Announcements To join clicker to class today Turn

- Slides: 5

Announcements To join clicker to class today: – Turn on the Clicker (the red LED comes on). – Push “Join” button followed by “ 20” followed by the “Send” button (switches to flashing green LED if successful). ● ● If you gave me an exam to doublecheck, last week, you can pick it up at the end of class. Will be starting chapter 8 Today. Reading and problems have been e-mailed to you and posted on class web site. Quiz Today will cover all of Chapter 7 except for section 7. 5. Wear appropriate clothes to lab (no sandals, shorts, etc. . . )

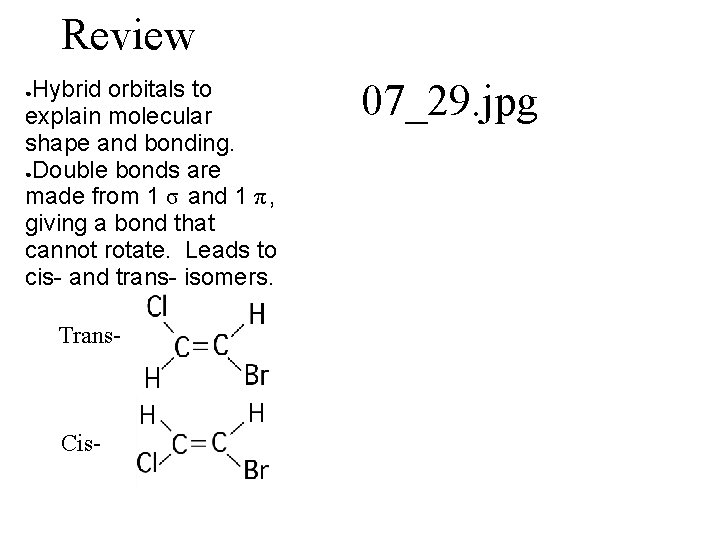
Review Hybrid orbitals to explain molecular shape and bonding. ●Double bonds are made from 1 σ and 1 π, giving a bond that cannot rotate. Leads to cis- and trans- isomers. ● Trans- Cis- 07_29. jpg

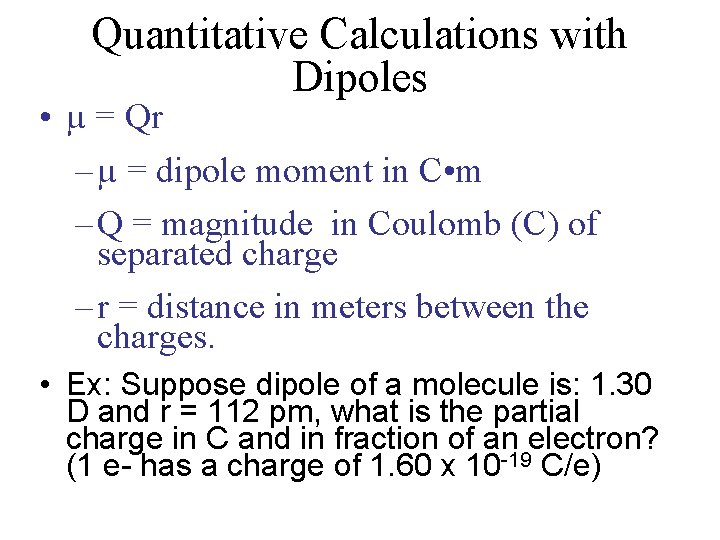
Quantitative Calculations with Dipoles • µ = Qr – µ = dipole moment in C • m – Q = magnitude in Coulomb (C) of separated charge – r = distance in meters between the charges. • Ex: Suppose dipole of a molecule is: 1. 30 D and r = 112 pm, what is the partial charge in C and in fraction of an electron? (1 e- has a charge of 1. 60 x 10 -19 C/e)

Chapter 8 – Gases • Pressure • Ideal Gas Law (PV = n. RT, solutions for P, V, n, T, density and molar mass) • Dalton’s Law of Partial Pressures • Henry’s Law of gas solubility • Kinetic Molecular Theory of Gases (molecular speeds, diffusion and effusion) • Real Gases/Non-Ideal behavior (van der Waals equation)

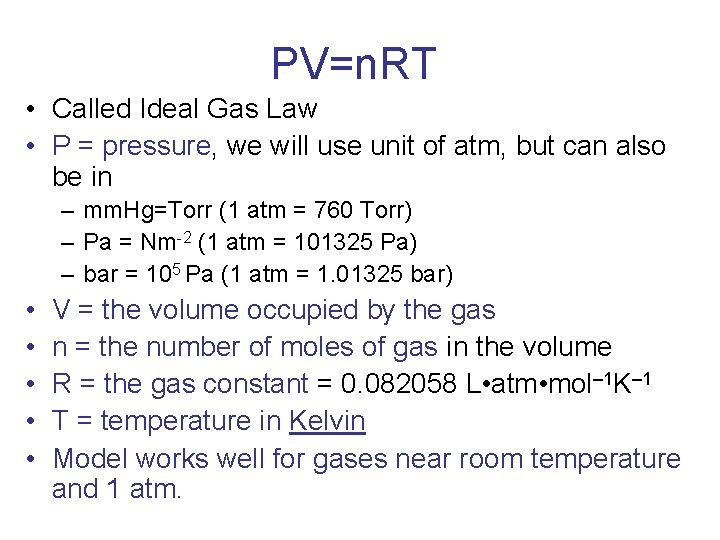
PV=n. RT • Called Ideal Gas Law • P = pressure, we will use unit of atm, but can also be in – mm. Hg=Torr (1 atm = 760 Torr) – Pa = Nm-2 (1 atm = 101325 Pa) – bar = 105 Pa (1 atm = 1. 01325 bar) • • • V = the volume occupied by the gas n = the number of moles of gas in the volume R = the gas constant = 0. 082058 L • atm • mol– 1 K– 1 T = temperature in Kelvin Model works well for gases near room temperature and 1 atm.