ANTENATAL AND NEWBORN SCREENING RESOURCE Click image to
ANTENATAL AND NEWBORN SCREENING RESOURCE Click image to watch animated version. Internet access is required. 3 December 2018 Click to start More information and resources for each programme: www. gov. uk/guidance/nhs-population-screening-education-and-training For the latest news, subscribe to the blog: phescreening. blog. gov. uk © Public Health England
MAIN MENU Use the buttons below to select each chapter. What is screening? Personal informed choice Infectious diseases in pregnancy Newborn blood spot Fetal anomaly Newborn and infant physical examination Sickle cell and thalassaemia Newborn hearing Main menu Chapter < > This button will bring you back to this page. This button will take you back to the start of the chapter.
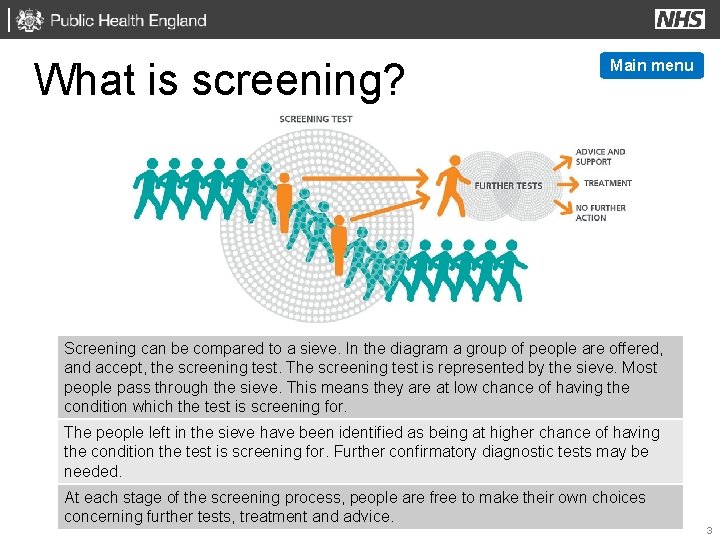
What is screening? Main menu Screening can be compared to a sieve. In the diagram a group of people are offered, and accept, the screening test. The screening test is represented by the sieve. Most people pass through the sieve. This means they are at low chance of having the condition which the test is screening for. The people left in the sieve have been identified as being at higher chance of having the condition the test is screening for. Further confirmatory diagnostic tests may be needed. At each stage of the screening process, people are free to make their own choices concerning further tests, treatment and advice. 3

Personal informed choice Main menu There are 6 antenatal and newborn screening programmes. It is very important that women and their partners have all the information they need about each programme to enable them to make a personal informed choice. 1. Determine knowledge of the conditions being screened for. 2. Check they have read ‘Screening tests for you and your baby’. 3. Explain that screening is optional. 4. Check understanding of screening versus diagnosis. 5. Describe screening test offered, how it is done, timing of test. 6. Explain the likelihood and meaning of increased/higher or lower chance screening results. 7. Explain and agree how and when results will be given. 8. Explain that confirmatory/repeat testing is occasionally required. 9. Discuss the meaning and implications of the possible test result. 10. Discuss the possibility that screening can provide information about other conditions. 11. Document decision. Click on image to download leaflet in English and other languages. 4
Infectious diseases in pregnancy screening (IDPS) Main menu Screening is offered and recommended for syphilis, human immunodeficiency virus (HIV) and hepatitis B (HBV) to significantly reduce the risk of vertical transmission of infection (mother to child). Click on the buttons for more information. Syphilis HIV Hepatitis B (HBV) If the woman declines screening for any of the 3 infections she should be referred as soon as possible to the screening team who will re-offer screening, ideally before 20 weeks gestation or within 2 weeks if ≥ 24 weeks gestation. A negative result means a woman is ‘negative now’ and does not confer protection throughout pregnancy. Advise women about protecting themselves from infection and to report symptoms as soon as possible. Repeat tests are recommended if the woman changes partner/s, injects drugs, is a sex worker, has an infected partner/s, has a bisexual partner/s or if she or her partner/s are diagnosed with another sexually transmitted infection (STI). 5

Syphilis < > Chapter Syphilis is transmitted through sexual contact. It is caused by the bacterium Treponema Pallidum. If untreated, it can result in poor pregnancy and neonatal outcomes including late miscarriage and stillbirth and can affect children’s teeth, bones vision and hearing. Screening must be offered and recommended early in pregnancy regardless of history of previous infections or treatment. Screening identifies women with a current infection and early treatment with antibiotic injections greatly reduces the risk of vertical transmission. Screen positive women should be seen by the screening team within 10 days of their result for counselling and referral to specialist services. Advise women about protecting themselves from infection and to report symptoms as soon as possible. They can request retesting at any time if they think they are at risk of infection. Babies born to women treated for syphilis in pregnancy should be followed up by paediatricians at birth in line with the British Association for Sexual Health and HIV – BASHH guidelines. 6
HIV < > Chapter HIV is a blood borne virus and is transmitted through sexual contact, contaminated blood (such as needle sharing), vertical transmission of infection (mother to child) during pregnancy, delivery and through breastfeeding. Transmission from mother to child can be greatly reduced to less than 0. 5% with a combination of interventions that include adherence to anti-retroviral therapy (ART), appropriate management of pregnancy and delivery and avoidance of breastfeeding. Screen positive and known positive women should be seen by the screening team within 10 days of their result for specialist multidisciplinary services treatment in line with British HIV Association (BHIVA) guidelines. Repeat tests are recommended if the woman changes partner, injects drugs, is a sex worker, has a bisexual partner or is diagnosed with a sexually transmitted infection (STI). A negative result means a woman is negative ‘now’ and does not confer protection throughout pregnancy. 7
Hepatitis B (HBV) < > Chapter Infection by HBV can result in an infectious inflammatory illness of the liver. Chronic infection can lead to cirrhosis and liver cancer. Screening identifies women who are infected and whose babies are at risk of contracting HBV is present in all body fluids. Transmission occurs through sexual contact, contact with blood, needle sharing with an infected person or from vertical transmission of infection (mother to child). All women who are known positive or newly diagnosed with hepatitis B should be seen by the screening team within 10 days of their result for counselling and referral to specialist services. The mode of delivery and breast feeding do not affect transmission if the baby receives a full schedule of vaccinations in their first year. 8

Hepatitis B (HBV) A full course of vaccination for babies born to women with hepatitis B during their first year of life greatly reduces the risk of vertical transmission of HBV infection. < Chapter Click on image to download leaflet. The schedule for at risk babies is: 1. An initial single hepatitis B vaccine within 24 hours of birth +/- hepatitis B immunoglobulin (HBIG) if the mother has higher infectivity. 2. A second single dose at 4 weeks. 3. The next three doses are given as a combined vaccine at 8, 12 and 16 weeks as part of routine childhood immunisation. 4. A booster single dose is given at 12 months, with a blood test to check for the child’s infection status (HBs. Ag). Complete the ‘red book’ hepatitis B page and inform the child health record department, GP and health visitor of the mother’s HBV status and need for completion of the childhood vaccination schedule. Family members and siblings require follow-up by the GP. 9

Fetal anomaly screening programme (FASP) Main menu FASP offers screening for all eligible pregnant women to check the baby for structural fetal anomalies, Down’s syndrome, Edwards’ syndrome and Patau’s syndrome. Click on the buttons for more information. Eligibility criteria Down’s syndrome (T 21) Edwards’ syndrome (T 18) and Patau’s syndrome (T 13) Screening choices Nuchal translucency (NT) Diagnostic testing 18+0 to 20+6 week ultrasound scan Conditions screened for 10
Eligibility criteria < > Chapter The eligibility criteria for entry into the first trimester screening programme is a fetal crown rump length (CRL) measurement between 45. 0 mm and 84. 0 mm (which equates to 11+2 days to 14+1 weeks of pregnancy). Women who book too late for first trimester testing or when a Nuchal Translucency (NT) measurement cannot be obtained in the first trimester can be offered second trimester quadruple screening for Down’s syndrome only from 14+2 until 20+0 weeks of pregnancy. 11

Down’s syndrome (T 21) < > Chapter Chromosomal condition – presence of 3 copies of chromosome 21 (trisomy 21). Down’s syndrome occurs in 10 per 10, 000 live births. Babies with Down’s syndrome are born to mothers of all ages but the chance of having a baby with the condition increases with maternal age. Changes can occur in the sperm or egg cells, which can lead to the baby having an extra chromosome. It does not usually run in families, with less than 5% of cases being hereditary. People with Down’s syndrome have learning difficulties but there is a wide spectrum just as there is a wide spectrum of abilities in the general population. Some health problems are more common in people with Down’s syndrome. These include heart conditions and problems with hearing and vision. Many health problems can be treated but unfortunately around 5% of babies will not live past their first birthday. For babies without serious health problems, survival is similar to that of other children and most people with Down’s syndrome will live into their 60 s or longer. Screening for Down’s syndrome should be offered to all pregnant women who book by 20+0 weeks of pregnancy. Screening cannot identify what level of disability the baby will have. 12
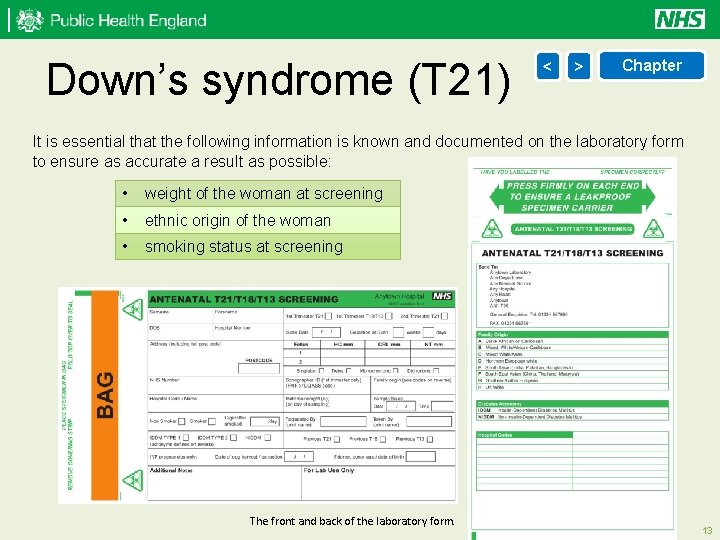
Down’s syndrome (T 21) < > Chapter It is essential that the following information is known and documented on the laboratory form to ensure as accurate a result as possible: • weight of the woman at screening • ethnic origin of the woman • smoking status at screening The front and back of the laboratory form. 13

Edwards’ syndrome (T 18) and Patau’s syndrome (T 13) < > Chapter Babies with Edwards’ syndrome and Patau’s syndrome are born to mothers of all ages, but the chance of having a baby with one of the conditions increases with maternal age. Babies with Edwards’ syndrome have an extra copy of chromosome 18 in all or some cells. Edwards’ syndrome occurs in 3 per 10, 000 live births. All babies born with Edwards’ syndrome and Patau’s syndrome will have a learning disability and a wide range of physical challenges, which can be extremely serious. They may have problems with their heart, respiratory system, kidneys and digestive system. Around half of babies with Patau’s syndrome will also have a cleft lip and palate. Babies with Patau’s syndrome have an extra copy of chromosome 13 in all or some cells. Patau’s syndrome occurs in 2 per 10, 000 live births. Sadly the survival rates are low and of those babies born alive only around 10% live past their first birthday. Some babies may survive to adulthood but this is rare. Babies with Edwards’ syndrome and Patau’s syndrome will have a low birthweight. Despite their difficulties children can slowly make progress in their development. Older children with either condition would need to attend a specialist school. 14

Screening choices and chances of an affected pregnancy < > Chapter The policy is to offer screening to assess the chance of the baby being born with Down’s syndrome and/or Edwards’ syndrome and Patau’s syndrome. The test of choice for both singleton and twin pregnancies is first trimester combined screening, which combines elements of an ultrasound scan and a maternal blood test. Women who book too late for first trimester combined testing or when a nuchal translucency (NT) measurement cannot be obtained (despite twice on the couch) in the first trimester, can be offered a screening test for Down’s syndrome in the second trimester. Women can choose: T 21 T 18/T 13 X To have screening for Down’s syndrome. X To have screening for Edwards’ syndrome/Patau’s syndrome. To have screening for Down’s syndrome and Edwards’s syndrome/Patau’s syndrome. X X Not to have screening. Document accurately and clearly the woman’s choices on the laboratory request form and in the pregnancy records. 15

Screening choices and chances of an affected pregnancy < > Chapter A woman with a screening chance result of 1 in 150 or greater (1 in 2 to 1 in 150) of having a pregnancy affected by Down’s syndrome or, Edwards’ syndrome/Patau’s syndrome in the first trimester is considered to be in the ‘higher chance’ group and offered an invasive test. For women having screening using the combined test, depending on their screening choice, up to 2 chances will be reported: • a risk for T 21 and a combined chance for T 18/T 13 • a risk for T 21 only or a combined chance for T 18/T 13 only It is important to note that regardless of choice of screening, the diagnostic test will give results for all 3 conditions: Down’s syndrome (T 21), Edwards’ syndrome (T 18) and Patau’s syndrome (T 13). Women must be made aware of this at the initial screening discussion and before making a choice as to which screening they wish to accept. 16
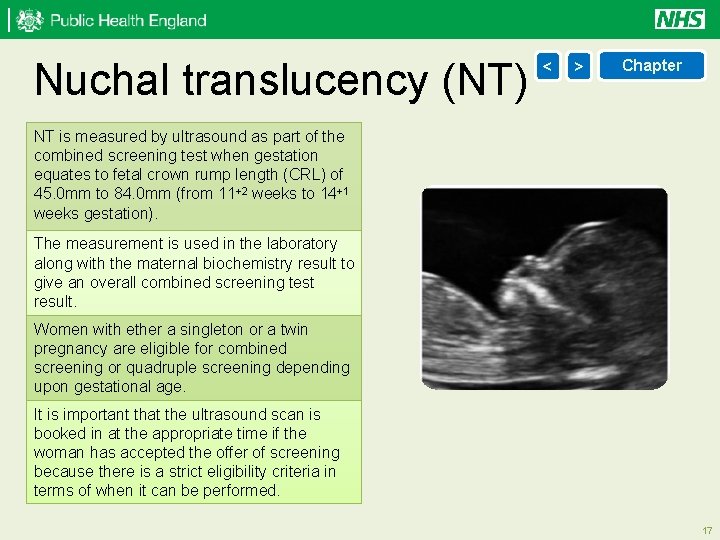
Nuchal translucency (NT) < > Chapter NT is measured by ultrasound as part of the combined screening test when gestation equates to fetal crown rump length (CRL) of 45. 0 mm to 84. 0 mm (from 11+2 weeks to 14+1 weeks gestation). The measurement is used in the laboratory along with the maternal biochemistry result to give an overall combined screening test result. Women with ether a singleton or a twin pregnancy are eligible for combined screening or quadruple screening depending upon gestational age. It is important that the ultrasound scan is booked in at the appropriate time if the woman has accepted the offer of screening because there is a strict eligibility criteria in terms of when it can be performed. 17
Diagnostic testing < > Chapter Chorionic Villus Sampling (CVS) is an abdominal, or sometimes cervical, invasive procedure performed under continuous ultrasound guidance. CVS can be performed from 10 weeks, but is usually only performed from 11 weeks of pregnancy, to obtain a sample of placental tissue for chromosomal or genetic analysis. Up to 1 out of every 100 women who have a CVS will miscarry. View illustration of CVS Amniocentesis is an invasive abdominal procedure performed under continuous ultrasound guidance. Amniocentesis is performed from about 15 completed weeks onwards to obtain a sample of amniotic fluid surrounding the fetus. The sample of amniotic fluid is sent for chromosomal or genetic analysis. Up to 1 out of every 100 women who have an amniocentesis will miscarry. View illustration of amniocentesis 18
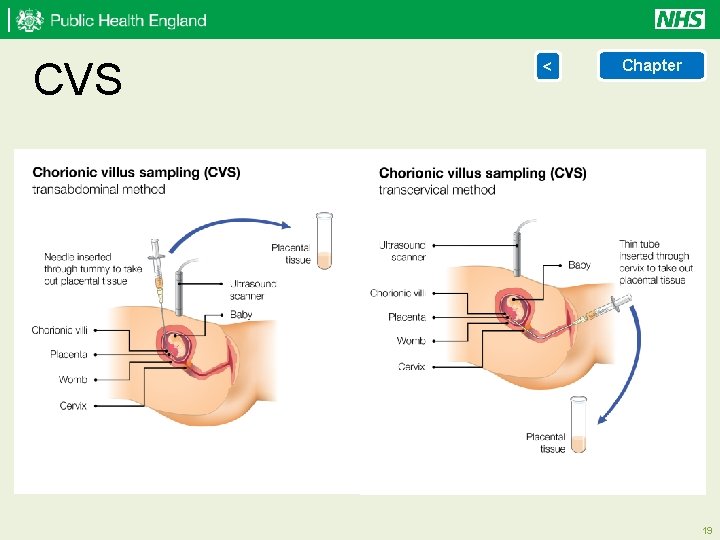
CVS < Chapter 19
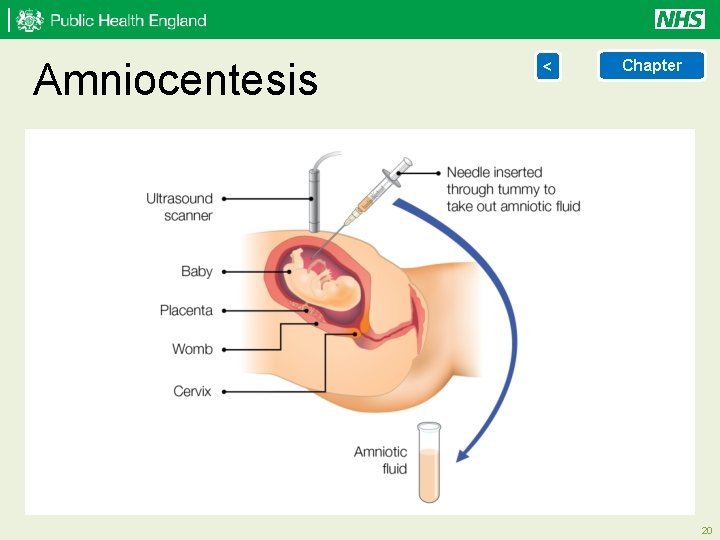
Amniocentesis < Chapter 20

18+0 to 20+6 week < > Chapter ultrasound scan The fetal anomaly programme recommends the offer of a mid-pregnancy scan which is undertaken between 18+0 to 20+6 weeks of pregnancy to screen for major 11 fetal anomalies. Women who wish to have a fetal anomaly ultrasound scan, but do not wish to be informed if abnormalities are found, should be advised that all significant findings seen on the scan will be reported and therefore they should consider not having fetal anomaly ultrasound screening. The limitations of the test must be made clear. It will not detect all abnormalities. If the first examination is sub-optimal and the sonographer is suspicious of a possible fetal anomaly, a second opinion should be sought and a referral made for further investigation of the anomaly suspected. A single repeat scan must be offered and completed by 23+0 weeks gestation in cases where the image quality of the first examination is compromised by: • increased maternal body mass index (BMI) • uterine fibroids • abdominal scarring • sub-optimal fetal position 21
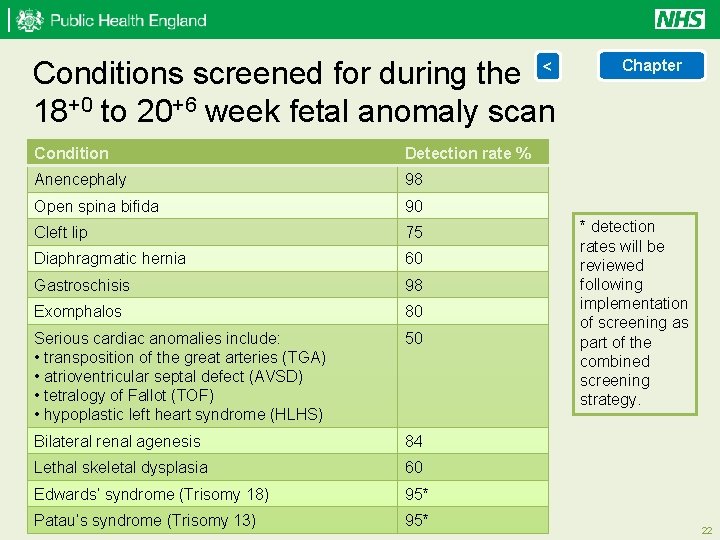
Conditions screened for during the < 18+0 to 20+6 week fetal anomaly scan Condition Detection rate % Anencephaly 98 Open spina bifida 90 Cleft lip 75 Diaphragmatic hernia 60 Gastroschisis 98 Exomphalos 80 Serious cardiac anomalies include: • transposition of the great arteries (TGA) • atrioventricular septal defect (AVSD) • tetralogy of Fallot (TOF) • hypoplastic left heart syndrome (HLHS) 50 Bilateral renal agenesis 84 Lethal skeletal dysplasia 60 Edwards’ syndrome (Trisomy 18) 95* Patau’s syndrome (Trisomy 13) 95* Chapter * detection rates will be reviewed following implementation of screening as part of the combined screening strategy. 22
Sickle cell and thalassaemia (SCT) screening programme Main menu Antenatal screening identifies women who are carriers or affected by sickle cell or thalassaemia. If a woman has a positive screening test the baby’s biological father is offered screening to assess risk to the baby. Women and couples at risk of having an affected baby are given information, advice and counselling to help them decide whether to have prenatal diagnosis (PND). Click on the buttons for more information. Sickle cell disease Thalassemia conditions Prenatal diagnosis (PND) Genetic inheritance What will screening in pregnancy detect? The test Family Origin Questionnaire (FOQ) 23

Sickle cell disease (SCD) < > Chapter SCD is an inherited condition which affects the quality of haemoglobin. In an individual with SCD the red blood cell becomes misshapen and rigid, resembling the shape of a sickle, when the haemoglobin is de-oxygenated. This process is called sickling and causes a wide range of clinical complications, including chronic anaemia, jaundice, painful crises, organ damage, infections and stroke in children and adults. Inheritance of an altered gene from both parents results in the disease and inheritance of only one altered gene results in a healthy carrier, sometimes called a trait. The likelihood of a person being a carrier depends on ancestry. SCD is most common in West Africa and India. Antenatal screening identifies approximately 10 per 1, 000 pregnant women as carriers or affected by the disease. Babies with SCD need prophylactic antibiotics and regular immunisations to prevent infections. Parents need specialist support to understand the condition and prevent or minimise the effects of a sickle cell crisis. Babies need regular specialist outpatient reviews and easy, direct access to specialist medical care when unwell. All pregnant women with SCD should receive specialist obstetric and haematological care 24

Thalassaemia conditions < > Chapter Alpha and beta thalassaemia major are inherited conditions that affect the quantity of haemoglobin. This results in severe, life-threatening anaemia. Alpha thalassaemia major is incompatible with life. There are other less serious thalassaemia conditions which can be detected by the screening programme. Inheritance of an altered gene from both parents results in a condition and inheritance of only one altered gene results in a healthy carrier. The likelihood of a person being a carrier depends on ancestry. Thalassaemia major is more common in Asia and Mediterranean countries. Approximately 6 per 1, 000 pregnant women are identified as carriers by the antenatal programme. People with beta thalassaemia major usually require regular blood transfusions every 3 to 4 weeks to sustain life and iron chelation therapy to prevent further illness. All pregnant women with thalassaemia should receive specialist obstetric and haematological care. 25
Prenatal diagnosis (PND) < > Chapter Screening for sickle cell and thalassaemia should be offered when a woman first presents in pregnancy so that PND can be offered by 12 weeks gestation and PND performed by 12+6 weeks gestation. Direct access to counselling and offer of PND should be available to people with a known risk of having a baby with a major haemoglobin disorder. Couples and women at risk of having a baby with SCD or thalassaemia major are defined as: • both biological parents being carriers of a significant haemoglobin variant • the woman is a carrier of a significant haemoglobin gene variant and the screening status of the biological father is unknown 26
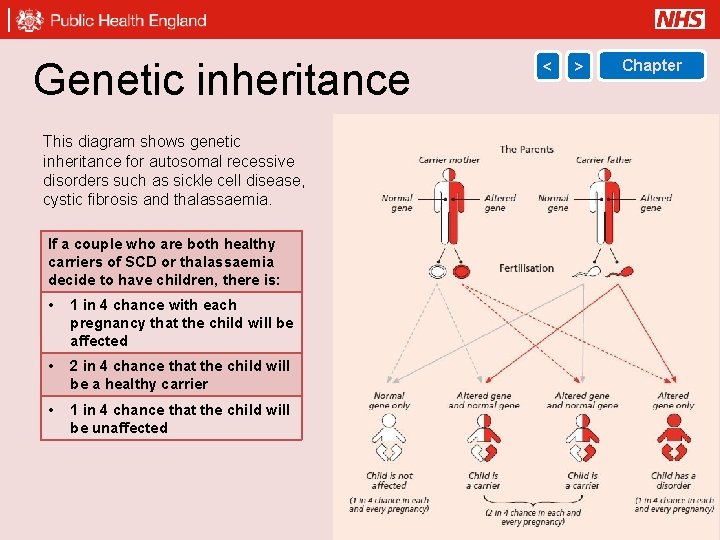
Genetic inheritance < > Chapter This diagram shows genetic inheritance for autosomal recessive disorders such as sickle cell disease, cystic fibrosis and thalassaemia. If a couple who are both healthy carriers of SCD or thalassaemia decide to have children, there is: • 1 in 4 chance with each pregnancy that the child will be affected • 2 in 4 chance that the child will be a healthy carrier • 1 in 4 chance that the child will be unaffected 27
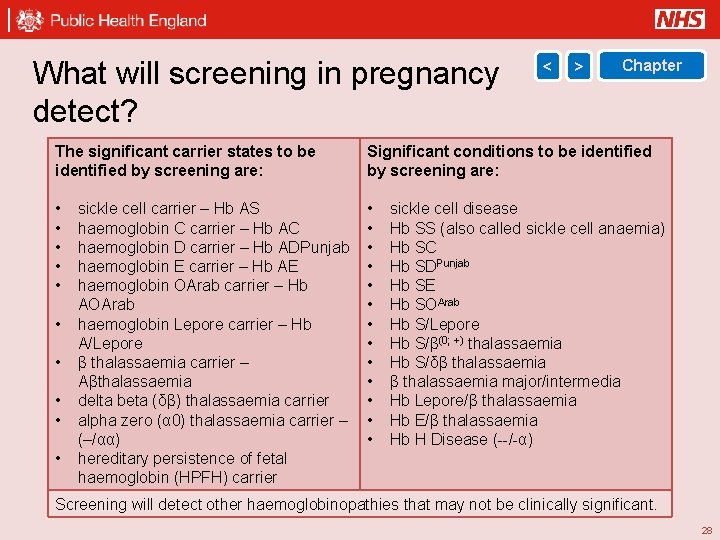
What will screening in pregnancy detect? < > Chapter The significant carrier states to be identified by screening are: Significant conditions to be identified by screening are: • • • • • • sickle cell carrier – Hb AS haemoglobin C carrier – Hb AC haemoglobin D carrier – Hb ADPunjab haemoglobin E carrier – Hb AE haemoglobin OArab carrier – Hb AOArab haemoglobin Lepore carrier – Hb A/Lepore β thalassaemia carrier – Aβthalassaemia delta beta (δβ) thalassaemia carrier alpha zero (α 0) thalassaemia carrier – (–/αα) hereditary persistence of fetal haemoglobin (HPFH) carrier sickle cell disease Hb SS (also called sickle cell anaemia) Hb SC Hb SDPunjab Hb SE Hb SOArab Hb S/Lepore Hb S/β(0; +) thalassaemia Hb S/δβ thalassaemia major/intermedia Hb Lepore/β thalassaemia Hb E/β thalassaemia Hb H Disease (--/-α) Screening will detect other haemoglobinopathies that may not be clinically significant. 28
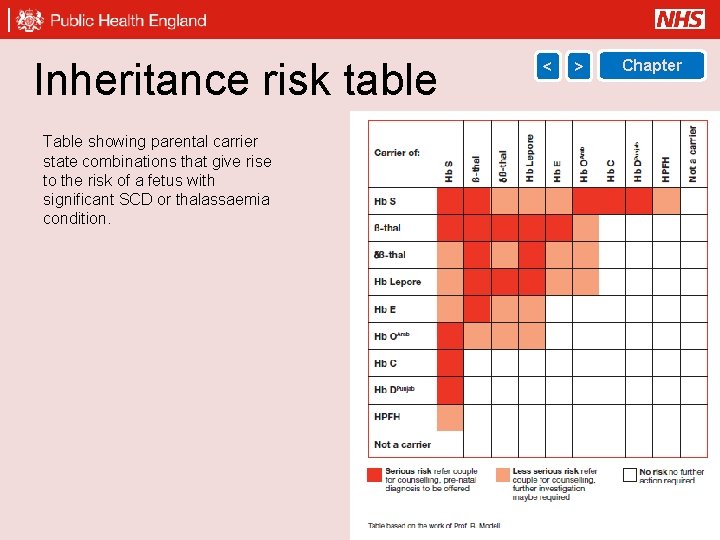
Inheritance risk table < > Chapter Table showing parental carrier state combinations that give rise to the risk of a fetus with significant SCD or thalassaemia condition. 29

The test < > Chapter There are 2 approaches based on the known prevalence of the conditions across the country. Low prevalence trusts The full blood count will screen all women for thalassaemia. The family origin questionnaire (FOQ) is used as an initial screening tool to identify women, or the baby’s biological father, at high risk of being a carrier for sickle cell, and other haemoglobin variants. Where either parent falls into a high risk group, a screening blood test for haemoglobin variants must be offered to the woman. High prevalence trusts All women must be offered a screening blood test for sickle cell, thalassaemia and other haemoglobin variants, irrespective of family origins. The FOQ is relevant in the interpretation of red blood cell indices, particularly in screening groups at high risk of alpha zero Thalassaemia. 30
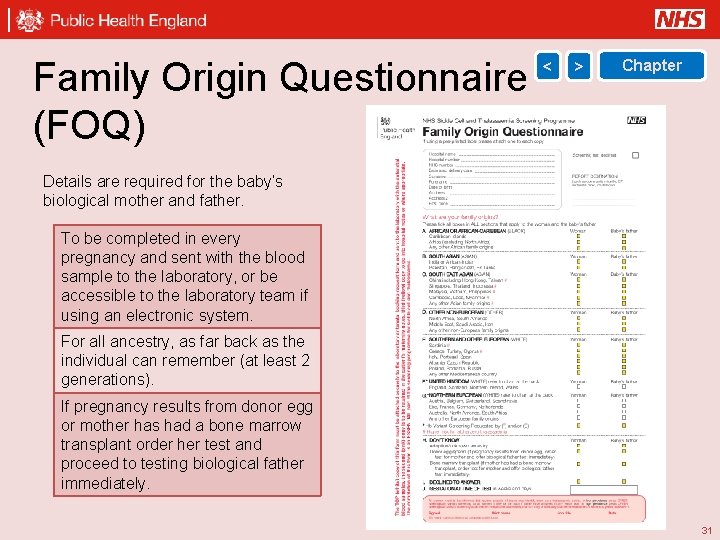
Family Origin Questionnaire (FOQ) < > Chapter Details are required for the baby’s biological mother and father. To be completed in every pregnancy and sent with the blood sample to the laboratory, or be accessible to the laboratory team if using an electronic system. For all ancestry, as far back as the individual can remember (at least 2 generations). If pregnancy results from donor egg or mother has had a bone marrow transplant order her test and proceed to testing biological father immediately. 31
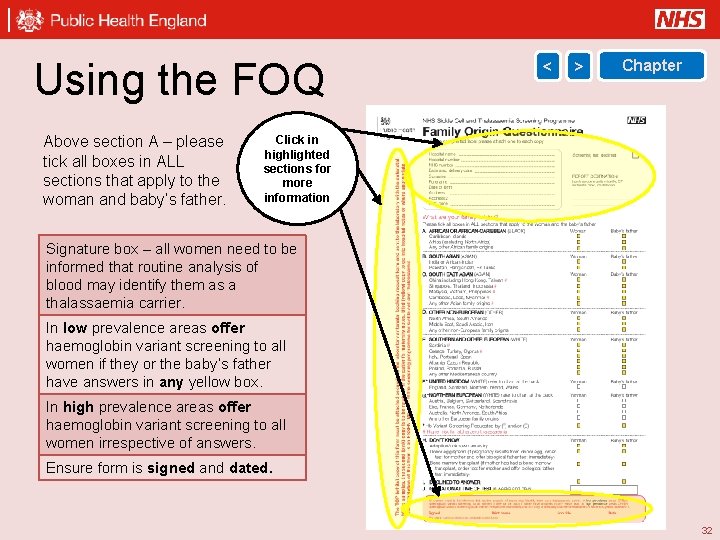
Using the FOQ Above section A – please tick all boxes in ALL sections that apply to the woman and baby’s father. < > Chapter Click in highlighted sections for more information Signature box – all women need to be informed that routine analysis of blood may identify them as a thalassaemia carrier. In low prevalence areas offer haemoglobin variant screening to all women if they or the baby’s father have answers in any yellow box. In high prevalence areas offer haemoglobin variant screening to all women irrespective of answers. Ensure form is signed and dated. 32
Using the FOQ < > Chapter Above section A Please tick all boxes in all sections that apply to the woman and baby’s father. 33
Using the FOQ < > Chapter Top copy (white) must be attached securely to the laboratory antenatal booking request form and sent to the laboratory with the blood samples. Second copy (pink) is to be retained in the patients maternity notes. Third copy (yellow) copy to go into hospital notes where appropriate. 34
Using the FOQ < Chapter Signature box – all women need to be informed that routine analysis of blood may identify them as a thalassaemia carrier. In low prevalence areas offer haemoglobin variant screening to all women if they or the baby’s father have answers in any yellow box. In high prevalence areas offer haemoglobin variant screening to all women irrespective of answers. Ensure form is signed and dated. 35

Newborn hearing screening programme (NHSP) > Main menu NHSP aims to identify moderate, severe and profound deafness and hearing impairment in newborn babies. Early identification of hearing impairment gives children a better chance of developing speech and language skills, and of making the most of social and emotional interaction from an early age. All babies born in England should be offered a hearing screen by 4 to 5 weeks of age. Screening should ideally be offered within days of birth but must be offered to all babies up to 3 months of (corrected) age. 1 to 2 babies in every 1, 000 are born with permanent hearing loss in one or both ears, increasing to 1 in 100 babies who have spent more than 48 hours in a neonatal intensive care. Moderate to severe hearing loss can significantly affect a baby's development. The hearing screening test is offered either: • in hospital prior to discharge • at home at about 5 to 12 days of age • as an outpatient 36
Newborn hearing tests < Chapter Screening usually takes place while the baby is settled or asleep. Two types of test are used: 1. Automated oto-acoustic emissions (AOAE): A soft clicking sound is played into the baby’s outer ear. When a hearing ear receives sound, the cochlea in the inner ear produces a response and this is collected by the microphone in the screening equipment. 2. Automated auditory brainstem responses (AABR): offered as a second test if no clear response is obtained from AOAE. Checks for responses from the baby’s auditory nerve. Soft clicking sounds played into the baby’s outer ear via soft baby ear muffs, trigger electrical impulses, which are collected into the screening equipment by 3 small sensors placed on the baby’s high forehead, back of shoulder and nape of the neck. If no clear response is obtained from AABR, babies are referred for an audiological assessment and should be seen within 4 weeks of screen completion. Parents should be encouraged to bring their baby for this appointment, so that any hearing loss can be detected early. 37
Newborn blood spot (NBS) screening programme Main menu NBS screening enables early identification, referral and treatment of babies with 9 rare but serious conditions. The programme helps to improve their health and prevent severe disability or even death. Click on the buttons for more information. Taking a sample Sample quality Storage of cards Special circumstances Sickle cell disease (SCD) Congenital hypothyroidism (CHT) Cystic fibrosis (CF) Phenylketonuria (PKU) MCADD Maple syrup urine disease (MSUD) Isovaleric acidaemia (IVA) Glutaric aciduria type 1 (GA 1) Homocystinuria (HCU) 38

Taking a sample < > Chapter Before collecting the blood spot, please check: Expiry date NHS number Date of Birth Sample date Complete the blood spot card and ask parents to check that the details on the card are correct. 39
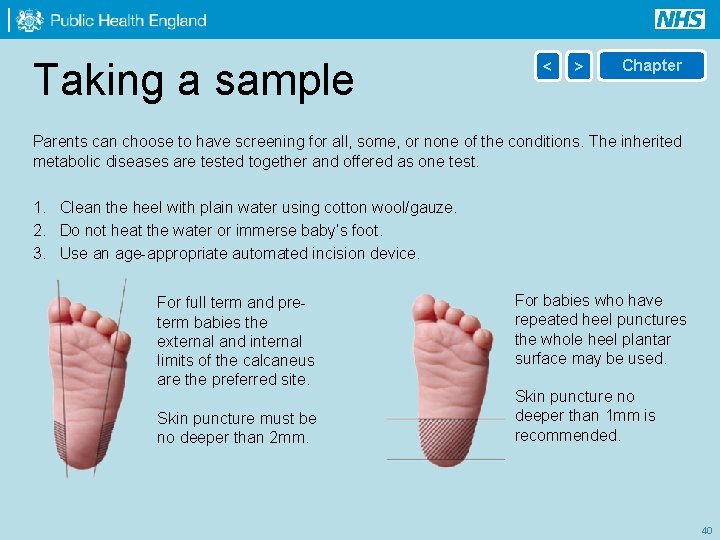
Taking a sample < > Chapter Parents can choose to have screening for all, some, or none of the conditions. The inherited metabolic diseases are tested together and offered as one test. 1. Clean the heel with plain water using cotton wool/gauze. 2. Do not heat the water or immerse baby’s foot. 3. Use an age-appropriate automated incision device. For full term and preterm babies the external and internal limits of the calcaneus are the preferred site. Skin puncture must be no deeper than 2 mm. For babies who have repeated heel punctures the whole heel plantar surface may be used. Skin puncture no deeper than 1 mm is recommended. 40

Taking a sample < > Chapter Puncture the heel and wait for the blood to flow and a hanging drop to form. Do: • drop one spot of blood onto each circle • let blood seep through from front to back • air-dry the blood spots before posting Do not: • allow the heel to make contact with the card • squeeze the baby’s heel, allow blood to flow naturally • compress the blood spots Despatch on same day or within 24 hours. Inform parents that they will receive the results within 6 weeks or sooner if results are positive. If parents do not receive results they should ask their health visitor. 41
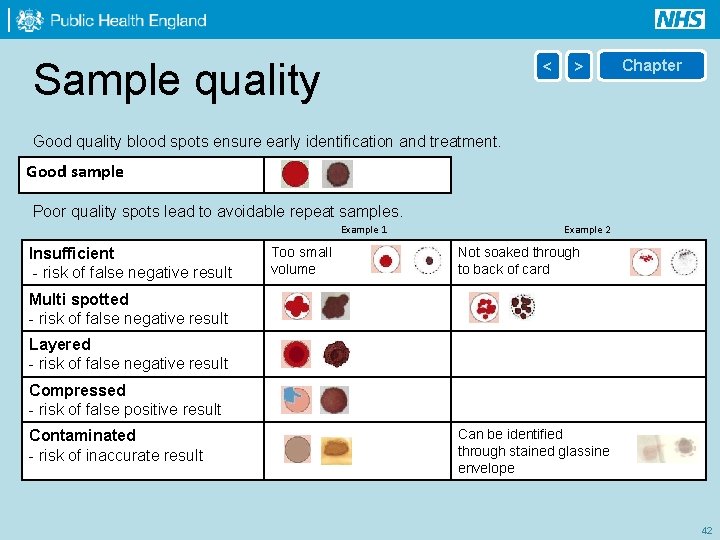
Sample quality < > Chapter Good quality blood spots ensure early identification and treatment. Good sample Poor quality spots lead to avoidable repeat samples. Example 1 Insufficient - risk of false negative result Too small volume Example 2 Not soaked through to back of card Multi spotted - risk of false negative result Layered - risk of false negative result Compressed - risk of false positive result Contaminated - risk of inaccurate result Can be identified through stained glassine envelope 42
Sample quality < > Chapter Click image to watch video. Internet access is required. 43
Storage of cards Residual blood spots are dried blood spots that are ‘left over’ after screening. < Chapter > book me hand Program Laboratories store blood spot cards after screening. Stored cards have several potential uses including research. Parents must be asked for their consent to future contact about research that could identify their baby. Examples of the potential uses are on NHS. UK. Click on the image to view. Code of for practice blood residual spots Click on images to view. 44

Special circumstances < > Chapter If a baby is admitted to a neonatal unit a single circle blood spot sample should be taken on admission/prior to blood transfusion to screen for sickle cell disease. Mark the blood spot card ‘pre-transfusion’ and send to the laboratory with the routine day 5 sample. 3 clear days are needed between a blood transfusion and the routine day 5 sample. If a baby is having multiple transfusions take the sample by day 8 at the latest. A post transfusion repeat will be needed after 3 clear days. Babies born at less than 32+0 weeks need a repeat sample for CHT taken at 28 days of age or discharge home, whichever is sooner. If screening is not complete make parents aware and ensure that this information is communicated when care is transferred. 45
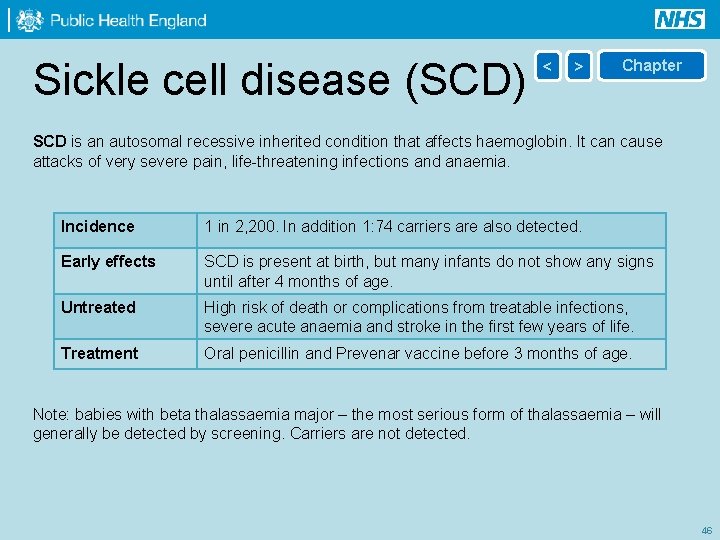
Sickle cell disease (SCD) < > Chapter SCD is an autosomal recessive inherited condition that affects haemoglobin. It can cause attacks of very severe pain, life-threatening infections and anaemia. Incidence 1 in 2, 200. In addition 1: 74 carriers are also detected. Early effects SCD is present at birth, but many infants do not show any signs until after 4 months of age. Untreated High risk of death or complications from treatable infections, severe acute anaemia and stroke in the first few years of life. Treatment Oral penicillin and Prevenar vaccine before 3 months of age. Note: babies with beta thalassaemia major – the most serious form of thalassaemia – will generally be detected by screening. Carriers are not detected. 46
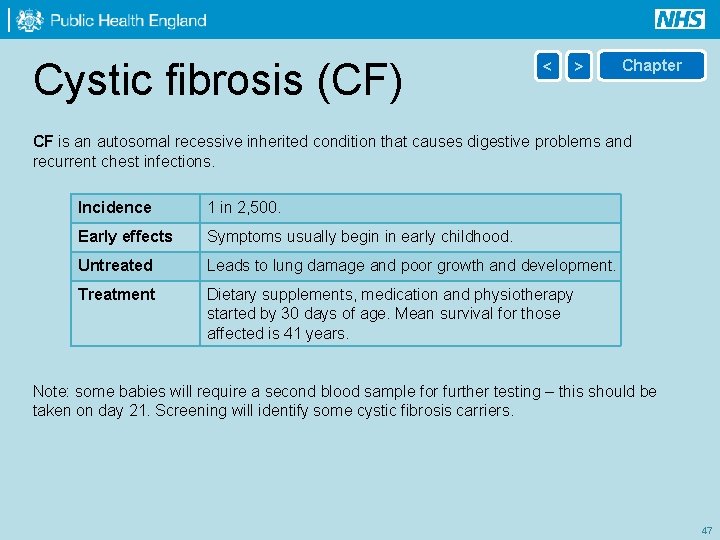
Cystic fibrosis (CF) < > Chapter CF is an autosomal recessive inherited condition that causes digestive problems and recurrent chest infections. Incidence 1 in 2, 500. Early effects Symptoms usually begin in early childhood. Untreated Leads to lung damage and poor growth and development. Treatment Dietary supplements, medication and physiotherapy started by 30 days of age. Mean survival for those affected is 41 years. Note: some babies will require a second blood sample for further testing – this should be taken on day 21. Screening will identify some cystic fibrosis carriers. 47
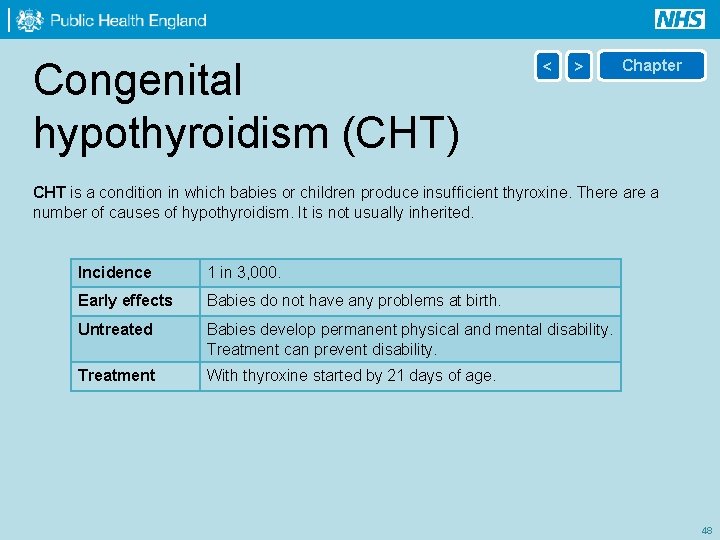
Congenital hypothyroidism (CHT) < > Chapter CHT is a condition in which babies or children produce insufficient thyroxine. There a number of causes of hypothyroidism. It is not usually inherited. Incidence 1 in 3, 000. Early effects Babies do not have any problems at birth. Untreated Babies develop permanent physical and mental disability. Treatment can prevent disability. Treatment With thyroxine started by 21 days of age. 48
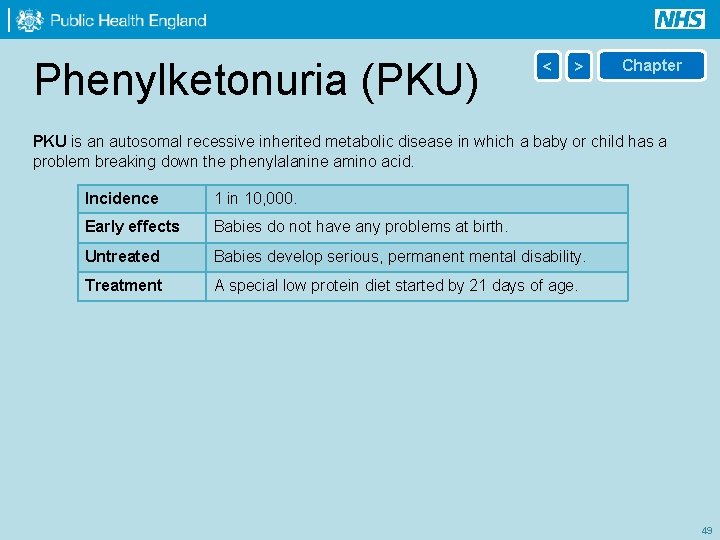
Phenylketonuria (PKU) < > Chapter PKU is an autosomal recessive inherited metabolic disease in which a baby or child has a problem breaking down the phenylalanine amino acid. Incidence 1 in 10, 000. Early effects Babies do not have any problems at birth. Untreated Babies develop serious, permanent mental disability. Treatment A special low protein diet started by 21 days of age. 49
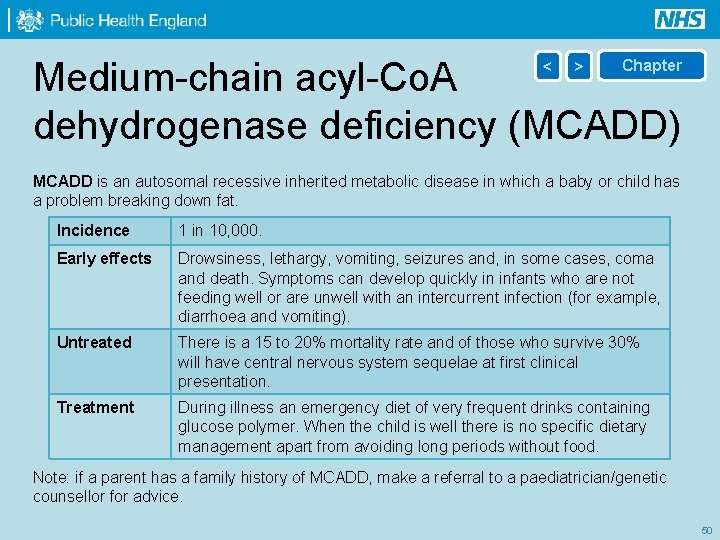
Medium-chain acyl-Co. A dehydrogenase deficiency (MCADD) < > Chapter MCADD is an autosomal recessive inherited metabolic disease in which a baby or child has a problem breaking down fat. Incidence 1 in 10, 000. Early effects Drowsiness, lethargy, vomiting, seizures and, in some cases, coma and death. Symptoms can develop quickly in infants who are not feeding well or are unwell with an intercurrent infection (for example, diarrhoea and vomiting). Untreated There is a 15 to 20% mortality rate and of those who survive 30% will have central nervous system sequelae at first clinical presentation. Treatment During illness an emergency diet of very frequent drinks containing glucose polymer. When the child is well there is no specific dietary management apart from avoiding long periods without food. Note: if a parent has a family history of MCADD, make a referral to a paediatrician/genetic counsellor for advice. 50

Maple syrup urine disease (MSUD) < > Chapter MSUD is an autosomal recessive inherited metabolic disease in which a baby or child has a problem breaking down leucine, isoleucine and valine amino acids. Incidence 1 in 150, 000. Early effects Many babies with MSUD become unwell when they are a few days old, with poor feeding, vomiting and excessive sleepiness. Untreated Leads to a coma and permanent brain damage or death in some cases. In older children a minor illness (such as a gastric upset) can lead to serious problems. Treatment A special low protein diet is used to prevent the build-up of harmful amino acids in the blood. A different regime is required when the child is ill, and they may need to be hospitalised. 51
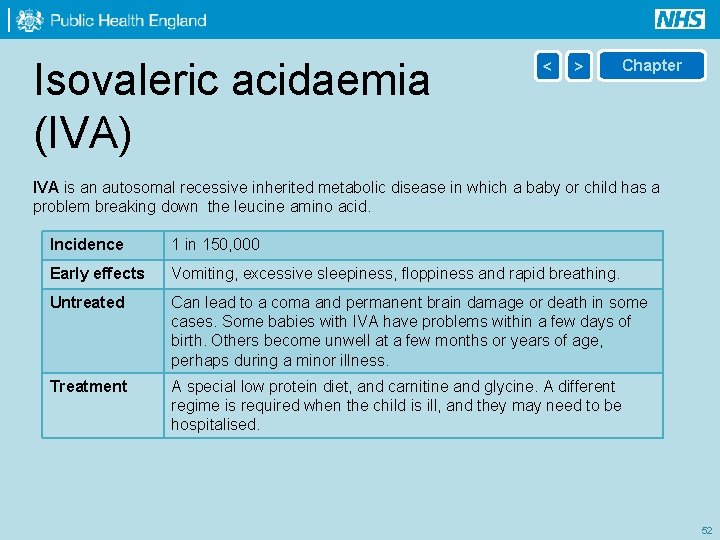
Isovaleric acidaemia (IVA) < > Chapter IVA is an autosomal recessive inherited metabolic disease in which a baby or child has a problem breaking down the leucine amino acid. Incidence 1 in 150, 000 Early effects Vomiting, excessive sleepiness, floppiness and rapid breathing. Untreated Can lead to a coma and permanent brain damage or death in some cases. Some babies with IVA have problems within a few days of birth. Others become unwell at a few months or years of age, perhaps during a minor illness. Treatment A special low protein diet, and carnitine and glycine. A different regime is required when the child is ill, and they may need to be hospitalised. 52
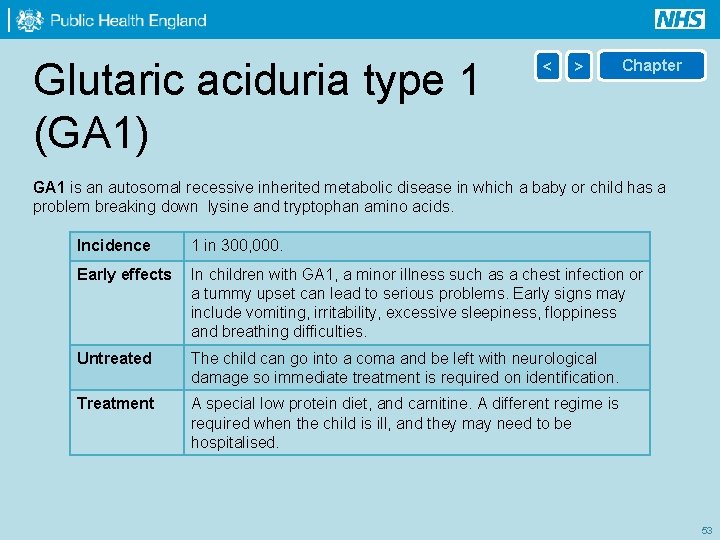
Glutaric aciduria type 1 (GA 1) < > Chapter GA 1 is an autosomal recessive inherited metabolic disease in which a baby or child has a problem breaking down lysine and tryptophan amino acids. Incidence 1 in 300, 000. Early effects In children with GA 1, a minor illness such as a chest infection or a tummy upset can lead to serious problems. Early signs may include vomiting, irritability, excessive sleepiness, floppiness and breathing difficulties. Untreated The child can go into a coma and be left with neurological damage so immediate treatment is required on identification. Treatment A special low protein diet, and carnitine. A different regime is required when the child is ill, and they may need to be hospitalised. 53
Homocystinuria (HCU) < Chapter HSU is an autosomal recessive inherited metabolic disease that prevents the breakdown of the homocysteine amino acid. Incidence 1 in 300, 000. Early effects Babies do not have any problems at birth. Untreated Most babies with HCU develop learning difficulties and eye problems as young children. It can lead to osteoporosis and blood clots and strokes. Treatment A special low protein diet and extra supplements and medicines. Note: newborn screening only detects the pyridoxine unresponsive form of HCU. 54
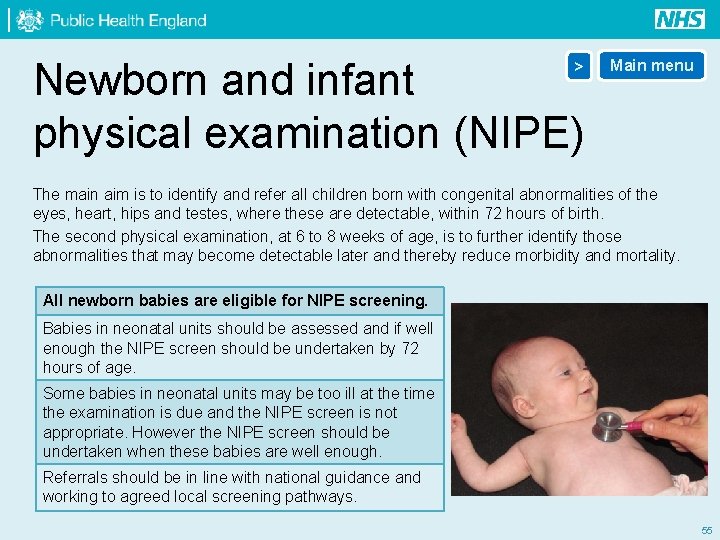
Newborn and infant physical examination (NIPE) > Main menu The main aim is to identify and refer all children born with congenital abnormalities of the eyes, heart, hips and testes, where these are detectable, within 72 hours of birth. The second physical examination, at 6 to 8 weeks of age, is to further identify those abnormalities that may become detectable later and thereby reduce morbidity and mortality. All newborn babies are eligible for NIPE screening. Babies in neonatal units should be assessed and if well enough the NIPE screen should be undertaken by 72 hours of age. Some babies in neonatal units may be too ill at the time the examination is due and the NIPE screen is not appropriate. However the NIPE screen should be undertaken when these babies are well enough. Referrals should be in line with national guidance and working to agreed local screening pathways. 55
Benefits of screening < Chapter Benefits Incidence Eyes Early detection of significant ocular anomalies to allow early treatment and reduce long-term morbidity. 2 or 3 in 10, 000 babies have problems with their eyes that require treatment. The prime purpose of screening is to identify congenital cataracts. Heart Early detection of significant congenital heart disease (CHD) to reduce morbidity and mortality. 4 to 10 in 1, 000 babies have a heart problem. Hips Early detection of developmental dysplasia of the hips (DDH) to reduce duration and burden of treatment and improve long-term outcome. 1 or 2 in 1, 000 babies have hip problems that require treatment. Testes Early detection of maldescent to allow timely operation to increase fertility, reduce risk of torsion and allow early detection of cancer. Around 1 in 100 baby boys have problems with their testes that require treatment. 56
- Slides: 56