Annual Supranational Laboratory Network Meeting Institut Pasteur Paris

Annual Supranational Laboratory Network Meeting Institut Pasteur, Paris, France October 24, 2005

Outline • • Status of the network/new SRLs "National Institutions, " To. R, Logo, coordination Financial issues Global Project coverage Issues in DRS New DRS Guidelines New TB control strategy, the Global Plan and implications for the SRLN Way forward …. .

Welcome to the network PAHO- Mexico- Susana Balandrao Instituto Nacional de Diagnostico y Referencia Epidemiologicos (National Diagnostic and Epidemiologic Reference Institute) (INDRE) in Mexico City PAHO- Argentina- Lucia Barrera National TB Reference Laboratory, Buenos Aires, Argentina EMRO- Egypt- Mushira Ismail National TB Reference Laboratory, Cairo, Egypt WPRO- Australia- Ivan Bastian and Richard Lumb Insitute of Medical and Veterinary Science, (IMVS), Adelaide, Australia **SEARO- *Candidate under evaluation Dhanida and Somsak Rienthong, Thailand NRL, Bureau of AIDS, TB and STIs Department of Disease Control Candidate SRLs nominated by WHO regional offices Based on regional needs
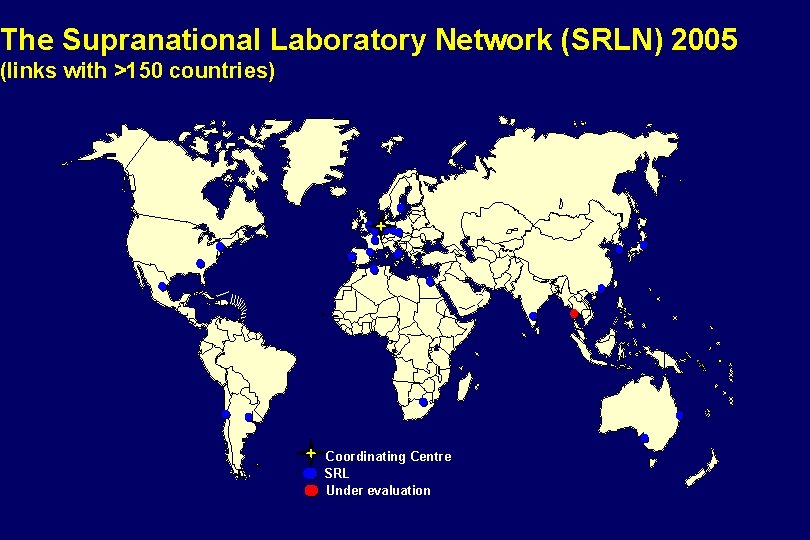
The Supranational Laboratory Network (SRLN) 2005 (links with >150 countries) Coordinating Centre SRL Under evaluation

The Supranational Laboratory Network (SRLN) 2005 • Coordinating Centre: Antwerp, Belgium • Global total: 25 laboratories, one candidate • Strong regional networks developing • 11 rounds of proficiency testing, • 150 reference laboratories have received panels Africa: 2 Americas: 5 Middle East: 1 Europe: 11 South Asia: 1 and 1 candidate Western Pacific: 5 • 4 annual network meetings • Link/subgroup/regional networks • Two ongoing SLD studies • Other studies planned

Legal Status WHO status: "National Institutions recognized by WHO. " Process to go through regional offices and national bodies. To. R: Developed, to be circulated November http: //www. who. int/csr/disease/influenza/en/TORNICs. pdf Logo: independent logo permitted by WHO legal department Coordination…. .
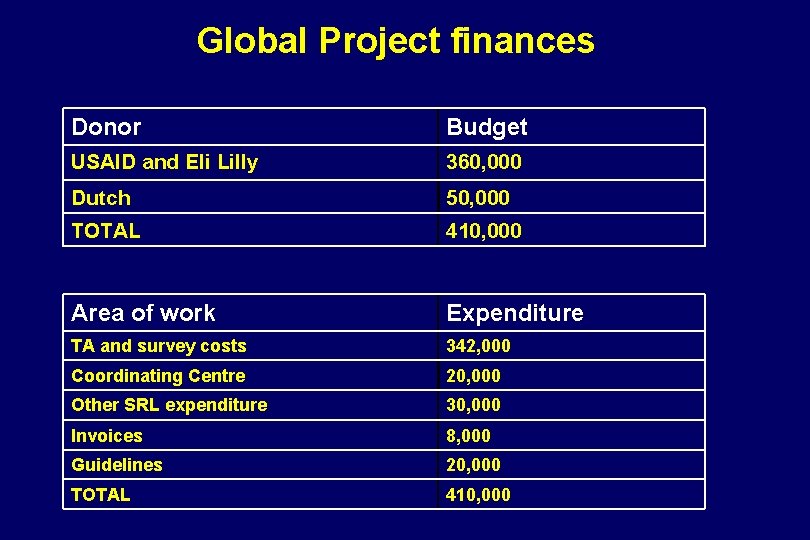
Global Project finances Donor Budget USAID and Eli Lilly 360, 000 Dutch 50, 000 TOTAL 410, 000 Area of work Expenditure TA and survey costs 342, 000 Coordinating Centre 20, 000 Other SRL expenditure 30, 000 Invoices 8, 000 Guidelines 20, 000 TOTAL 410, 000

Finances • Better coverage in protocol budget development, including budget for shipment • More surveys covered by GFATM and donors, freeing funds for laboratory TA, offset by increasing survey costs weak dollar. • More compensation expected with DOTS-Plus roll out • Possibilities of financing via "research"

Surveillance • The routine collection of data about disease frequency and distribution; the analysis of those data; and the dissemination of that information to those who need to know. • Information to answer three questions: – How big is the problem? – Where and to whom should limited resources go? – Is the problem getting better with my interventions?
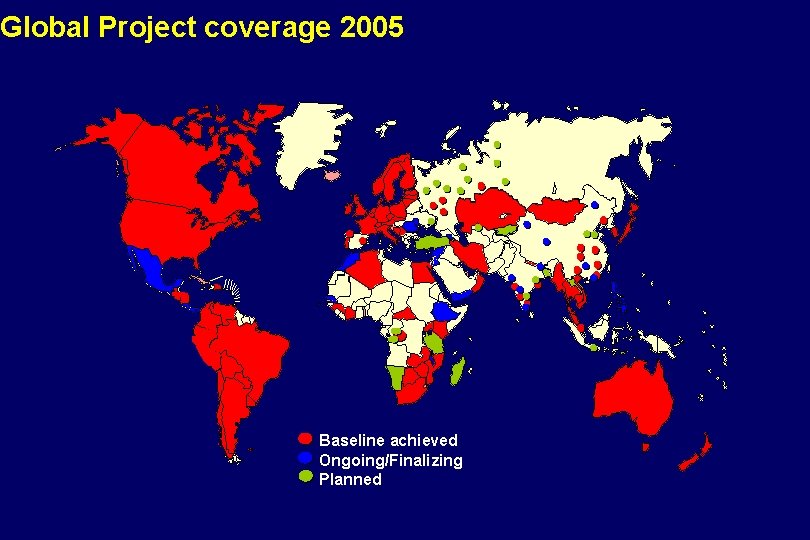
Global Project coverage 2005 Baseline achieved Ongoing/Finalizing Planned
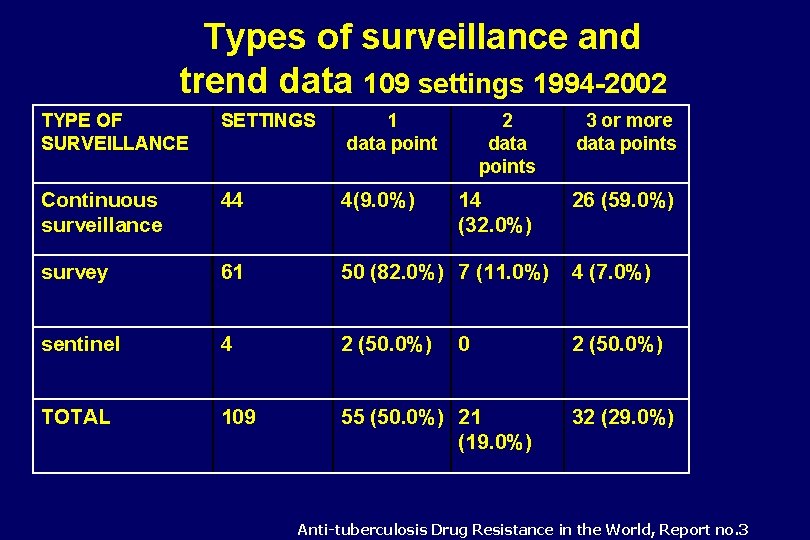
Types of surveillance and trend data 109 settings 1994 -2002 TYPE OF SURVEILLANCE SETTINGS 1 data point 2 data points 3 or more data points Continuous surveillance 44 4(9. 0%) 14 (32. 0%) 26 (59. 0%) survey 61 50 (82. 0%) 7 (11. 0%) 4 (7. 0%) sentinel 4 2 (50. 0%) TOTAL 109 55 (50. 0%) 21 (19. 0%) 0 32 (29. 0%) Anti-tuberculosis Drug Resistance in the World, Report no. 3
Progress in DR surveillance • 13 surveys ongoing • 33 surveys in preparation • 18 surveys in the pipeline } including HBC: China, India and Russia, and HB Africa • Trend/surveillance with >3 data points from 48 settings • Representative retreatment data from >10 high priority settings • Second line DST: 15+ settings where isolates with FLD resistance were tested for selected SLD resistance • DRS/HIV in context of routine survey/surveillance from 6 survey settings and undetermined number surveillance settings • Data from 5 Prison settings

Adding research questions to routine surveys • • • Acquisition R in high HIV prevalence settings MDR/HIV Rapid/genetic testing Routine testing of SLD OR (misclassification, transport systems, risk factors)
ISSUES in Anti-TB Drug resistance Surveillance and Testing Laboratory SAFETY of Culture and DST QUALITY of Culture and DST AVAILABILITY of Culture and DST NO standardized methods for 2 nd line DST, DST for some drugs unreliable Transport of strains expensive, legal/ethical implications SRLN bears much of the cost, and TA often limited to PT and initial assessments No capacity to determine TRUE acquired resistance New technologies must be combined with functional and safe conventional methods-Slow integration of new technologies- pilots with MGIT, starting to pilot genetic methods

New DRS Guidelines • • Flexible surveillance approach- fitting the needs of countries Retreatment cases Samples for repeat surveys Attention to clusters Classification of cases Ethical issues Small surveys for DOTS-Plus regimen design Update of SDRTB software * Required update for changes in 2 nd testing approach and combined HIV surveillance Principles: -Sample accurately represents population under study -Quality assured laboratory results -Differentiation between new and previously treated cases

WHO Stop TB Strategy 1. Pursuing quality DOTS Expansion 2. Addressing TB/HIV and MDR 3. Contribute to health systems strengthening 4. Engaging all care providers 5. Empowering patients and communities 6. Enabling and promoting research STAG, WHO Regional Advisers meeting, Versailles meeting, DOTS-Plus, DOTS Expansion, and TB/HIV workings groups: RECOGNIZE THE NEED FOR LABORATORY DEVELOPMENT
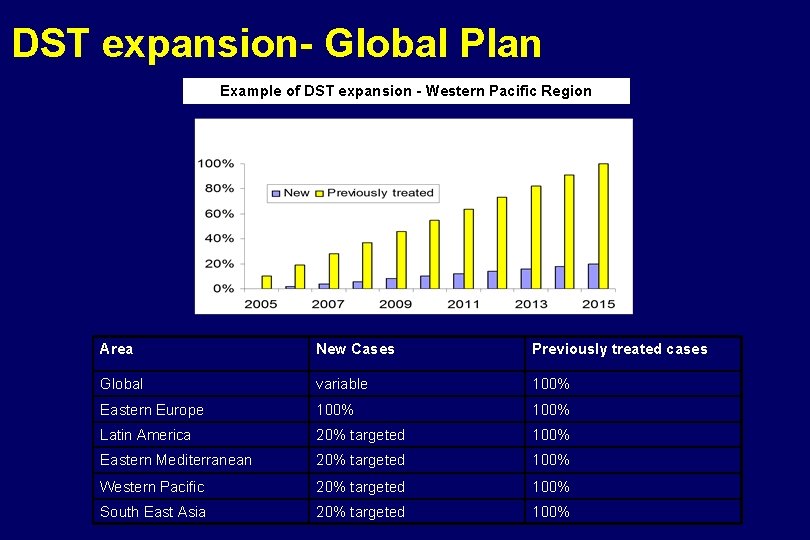
DST expansion- Global Plan Example of DST expansion - Western Pacific Region Area New Cases Previously treated cases Global variable 100% Eastern Europe 100% Latin America 20% targeted 100% Eastern Mediterranean 20% targeted 100% Western Pacific 20% targeted 100% South East Asia 20% targeted 100%
- Slides: 17