Anomalous Water Molecule Seen by Neutron Scattering T
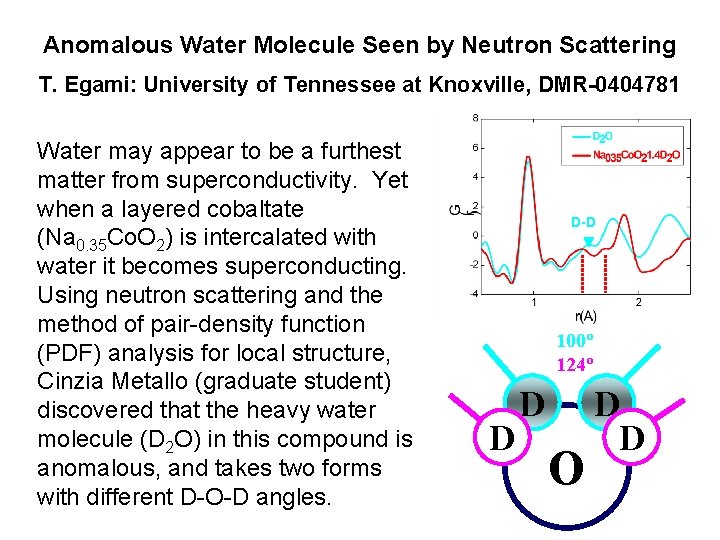
Anomalous Water Molecule Seen by Neutron Scattering T. Egami: University of Tennessee at Knoxville, DMR-0404781 Water may appear to be a furthest matter from superconductivity. Yet when a layered cobaltate (Na 0. 35 Co. O 2) is intercalated with water it becomes superconducting. Using neutron scattering and the method of pair-density function (PDF) analysis for local structure, Cinzia Metallo (graduate student) discovered that the heavy water molecule (D 2 O) in this compound is anomalous, and takes two forms with different D-O-D angles. D-D 100º 124º D D O D D

• While the mechanism of superconductivity in regular metals, such as aluminum, is well understood, that of complex metallic oxides, including high-temperature superconductivity in some cupper compounds, remains a deep mystery. What we and others found is that in these oxides local atomic structure and dynamics are different from the average, and they may be contributing to this strange phenomenon. Using neutron scattering as a main tool we try to cut into this mystery, as the example of cobaltate shows. • We have a high-school senior in our group! Eddie Buehler, a gifted senior in Farragut High (center, with several of our group members) studies molecular dynamics modeling of liquids as a special project with full credit.
- Slides: 2