Answer Key Ch 11 Review Sheet 1 What








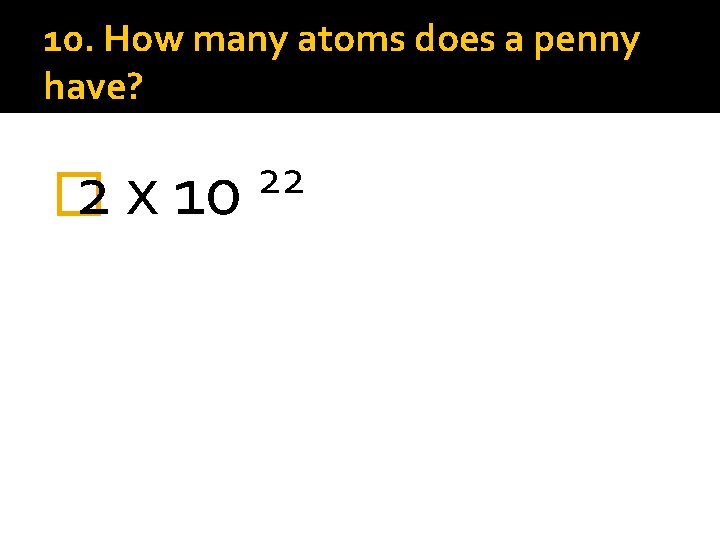











- Slides: 26

Answer Key Ch. 11 Review Sheet

1. What is an atom? �The smallest particle in which an element can be divided and still be the same substance

2. What particle did J. J. Thomson discover? � The electron

3. Where are electrons most likely to be found? � Electron Clouds

4. What did Dalton believe? �All substances are made of atoms �Atoms cannot be divided, created, or destroyed �Atoms join with other atoms to make new substances �Atoms of the same element are alike and atoms of different elements are different

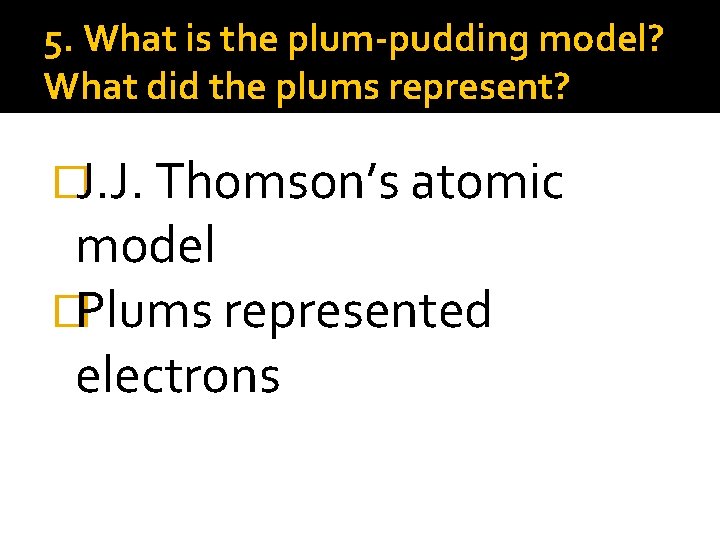
5. What is the plum-pudding model? What did the plums represent? �J. J. Thomson’s atomic model �Plums represented electrons

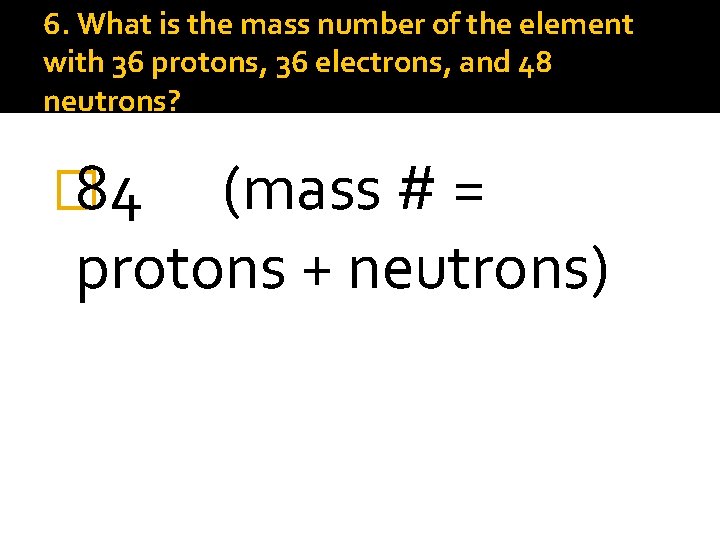
6. What is the mass number of the element with 36 protons, 36 electrons, and 48 neutrons? � 84 (mass # = protons + neutrons)

7. What part of an element has the least mass? � Electron

8. How did Democritus describe atoms? �Small, hard particles that could not be divided; atoms were made of a single material that could be formed into different shapes and sizes.

9. If hydrogen-1 has one proton, how many protons does hydrogen-2 have? � 1
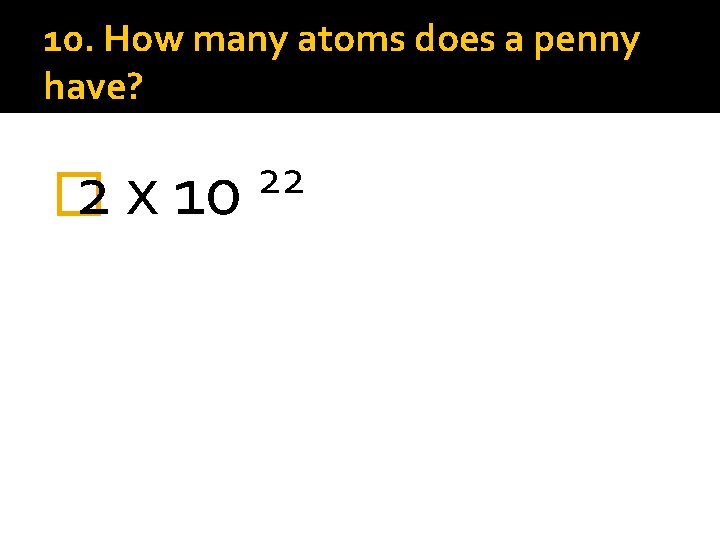
10. How many atoms does a penny have? � 2 x 10 22

11. What are isotopes? �Atoms of the same element that have different numbers of neutrons.

12. What are radioactive isotopes? �Atoms that have an unstable nucleus that will change over time.

13. What is an electron? � Negatively charged particle in an atom

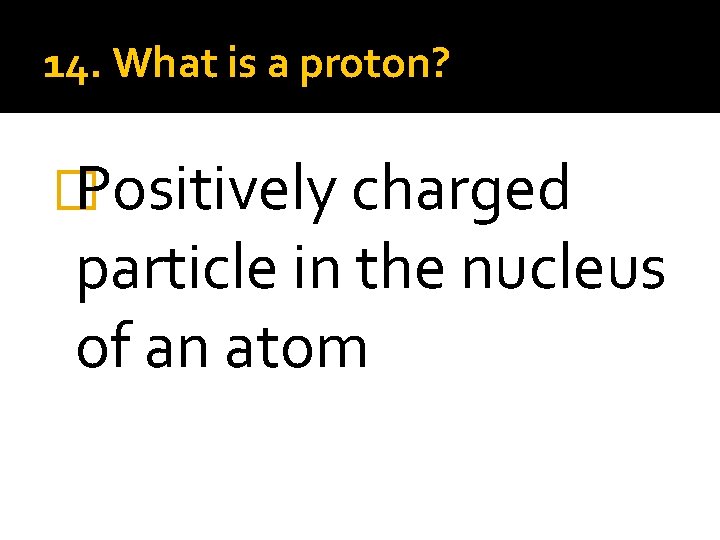
14. What is a proton? � Positively charged particle in the nucleus of an atom

15. What is a neutron? � A particle with no charge found in the nucleus of an atom

16. What is the atomic number? �Number of protons in the nucleus of an atom; it’s also the number of electrons in a neutral atom

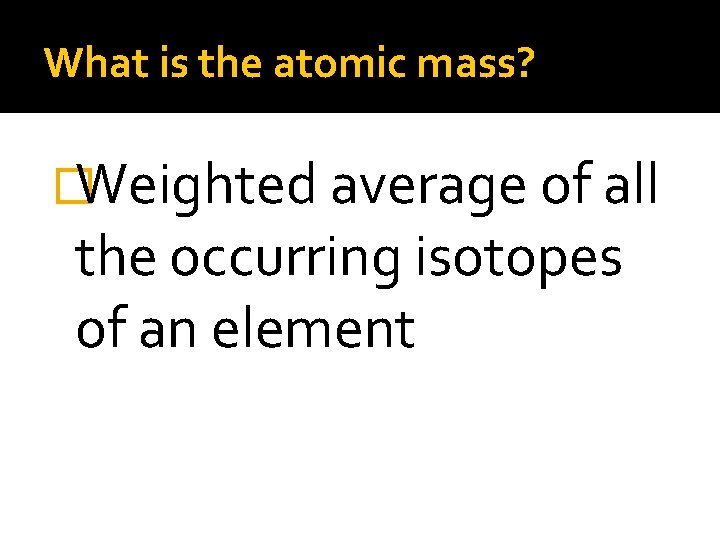
What is the atomic mass? �Weighted average of all the occurring isotopes of an element

18. Define electron cloud. � Region in an atom where electrons are likely to be found

19. Define ion. � A charged particle that forms when an atom loses or gains electrons

20. What is a gravitational force? � Force of attraction between two objects

21. What is a weak force? � Force in the nucleus of an unstable atom that allows the nucleus to change.

22. Define atomic mass unit � Unit of mass that describes the mass of an atom or molecule � (SI Unit)

23. What is a strong force? �Force that occurs in the nucleus in order to keep protons from repelling each other.

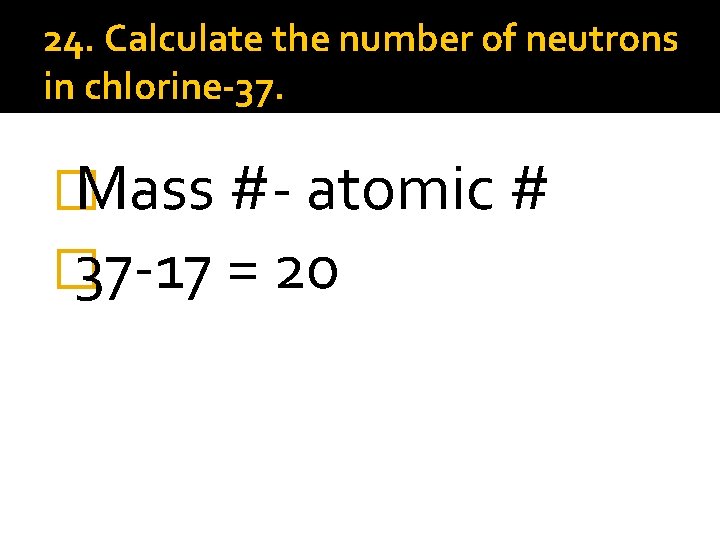
24. Calculate the number of neutrons in chlorine-37. � Mass #- atomic # � 37 -17 = 20

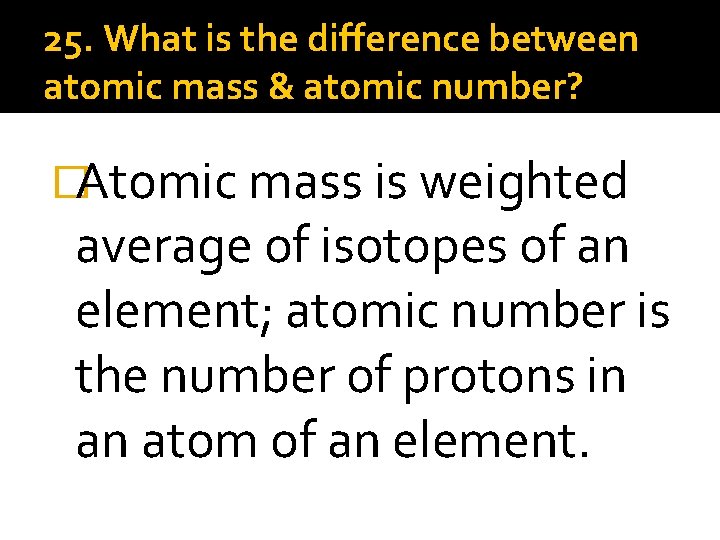
25. What is the difference between atomic mass & atomic number? �Atomic mass is weighted average of isotopes of an element; atomic number is the number of protons in an atom of an element.