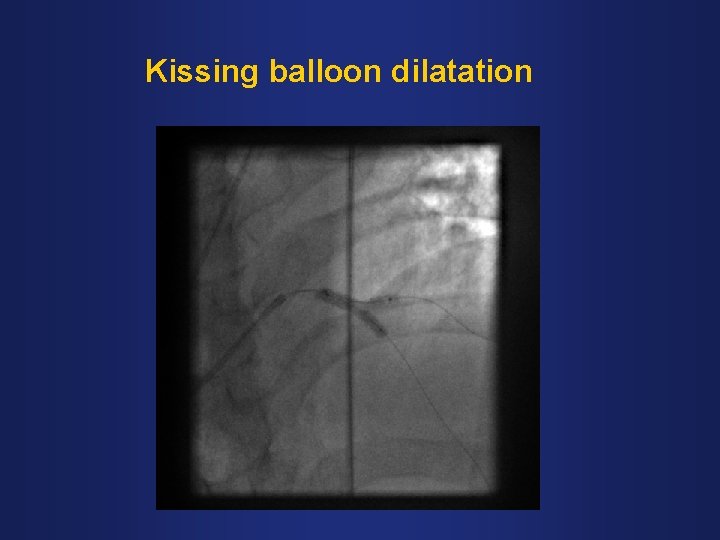
Kissing balloon dilatation

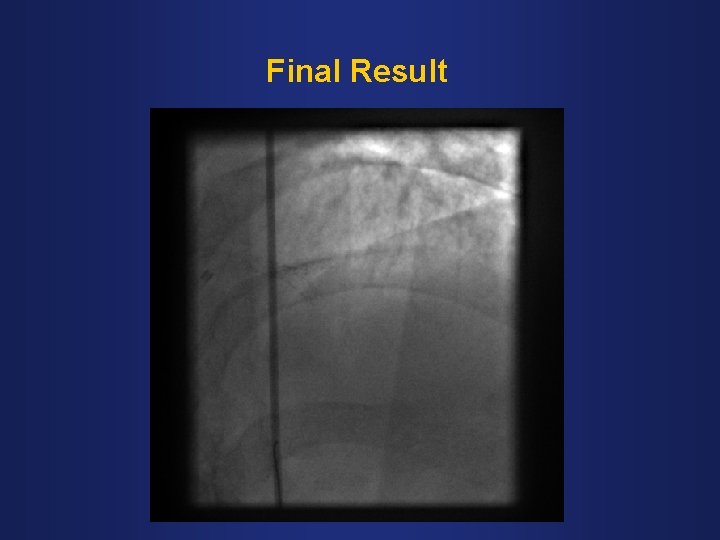
Final Result

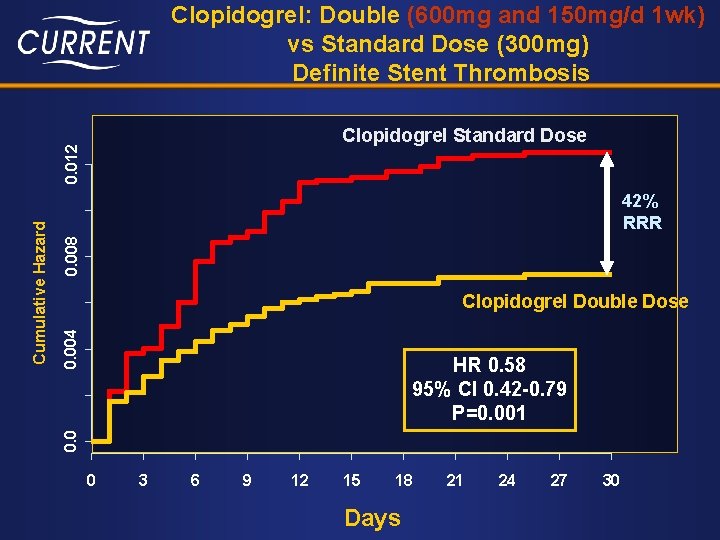
Clopidogrel: Double (600 mg and 150 mg/d 1 wk) vs Standard Dose (300 mg) Definite Stent Thrombosis 0. 008 42% RRR 0. 004 Clopidogrel Double Dose HR 0. 58 95% CI 0. 42 -0. 79 P=0. 001 0. 0 Cumulative Hazard 0. 012 Clopidogrel Standard Dose 0 3 6 9 12 15 18 Days 21 24 27 30

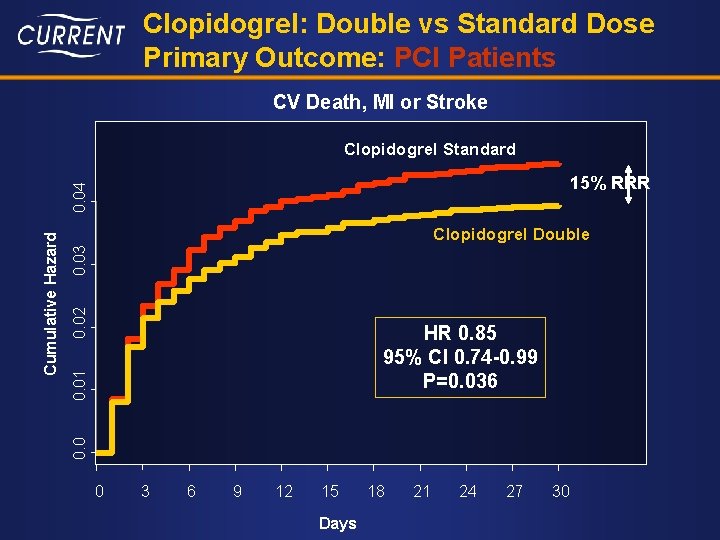
Clopidogrel: Double vs Standard Dose Primary Outcome: PCI Patients CV Death, MI or Stroke Clopidogrel Standard 0. 02 0. 03 Clopidogrel Double 0. 01 HR 0. 85 95% CI 0. 74 -0. 99 P=0. 036 0. 0 Cumulative Hazard 0. 04 15% RRR 0 3 6 9 12 15 Days 18 21 24 27 30

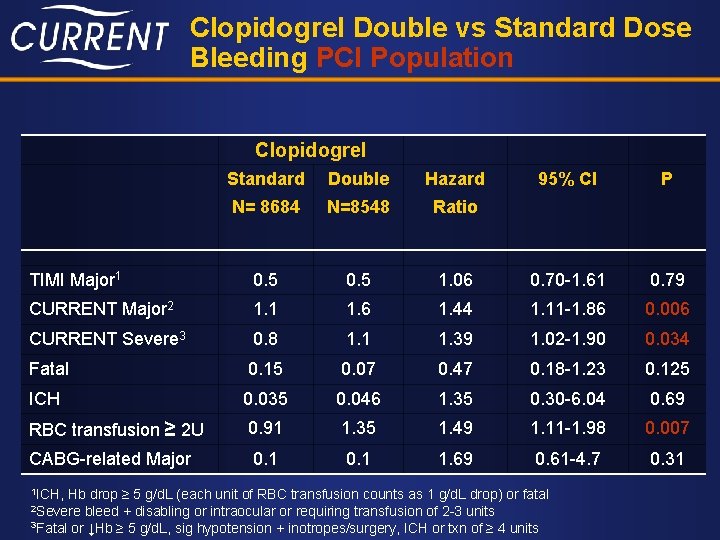
Clopidogrel Double vs Standard Dose Bleeding PCI Population Clopidogrel Standard Double Hazard 95% CI P N= 8684 N=8548 Ratio TIMI Major 1 0. 5 1. 06 0. 70 -1. 61 0. 79 CURRENT Major 2 1. 1 1. 6 1. 44 1. 11 -1. 86 0. 006 CURRENT Severe 3 0. 8 1. 1 1. 39 1. 02 -1. 90 0. 034 Fatal 0. 15 0. 07 0. 47 0. 18 -1. 23 0. 125 ICH 0. 035 0. 046 1. 35 0. 30 -6. 04 0. 69 RBC transfusion ≥ 2 U 0. 91 1. 35 1. 49 1. 11 -1. 98 0. 007 CABG-related Major 0. 1 1. 69 0. 61 -4. 7 0. 31 1 ICH, Hb drop ≥ 5 g/d. L (each unit of RBC transfusion counts as 1 g/d. L drop) or fatal bleed + disabling or intraocular or requiring transfusion of 2 -3 units 3 Fatal or ↓Hb ≥ 5 g/d. L, sig hypotension + inotropes/surgery, ICH or txn of ≥ 4 units 2 Severe

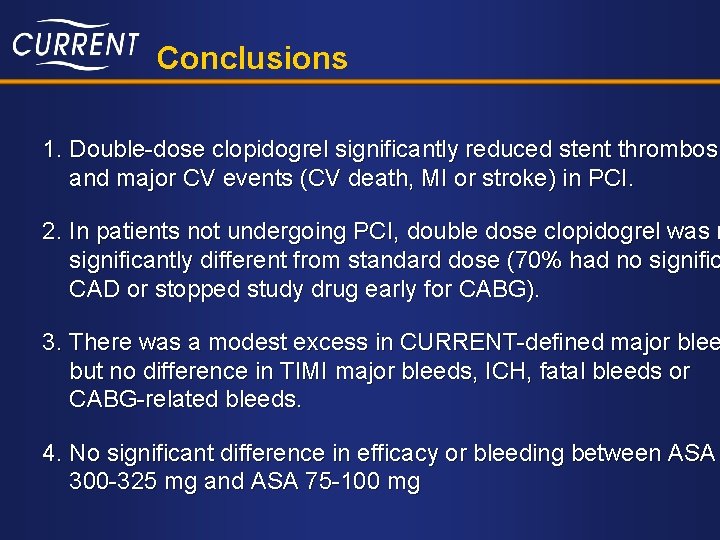
Conclusions 1. Double-dose clopidogrel significantly reduced stent thrombosi and major CV events (CV death, MI or stroke) in PCI. 2. In patients not undergoing PCI, double dose clopidogrel was n significantly different from standard dose (70% had no signific CAD or stopped study drug early for CABG). 3. There was a modest excess in CURRENT-defined major blee but no difference in TIMI major bleeds, ICH, fatal bleeds or CABG-related bleeds. 4. No significant difference in efficacy or bleeding between ASA 300 -325 mg and ASA 75 -100 mg

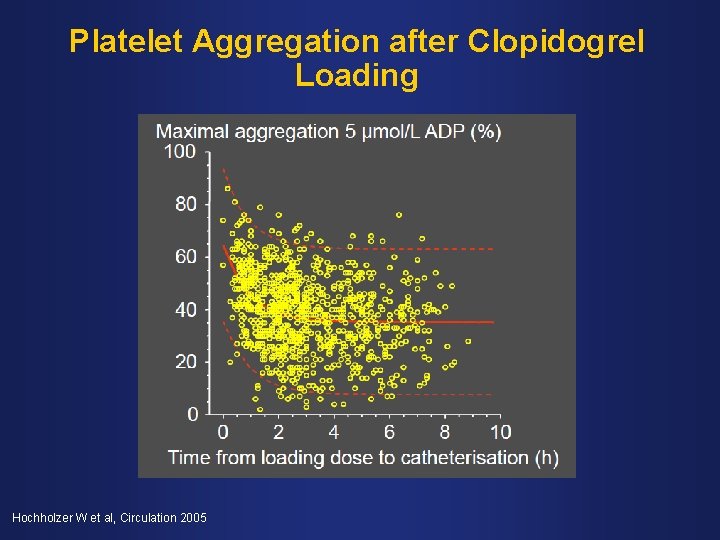
Platelet Aggregation after Clopidogrel Loading Hochholzer W et al, Circulation 2005

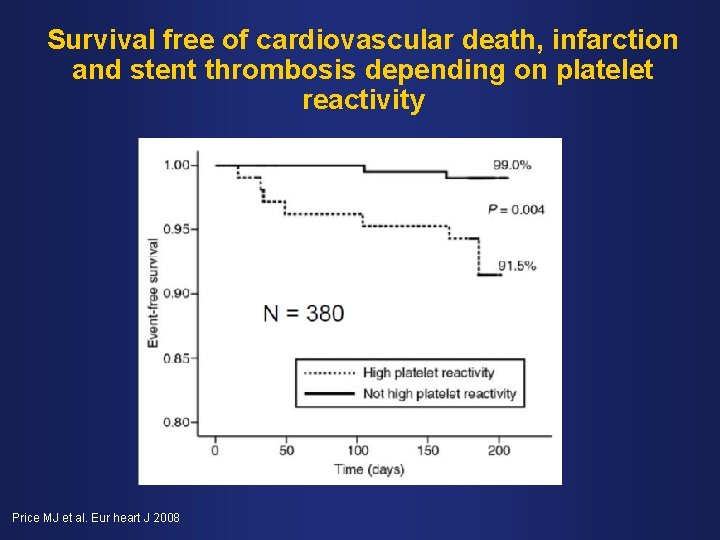
Survival free of cardiovascular death, infarction and stent thrombosis depending on platelet reactivity Price MJ et al. Eur heart J 2008


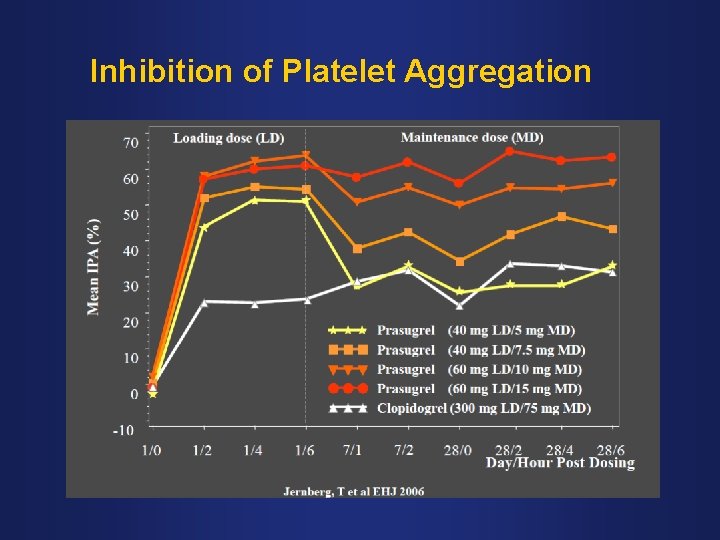
Inhibition of Platelet Aggregation

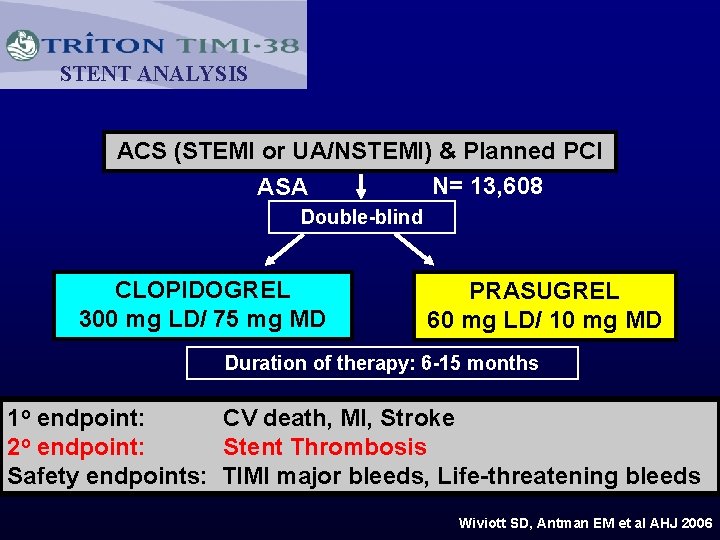
STENT ANALYSIS ACS (STEMI or UA/NSTEMI) & Planned PCI N= 13, 608 ASA Double-blind CLOPIDOGREL 300 mg LD/ 75 mg MD PRASUGREL 60 mg LD/ 10 mg MD Duration of therapy: 6 -15 months 1 o endpoint: CV death, MI, Stroke 2 o endpoint: Stent Thrombosis Safety endpoints: TIMI major bleeds, Life-threatening bleeds Wiviott SD, Antman EM et al AHJ 2006

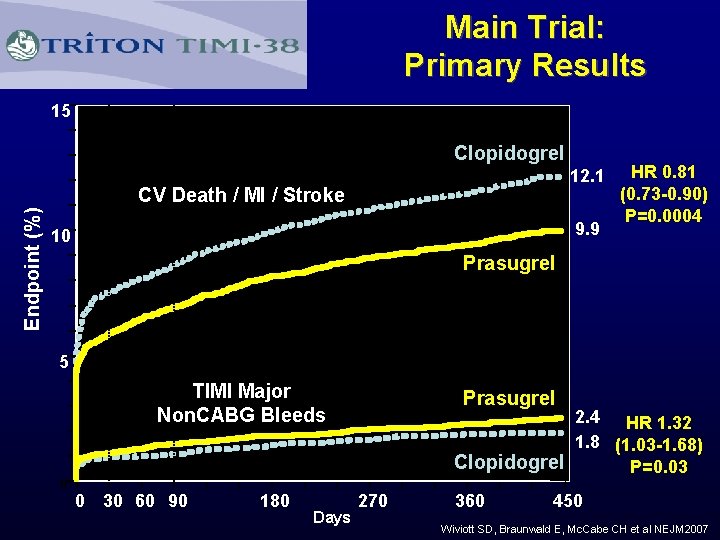
Main Trial: Primary Results 15 Clopidogrel 12. 1 Endpoint (%) CV Death / MI / Stroke 9. 9 10 HR 0. 81 (0. 73 -0. 90) P=0. 0004 Prasugrel 5 TIMI Major Non. CABG Bleeds Prasugrel 2. 4 HR 1. 32 1. 8 (1. 03 -1. 68) Clopidogrel P=0. 03 0 0 30 60 90 180 Days 270 360 450 Wiviott SD, Braunwald E, Mc. Cabe CH et al NEJM 2007

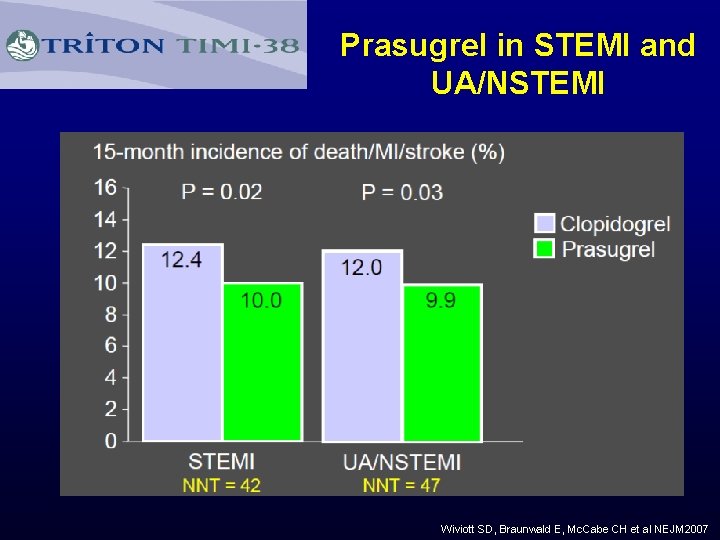
Prasugrel in STEMI and UA/NSTEMI Wiviott SD, Braunwald E, Mc. Cabe CH et al NEJM 2007

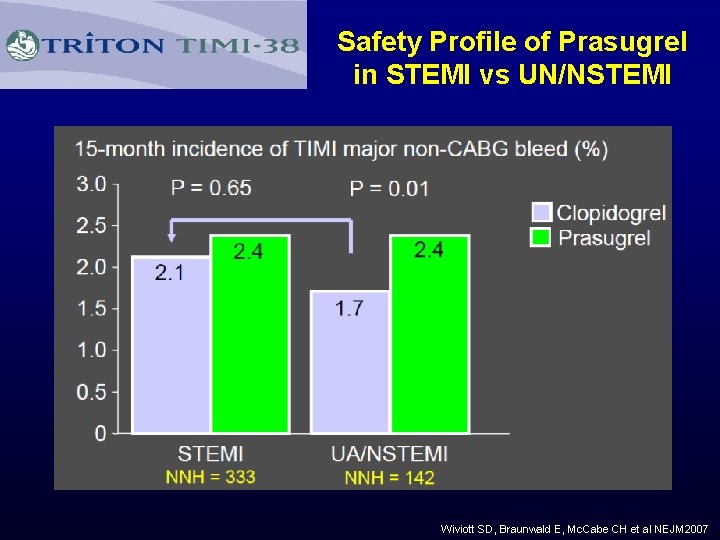
Safety Profile of Prasugrel in STEMI vs UN/NSTEMI Wiviott SD, Braunwald E, Mc. Cabe CH et al NEJM 2007

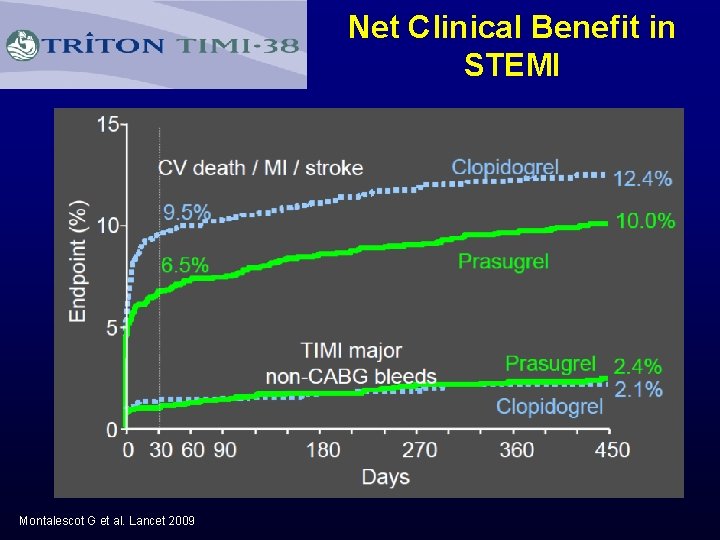
Net Clinical Benefit in STEMI Montalescot G et al. Lancet 2009

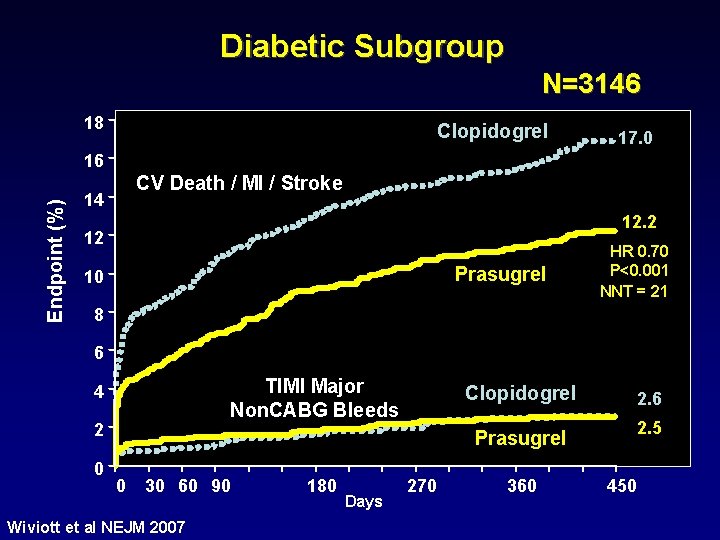
Diabetic Subgroup N=3146 18 Clopidogrel 17. 0 Endpoint (%) 16 CV Death / MI / Stroke 14 12. 2 12 Prasugrel 10 HR 0. 70 P<0. 001 NNT = 21 8 6 TIMI Major Non. CABG Bleeds 4 2 0 Clopidogrel 2. 6 2. 5 Prasugrel 0 30 60 90 Wiviott et al NEJM 2007 180 Days 270 360 450

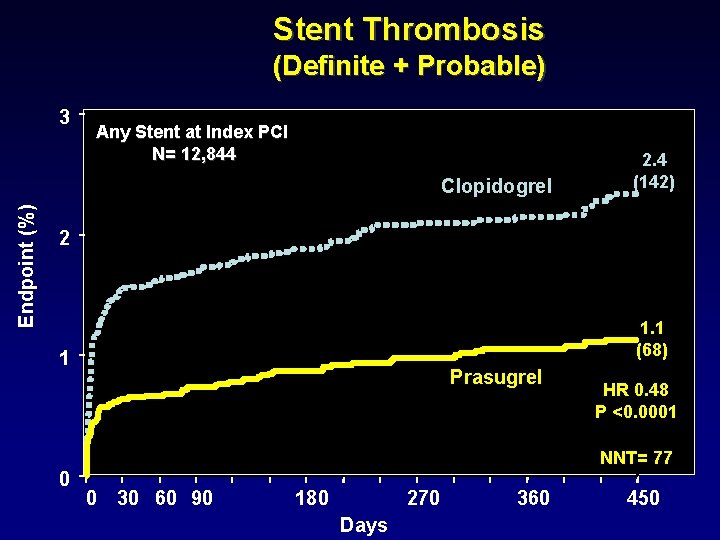
Stent Thrombosis (Definite + Probable) 3 Any Stent at Index PCI N= 12, 844 Endpoint (%) Clopidogrel 2. 4 (142) 2 1. 1 (68) 1 Prasugrel HR 0. 48 P <0. 0001 NNT= 77 0 0 30 60 90 180 270 Days 360 450

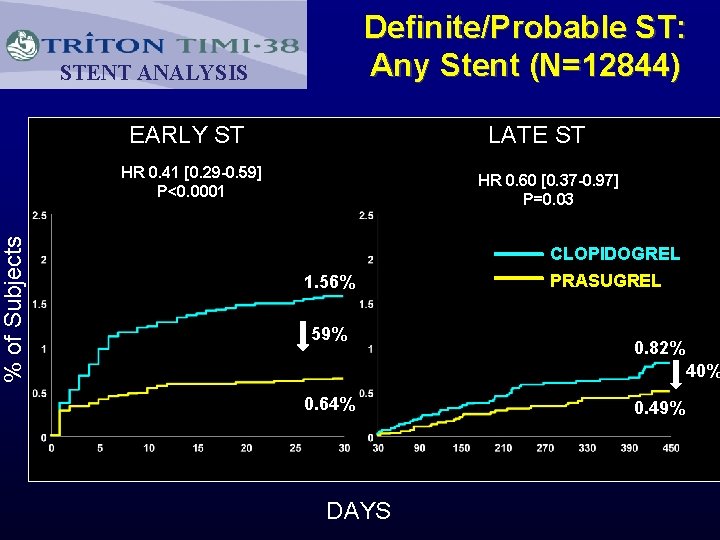
% of Subjects Definite/Probable ST: Any Stent (N=12844) STENT ANALYSIS EARLY ST LATE ST HR 0. 41 [0. 29 -0. 59] P<0. 0001 HR 0. 60 [0. 37 -0. 97] P=0. 03 CLOPIDOGREL 1. 56% 59% PRASUGREL 0. 82% 40% 0. 64% DAYS 0. 49%

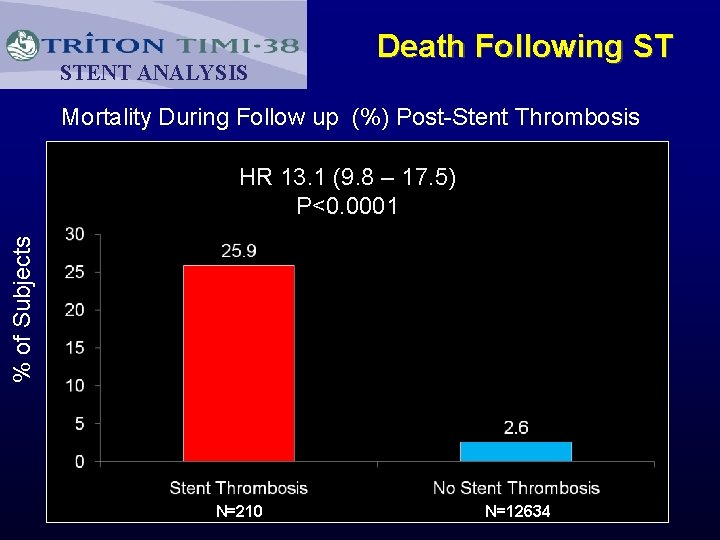
STENT ANALYSIS Death Following ST Mortality During Follow up (%) Post-Stent Thrombosis % of Subjects HR 13. 1 (9. 8 – 17. 5) P<0. 0001 N=210 N=12634

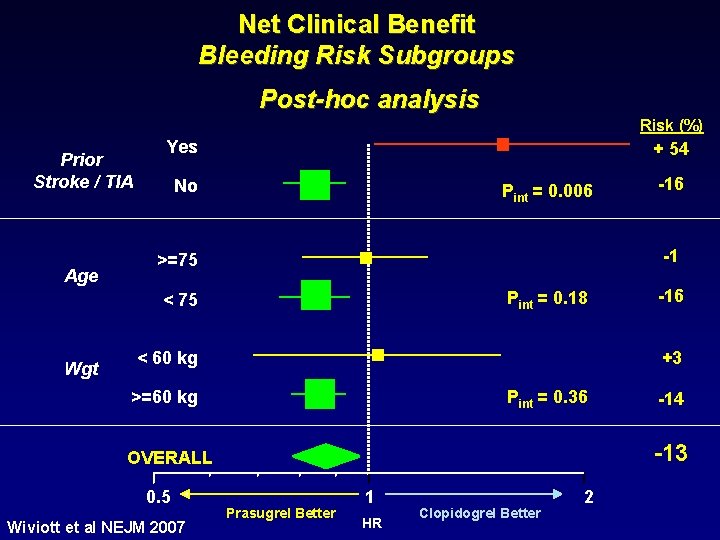
Net Clinical Benefit Bleeding Risk Subgroups Post-hoc analysis Risk (%) Prior Stroke / TIA Age Yes + 54 No Pint = 0. 006 -1 >=75 Pint = 0. 18 < 75 Wgt >=60 kg Pint = 0. 36 -14 -13 OVERALL Wiviott et al NEJM 2007 -16 +3 < 60 kg 0. 5 -16 Prasugrel Better 1 HR Clopidogrel Better 2

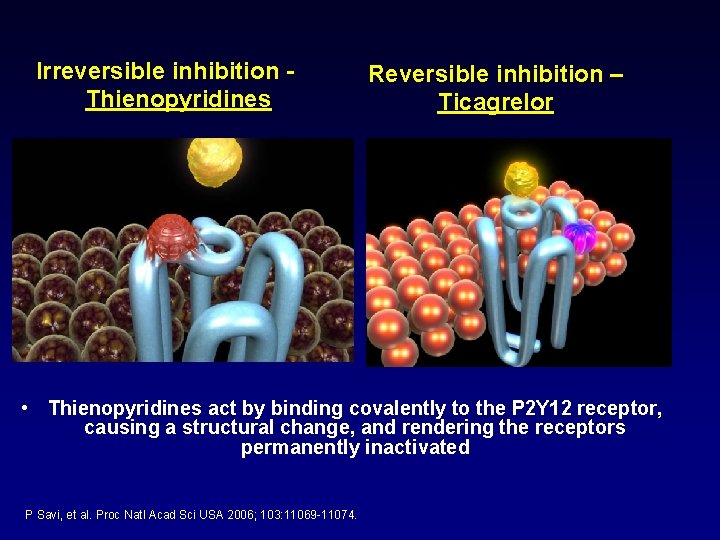
Irreversible inhibition Thienopyridines Reversible inhibition – Ticagrelor • Thienopyridines act by binding covalently to the P 2 Y 12 receptor, causing a structural change, and rendering the receptors permanently inactivated P Savi, et al. Proc Natl Acad Sci USA 2006; 103: 11069 -11074.

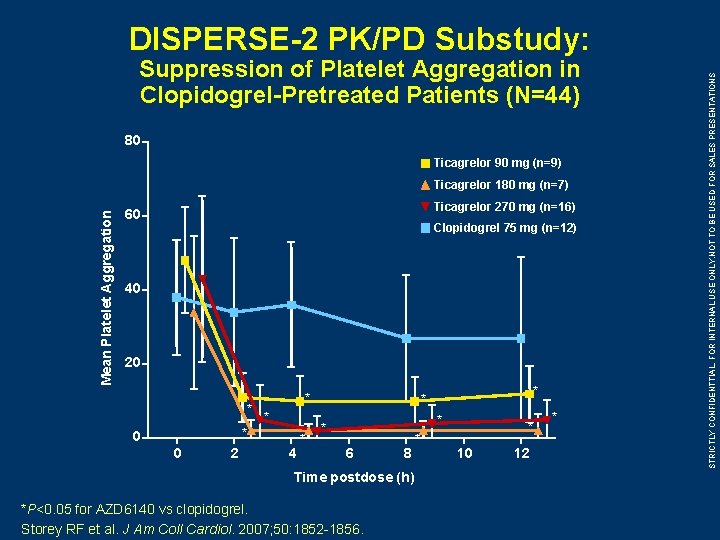
Suppression of Platelet Aggregation in Clopidogrel-Pretreated Patients (N=44) 80 Ticagrelor 90 mg (n=9) Mean Platelet Aggregation Ticagrelor 180 mg (n=7) Ticagrelor 270 mg (n=16) 60 Clopidogrel 75 mg (n=12) 40 20 * * 0 0 2 4 * * * 6 8 Time postdose (h) *P<0. 05 for AZD 6140 vs clopidogrel. Storey RF et al. J Am Coll Cardiol. 2007; 50: 1852 -1856. * * 10 12 * STRICTLY CONFIDENTTIAL, FOR INTERNAL USE ONLY. NOT TO BE USED FOR SALES PRESENTATIONS DISPERSE-2 PK/PD Substudy:

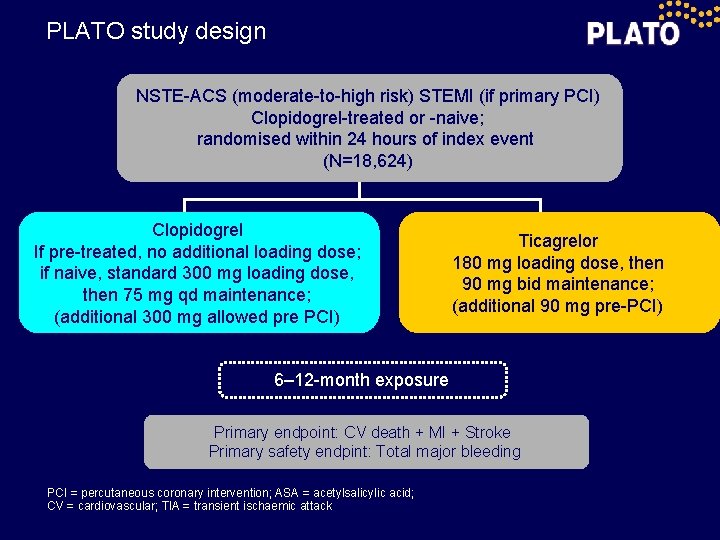
PLATO study design NSTE-ACS (moderate-to-high risk) STEMI (if primary PCI) Clopidogrel-treated or -naive; randomised within 24 hours of index event (N=18, 624) Clopidogrel If pre-treated, no additional loading dose; if naive, standard 300 mg loading dose, then 75 mg qd maintenance; (additional 300 mg allowed pre PCI) Ticagrelor 180 mg loading dose, then 90 mg bid maintenance; (additional 90 mg pre-PCI) 6– 12 -month exposure Primary endpoint: CV death + MI + Stroke Primary safety endpint: Total major bleeding PCI = percutaneous coronary intervention; ASA = acetylsalicylic acid; CV = cardiovascular; TIA = transient ischaemic attack

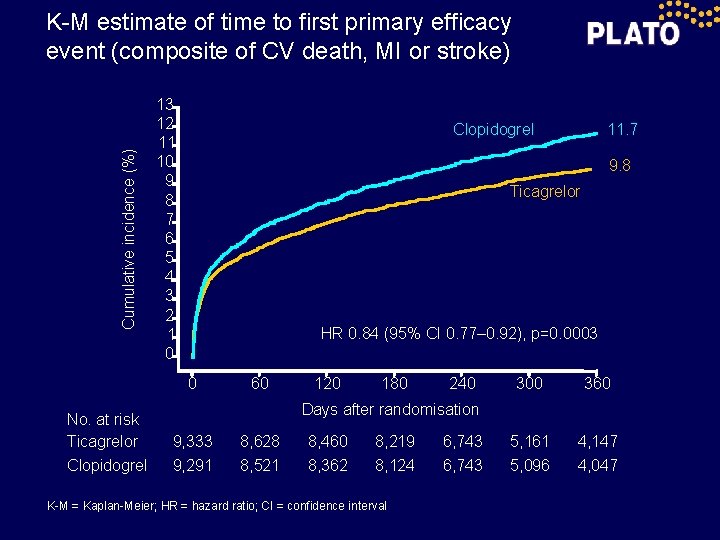
Cumulative incidence (%) K-M estimate of time to first primary efficacy event (composite of CV death, MI or stroke) 13 12 11 10 9 8 7 6 5 4 3 2 1 0 9. 8 Ticagrelor HR 0. 84 (95% CI 0. 77– 0. 92), p=0. 0003 0 No. at risk Ticagrelor Clopidogrel 11. 7 Clopidogrel 60 120 180 240 300 360 5, 161 5, 096 4, 147 4, 047 Days after randomisation 9, 333 9, 291 8, 628 8, 521 8, 460 8, 362 8, 219 8, 124 K-M = Kaplan-Meier; HR = hazard ratio; CI = confidence interval 6, 743

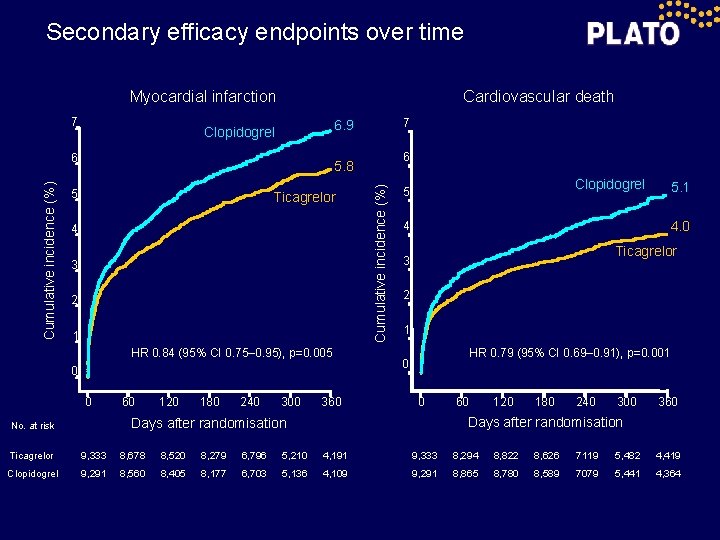
Secondary efficacy endpoints over time Cardiovascular death Myocardial infarction 7 6 6 5. 8 5 Ticagrelor 4 3 2 1 HR 0. 84 (95% CI 0. 75– 0. 95), p=0. 005 0 0 60 120 180 240 300 360 Cumulative incidence (%) 7 6. 9 Clopidogrel 5 4. 0 4 Ticagrelor 3 2 1 HR 0. 79 (95% CI 0. 69– 0. 91), p=0. 001 0 0 60 120 180 240 300 360 Days after randomisation No. at risk 5. 1 Ticagrelor 9, 333 8, 678 8, 520 8, 279 6, 796 5, 210 4, 191 9, 333 8, 294 8, 822 8, 626 7119 5, 482 4, 419 Clopidogrel 9, 291 8, 560 8, 405 8, 177 6, 703 5, 136 4, 109 9, 291 8, 865 8, 780 8, 589 7079 5, 441 4, 364

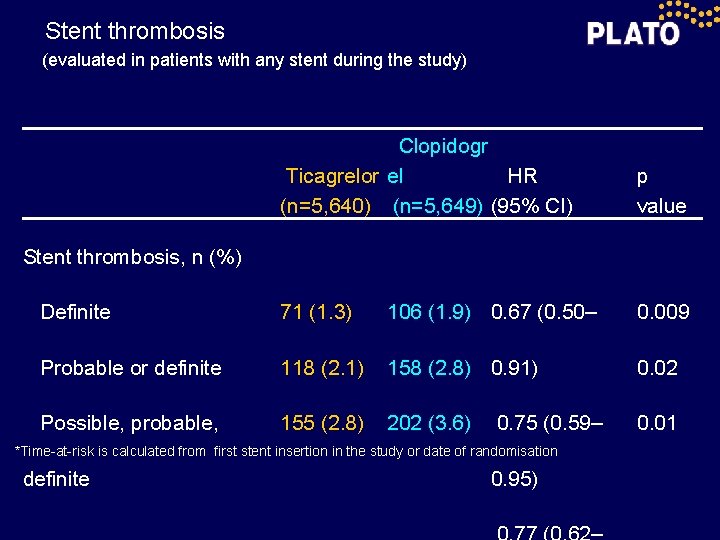
Stent thrombosis (evaluated in patients with any stent during the study) Clopidogr Ticagrelor el HR (n=5, 640) (n=5, 649) (95% CI) p value Definite 71 (1. 3) 106 (1. 9) 0. 67 (0. 50– 0. 009 Probable or definite 118 (2. 1) 158 (2. 8) 0. 91) 0. 02 Possible, probable, 155 (2. 8) 202 (3. 6) 0. 01 Stent thrombosis, n (%) 0. 75 (0. 59– *Time-at-risk is calculated from first stent insertion in the study or date of randomisation definite 0. 95)

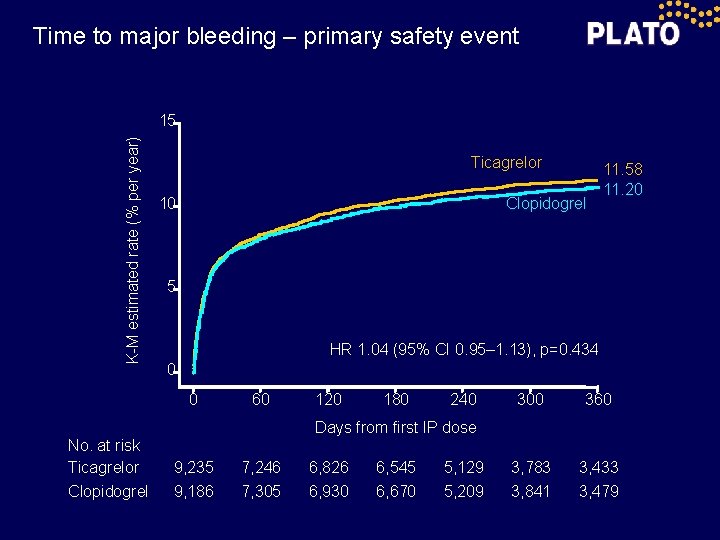
Time to major bleeding – primary safety event K-M estimated rate (% per year) 15 Ticagrelor 10 Clopidogrel 5 HR 1. 04 (95% CI 0. 95– 1. 13), p=0. 434 0 0 No. at risk Ticagrelor Clopidogrel 11. 58 11. 20 60 120 180 240 300 360 3, 783 3, 841 3, 433 3, 479 Days from first IP dose 9, 235 9, 186 7, 246 7, 305 6, 826 6, 930 6, 545 6, 670 5, 129 5, 209

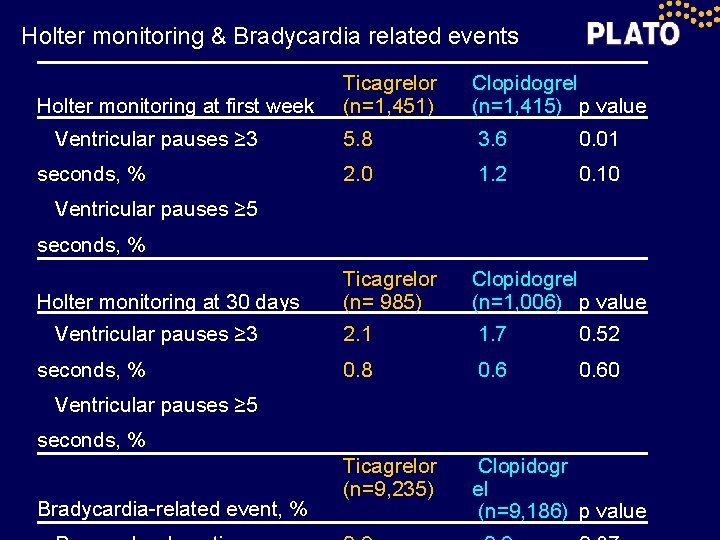
Holter monitoring & Bradycardia related events Holter monitoring at first week Ventricular pauses ≥ 3 seconds, % Ticagrelor (n=1, 451) Clopidogrel (n=1, 415) p value 5. 8 3. 6 0. 01 2. 0 1. 2 0. 10 Ticagrelor (n= 985) Clopidogrel (n=1, 006) p value 2. 1 1. 7 0. 52 0. 8 0. 60 Ticagrelor (n=9, 235) Clopidogr el (n=9, 186) p value Ventricular pauses ≥ 5 seconds, % Holter monitoring at 30 days Ventricular pauses ≥ 3 seconds, % Ventricular pauses ≥ 5 seconds, % Bradycardia-related event, %

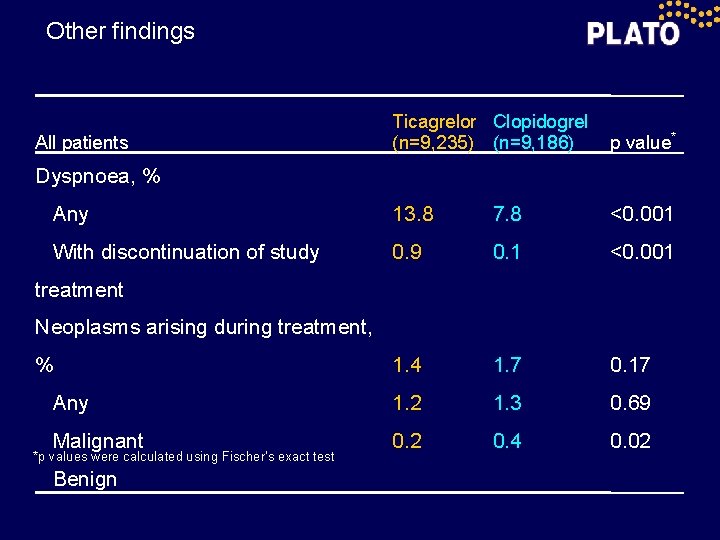
Other findings Ticagrelor Clopidogrel (n=9, 235) (n=9, 186) p value* Any 13. 8 7. 8 <0. 001 With discontinuation of study 0. 9 0. 1 <0. 001 1. 4 1. 7 0. 17 Any 1. 2 1. 3 0. 69 Malignant 0. 2 0. 4 0. 02 All patients Dyspnoea, % treatment Neoplasms arising during treatment, % *p values were calculated using Fischer’s exact test Benign

Conclusions • Reversible, more intense P 2 Y 12 receptor inhibition for one year with ticagrelor in comparison with clopidogrel in a broad population with ST- and non-ST-elevation ACS provides – Reduction in myocardial infarction and stent thrombosis – Reduction in cardiovascular and total mortality – No change in the overall risk of major bleeding

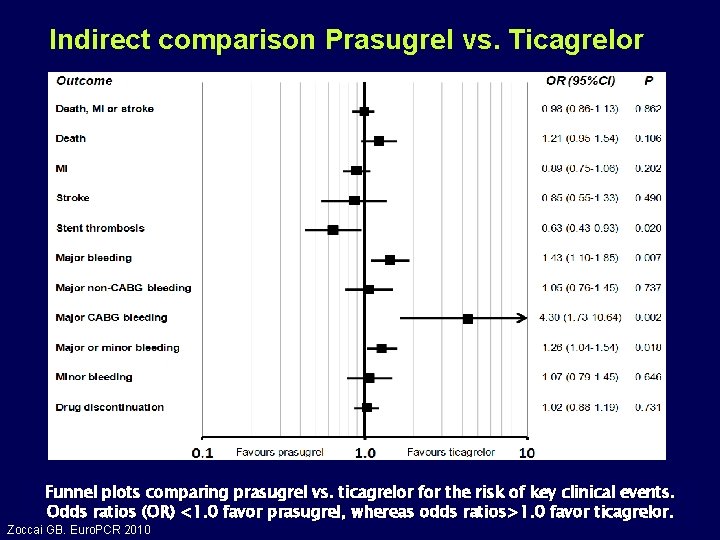
Indirect comparison Prasugrel vs. Ticagrelor Funnel plots comparing prasugrel vs. ticagrelor for the risk of key clinical events. Odds ratios (OR) <1. 0 favor prasugrel, whereas odds ratios>1. 0 favor ticagrelor. Zoccai GB. Euro. PCR 2010


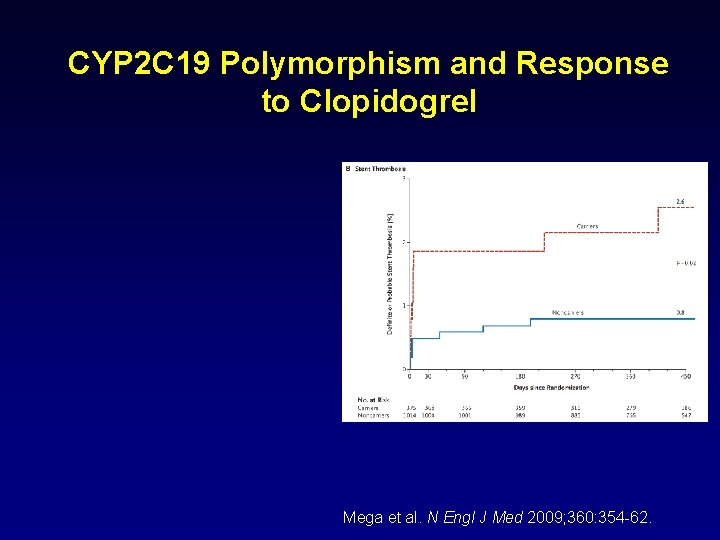
CYP 2 C 19 Polymorphism and Response to Clopidogrel Mega et al. N Engl J Med 2009; 360: 354 -62.

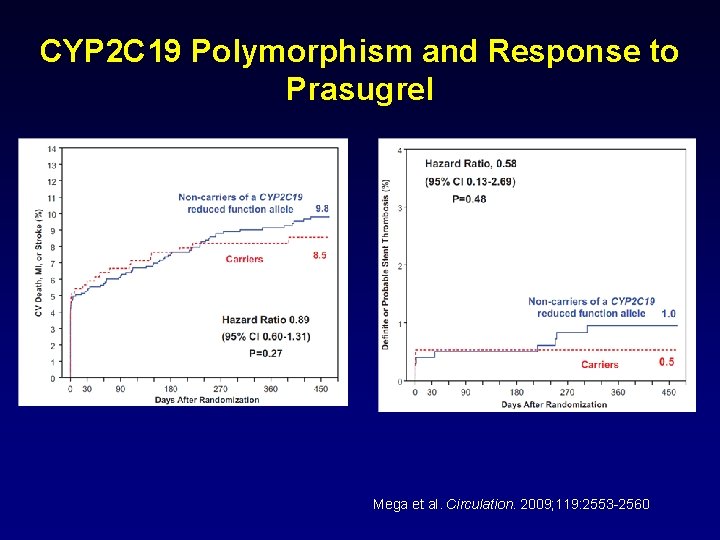
CYP 2 C 19 Polymorphism and Response to Prasugrel Mega et al. Circulation. 2009; 119: 2553 -2560

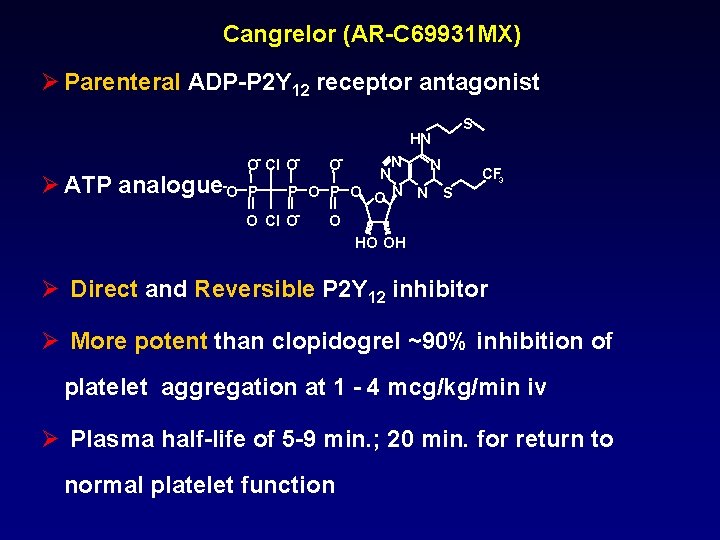
Cangrelor (AR-C 69931 MX) Ø Parenteral ADP-P 2 Y 12 receptor antagonist HN Ø ATP analogue O- Cl O- -O P O- N N N P O O N N S O Cl O- S CF 3 O HO OH Ø Direct and Reversible P 2 Y 12 inhibitor Ø More potent than clopidogrel ~90% inhibition of platelet aggregation at 1 - 4 mcg/kg/min iv Ø Plasma half-life of 5 -9 min. ; 20 min. for return to normal platelet function

CHAMPION Trial: Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition PCI Harrington et al. N Engl J Med 2009; 361: 2318 -29.

INNOVATE PCI: treatment with oral and intravenous Elinogrel in setting of non-urgent PCI • Second phase trial • Evaluation of clinical effectiveness, safety and tolerability Rao et al. ESC Congress 2010

ANTIAGREGANTS IN ACUTE CORONARY SYNDROME Karlis TRUSINSKIS Interventional Cardiologist Pauls Stradins Clinical University Hospital Riga, LATVIA
 Karlis trusinskis
Karlis trusinskis Acute coronary syndrome
Acute coronary syndrome Global registry of acute coronary events
Global registry of acute coronary events Karlis racenis
Karlis racenis Coronary steal
Coronary steal Ischemic heart disease classification
Ischemic heart disease classification Tipe investigasi dalam penelitian
Tipe investigasi dalam penelitian Interventional mri
Interventional mri Acute radiation syndrome
Acute radiation syndrome Firas mussa
Firas mussa Tricuspid valve
Tricuspid valve Coronary angiography equipment
Coronary angiography equipment Anemia or hypoproteinemia will ______ blood viscosity.
Anemia or hypoproteinemia will ______ blood viscosity. Atrioventricular groove
Atrioventricular groove Stent papyrus
Stent papyrus Right atrioventricular valve
Right atrioventricular valve Crash course circulatory system
Crash course circulatory system Fossa ovalis
Fossa ovalis Flow
Flow Cardiac plexus
Cardiac plexus Coronary sinusoids
Coronary sinusoids Coronary calcium score guidelines
Coronary calcium score guidelines Coronary artery disease pathophysiology
Coronary artery disease pathophysiology Sircumflex
Sircumflex Sinusoid anatomy
Sinusoid anatomy Heart wall
Heart wall Mesa coronary calcium score
Mesa coronary calcium score Unlocking of knee joint
Unlocking of knee joint Coronary artery disease
Coronary artery disease Qfr coronary
Qfr coronary Mesa coronary calcium score
Mesa coronary calcium score Cardiac cycle animation
Cardiac cycle animation Coronary sulcus
Coronary sulcus Coronary circulation
Coronary circulation Coronary personality
Coronary personality Coronary heart disease
Coronary heart disease Coronary blood flow
Coronary blood flow What is high quality cpr
What is high quality cpr Heart veins
Heart veins Superadded changes in coronary atherosclerosis
Superadded changes in coronary atherosclerosis Coronary circulation of heart
Coronary circulation of heart Pk papyrus covered coronary stent system
Pk papyrus covered coronary stent system Adjacent angles linear pair
Adjacent angles linear pair