Antenatal Maternal Stress Epigenetic Mechanisms of Developmental Programming
























- Slides: 44

Antenatal Maternal Stress: Epigenetic Mechanisms of Developmental Programming of Diseases Ravi Goyal, MD, Ph. D Assistant Professor Center for Perinatal Biology School of Medicine Loma Linda University

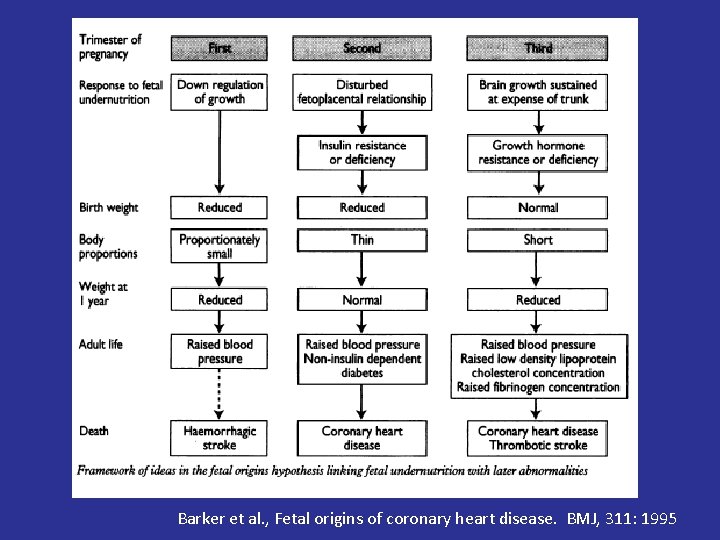
Developmental Origin Hypothesis The “Developmental Origin of Adult Health and Disease” proposes that under-nutrition in-utero permanently changes body functions and metabolism leading to an increased risk of diseases in adult life • Barker’s Hypothesis • Fetal programming of adult health and disease Barker et al, 1991

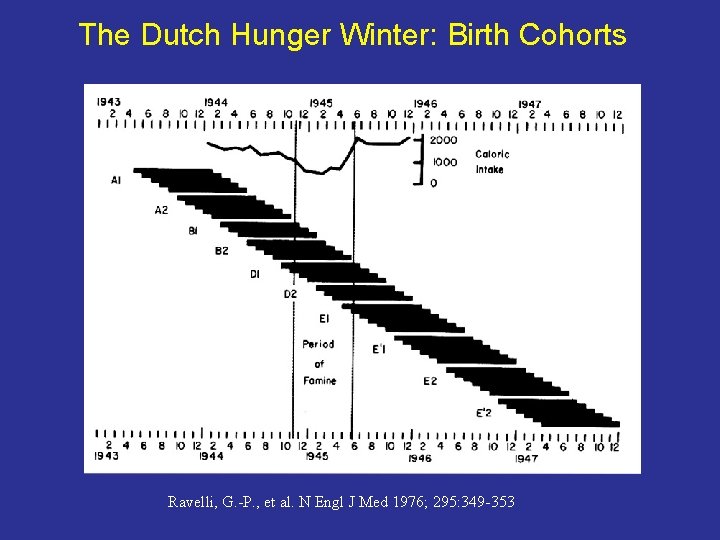
The Dutch Hunger Winter • World War II • The Dutch government (exile in London) called a general railway strike in the northern and western Netherlands • Germans retaliated • All food transport to western and northern Netherlands was interdicted

The Hunger Winter • Winter of 1944/1945 was unusually harsh • Canals rapidly froze over and became impassable for barges • Lasted 6 months, from November 1944 - May 5, 1945, when Holland was liberated from the German occupation

Adult rations - 400 -800 calories a day (less than a quarter of the recommended adult caloric intake)

The Dutch Hunger Winter: Birth Cohorts Ravelli, G. -P. , et al. N Engl J Med 1976; 295: 349 -353

Barker et al. , Fetal origins of coronary heart disease. BMJ, 311: 1995

Epidemiological Studies • Adult cohorts show that low infant weight is strongly associated with an increased disease risk – Barker DJP Lancet 1989 – Eriksson JG Diabetologia 2003; 46: 190 – Bhargava S New Eng J Med 2004; 350: 865

The effects of the famine • Increased incidence of – Hypertension – Type -2 DM – Cardiovascular mortality – Schizophrenia Baby born during the hunger winter – Obesity First 2 trimesters - 80% higher prevalence of overweight (p<0. 0005)

The Thrifty Phenotype As a result of poor nutritional conditions, a pregnant female can modify the development of her unborn child such that it will be prepared for survival in an environment in which resources are likely to be short, resulting in a thrifty phenotype (Hales & Barker, 1992)

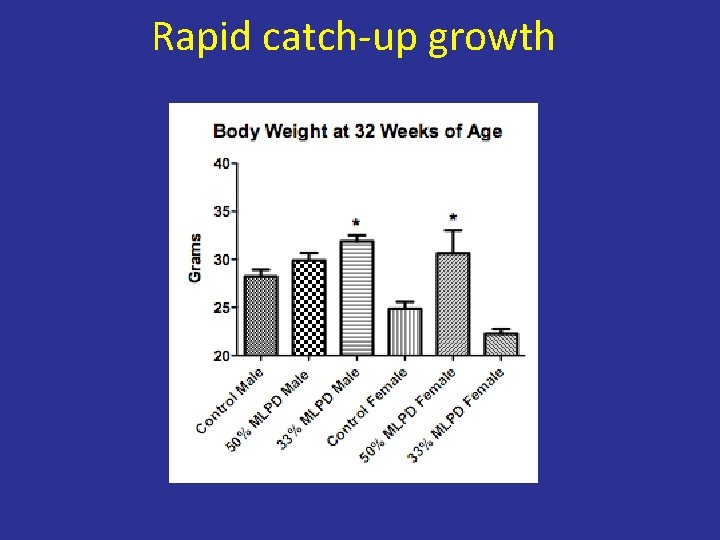
Our studies • Mice (FVB/NJ) • Control, 50% protein diet and 33% protein diet (isocaloric) • Mice consumed approximately equal amount of food

Research Questions • How does this developmental programming occurs? • What are the molecular mechanisms? • What are the genetic and epigenetic changes in specific signal transduction pathways?

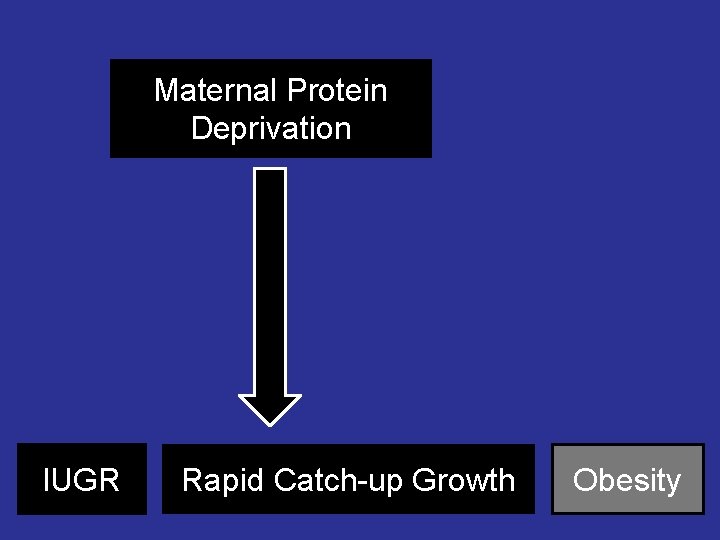
Maternal Protein Deprivation Baby born during the hunger winter IUGR

Birth Weight was reduced but not the litter Isocaloric diet with reduced size protein intake can lead to IUGR

What if a thrifty phenotype child is given excess nutrition? Rapid catch-up growth – Obesity – Hypertension – Diabetes

Maternal Protein Deprivation IUGR Rapid Catch-up Growth

Rapid catch-up growth

Rapid catch-up growth

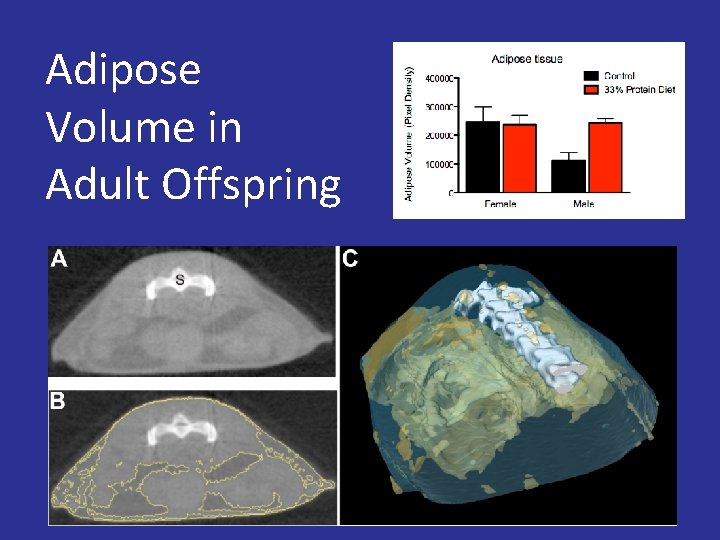
Maternal Protein Deprivation IUGR Rapid Catch-up Growth Obesity

Adipose Volume in Adult Offspring

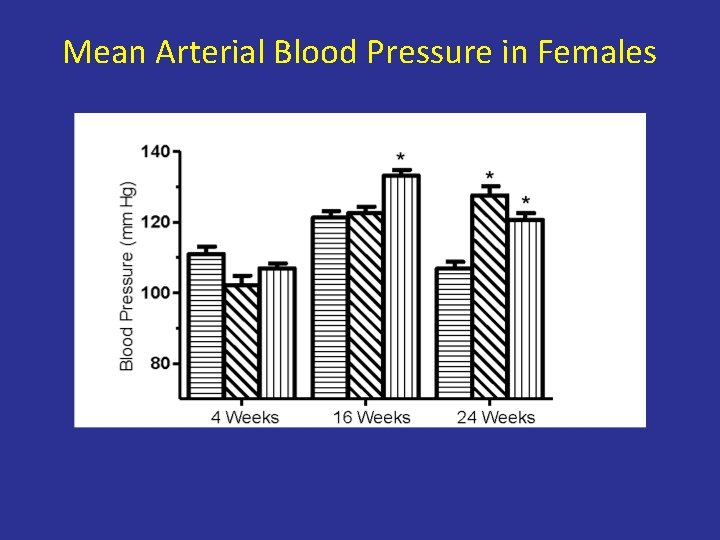
Maternal Protein Deprivation IUGR Rapid Catch-up Growth Hypertension

Mean Arterial Blood Pressure in Females

Mean Arterial Blood Pressure in Males

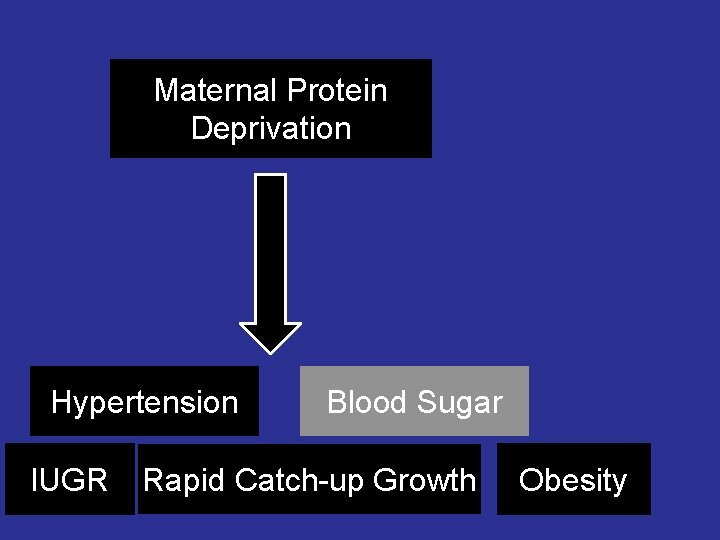
Maternal Protein Deprivation Hypertension IUGR Blood Sugar Rapid Catch-up Growth Obesity

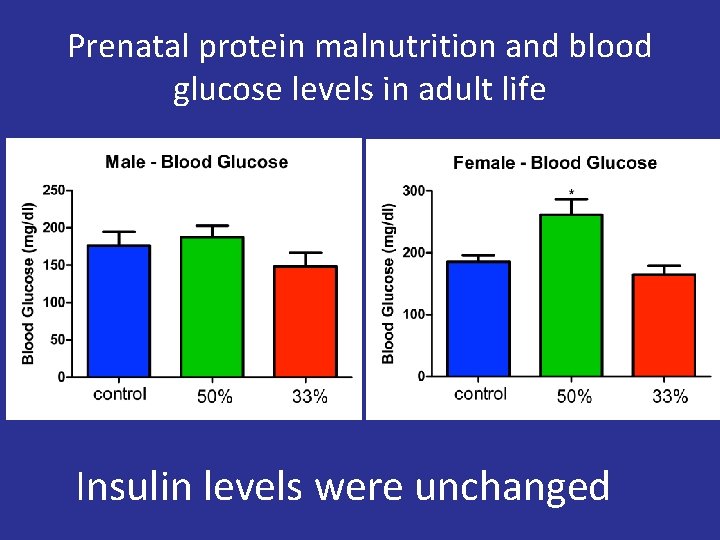
Prenatal protein malnutrition and blood glucose levels in adult life Insulin levels were unchanged

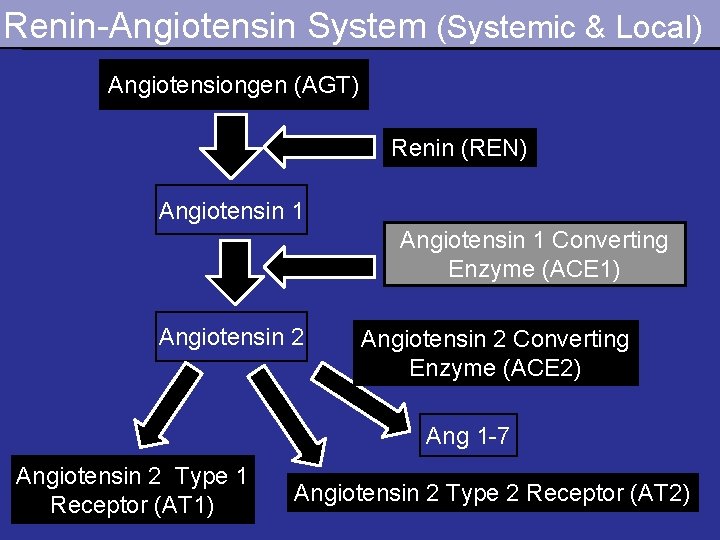
Maternal Protein Deprivation Altered gene expression Renin – Angiotensin System Hypertension

Renin-Angiotensin System (Systemic & Local) Angiotensiongen (AGT) Renin (REN) Angiotensin 1 Converting Enzyme (ACE 1) Angiotensin 2 Converting Enzyme (ACE 2) Ang 1 -7 Angiotensin 2 Type 1 Receptor (AT 1) Angiotensin 2 Type 2 Receptor (AT 2)

Angiotensin 1 Converting Enzyme (Brain)

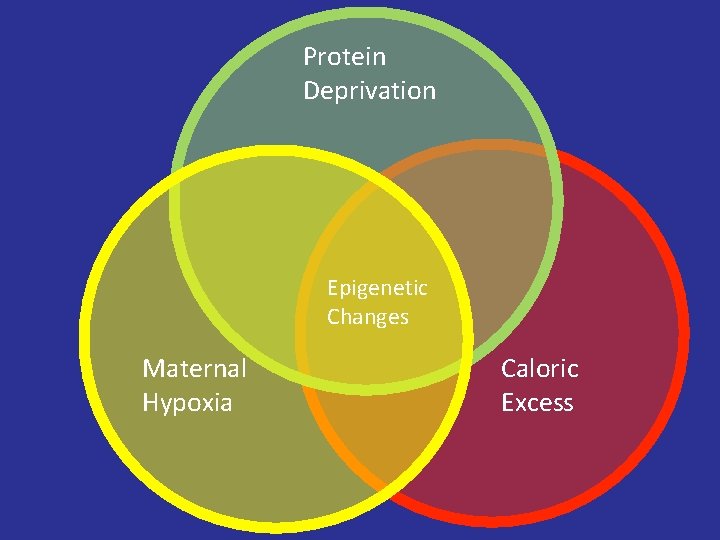
Protein Deprivation Epigenetic Changes Maternal Hypoxia Caloric Excess

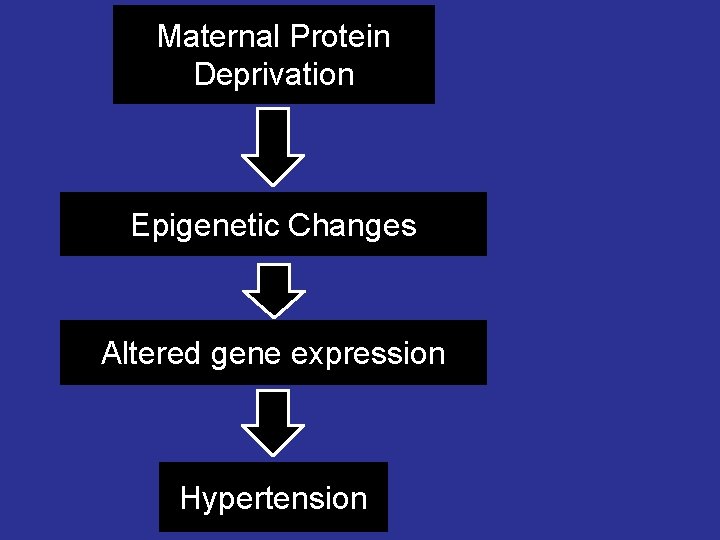
Maternal Protein Deprivation Epigenetic Changes Altered gene expression Hypertension

Epigenetics Heritable changes in phenotype or gene expression caused by mechanisms other than changes in DNA sequence per se Epigenetic Changes Transcriptional Regulation Translational Regulation DNA methylation Histone Modifications mi. RNA, ln. RNA-mediated regulation

DNA Methylation – Transcriptional Modification CH 3 CH 3 3’ CH 3 5’ Transcription Hindered – Less m. RNA Polymerase Methylated Cp. G Islands in Promoter Region Hypomethylated Cp. G Islands 3’ RNA Polymerase 5’ Higher amount of m. RNA

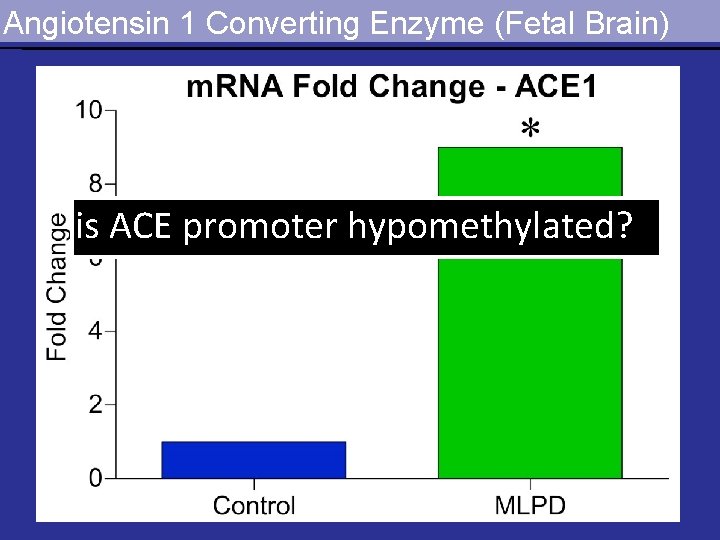
Angiotensin 1 Converting Enzyme (Fetal Brain) is ACE promoter hypomethylated?

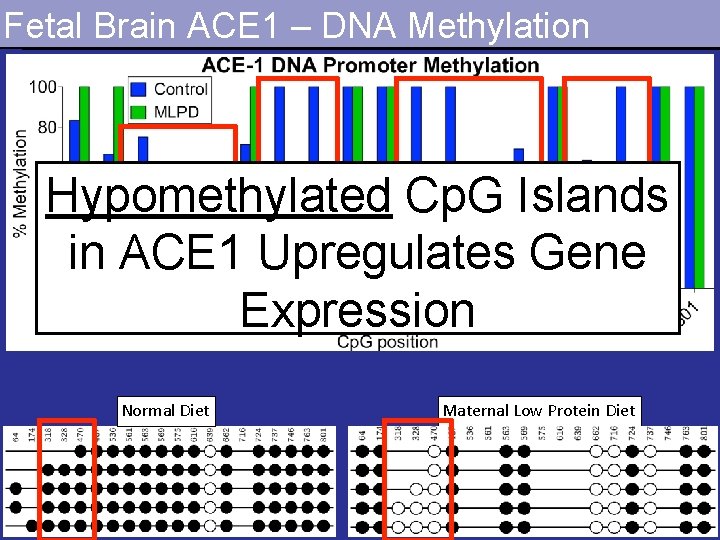
Fetal Brain ACE 1 – DNA Methylation Hypomethylated Cp. G Islands in ACE 1 Upregulates Gene Expression Normal Diet Maternal Low Protein Diet

Question to Consider • The programming occurs during fetal life and m. RNA are increased during fetal life, then why does blood pressure remains normal until adulthood?

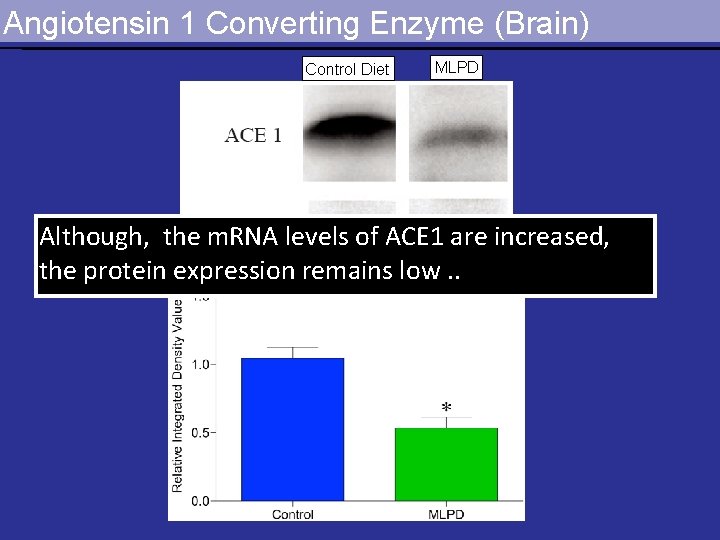
Angiotensin 1 Converting Enzyme (Brain) Control Diet MLPD Although, the m. RNA levels of ACE 1 are increased, the protein expression remains low. .

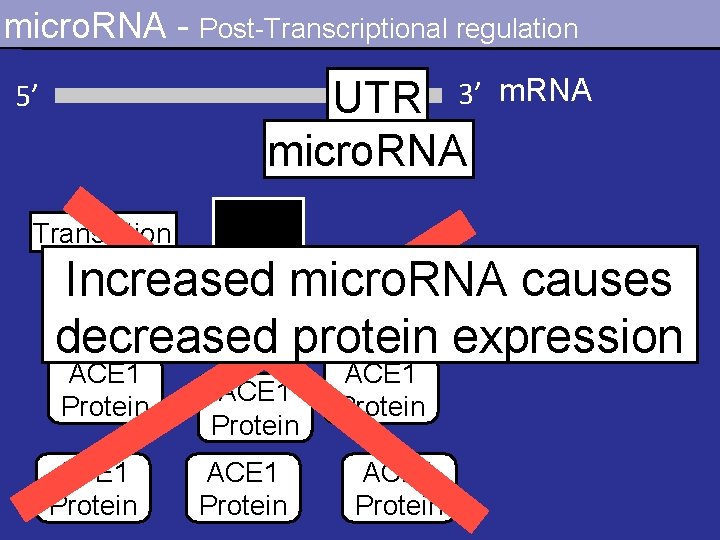
micro. RNA - Post-Transcriptional regulation UTR 3’ micro. RNA 5’ m. RNA Translation Increased micro. RNA causes decreased protein expression ACE 1 Protein ACE 1 Protein

Micro RNAs • identified mi. RNA putatively regulating ACE translation • mmu-mir-27 a and mmu-mir-27 b

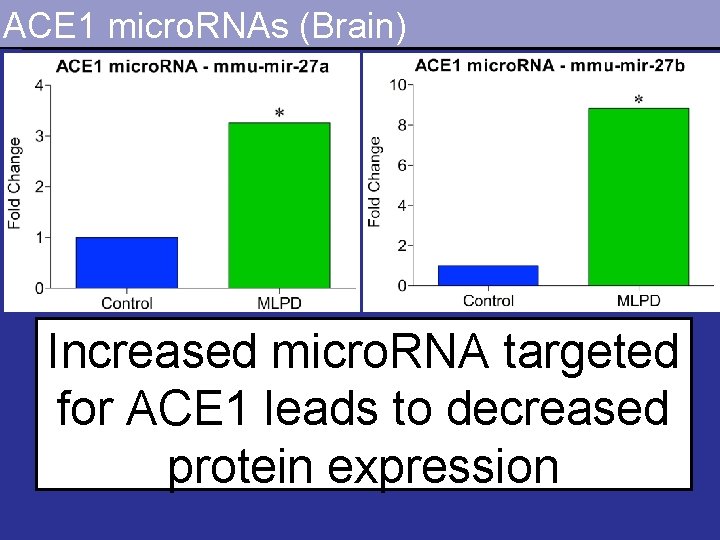
ACE 1 micro. RNAs (Brain) Increased micro. RNA targeted for ACE 1 leads to decreased protein expression

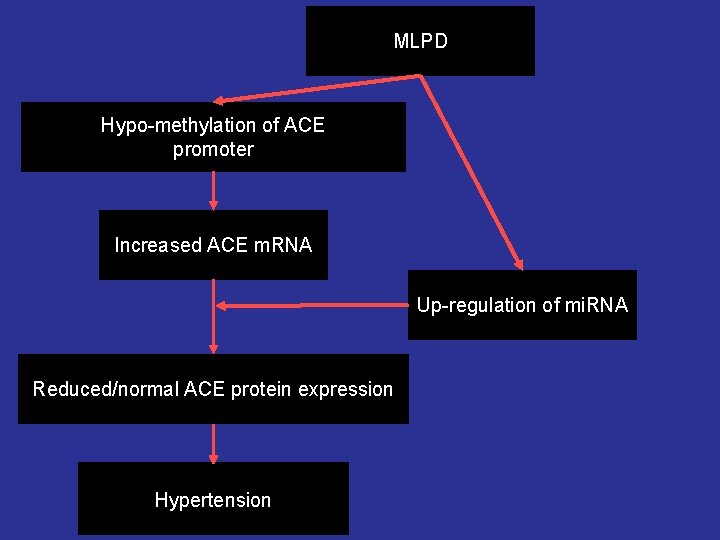
MLPD Hypo-methylation of ACE promoter Increased ACE m. RNA Up-regulation of mi. RNA Reduced/normal ACE protein expression Programming of Hypertension

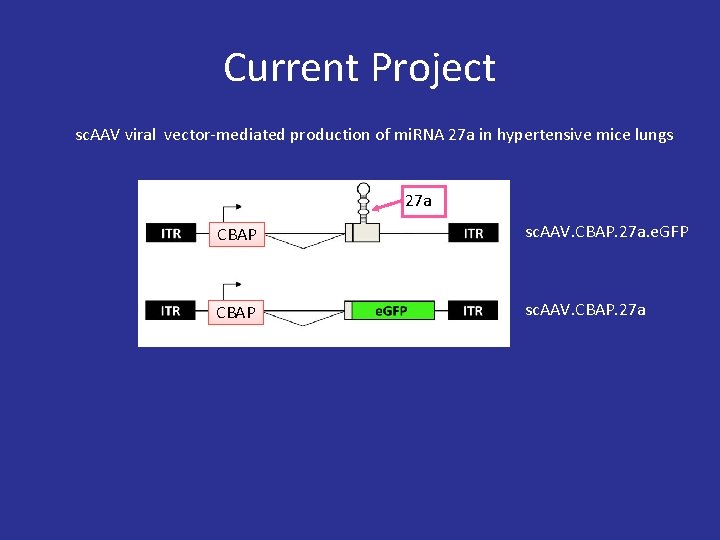
Current Project sc. AAV viral vector-mediated production of mi. RNA 27 a in hypertensive mice lungs 27 a CBAP sc. AAV. CBAP. 27 a. e. GFP CBAP sc. AAV. CBAP. 27 a

Conclusion Maternal Stress during Pregnancy Epigenetic Changes in Gene Products Physiological/Pathological Changes

Acknowledgements – Lawrence D. Longo, MD – Ciprian Georghe, MD, Ph. D – Dipali Goyal, BS – Andre Obenaus, Ph. D – Nina Chu, BS – Andrew Gallfy, BS – E Eun Jang, MD – Toni-An Wright, B. S.

Thank You