Announcements n n n Exam 1 Tonight 7








- Slides: 15

Announcements n n n Exam #1: Tonight!, 7: 00 -8: 30 pm, locations posted on website, bring ID Conflict Exam: 5: 00 -6: 30 pm, Noyes 217, bring ID No book homework due Friday! Lecture on Thursday! (New Material – Pre-lecture assignment still due at 8: 00 am Thursday) Exam scores posted late Wednesday evening in ATLAS Gradebook. Get graded exams back on Friday. Liquid nitrogen ice cream winner = highest section average!!!

Chemistry Joke of the Day n n Q: What do chemists call a benzene ring with iron atoms replacing the carbon atoms? A: A ferrous wheel.

Exam Format – 90 minutes total 15 Multiple Choice Questions n 5 possible choices n 50% of test grade n ~3 minutes to answer each (~45 min on this half of exam) 2 Free Response Questions n n n Write in answers – both qualitative and quantitative 50% of test grade ~45 minutes on this half of exam ***A periodic table & cover sheet with equations will be attached. ***

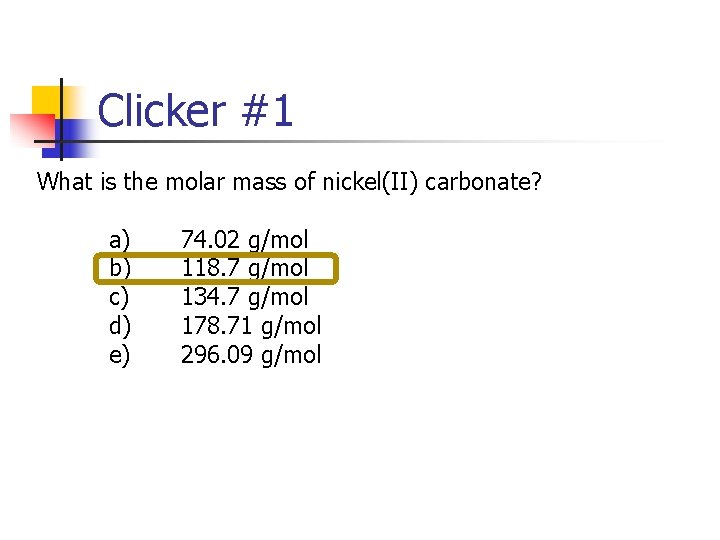
Clicker #1 What is the molar mass of nickel(II) carbonate? a) 74. 02 g/mol b) 118. 7 g/mol c) 134. 7 g/mol d) 178. 71 g/mol e) 296. 09 g/mol

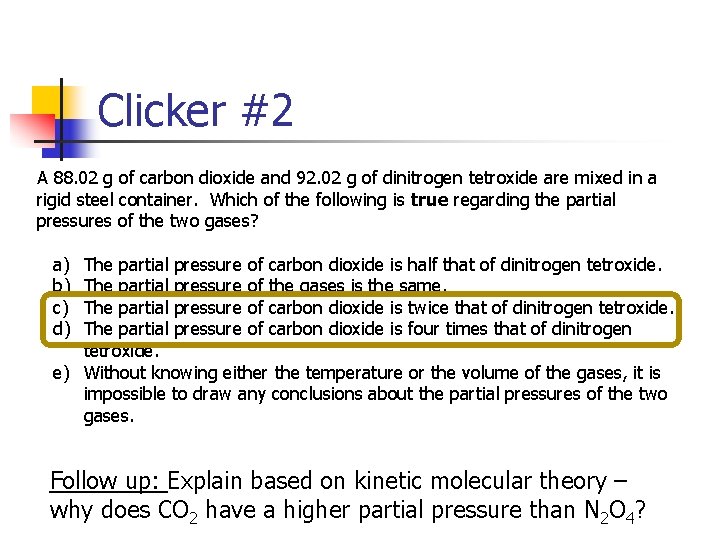
Clicker #2 A 88. 02 g of carbon dioxide and 92. 02 g of dinitrogen tetroxide are mixed in a rigid steel container. Which of the following is true regarding the partial pressures of the two gases? a) b) c) d) The partial pressure of carbon dioxide is half that of dinitrogen tetroxide. The partial pressure of the gases is the same. The partial pressure of carbon dioxide is twice that of dinitrogen tetroxide. The partial pressure of carbon dioxide is four times that of dinitrogen tetroxide. e) Without knowing either the temperature or the volume of the gases, it is impossible to draw any conclusions about the partial pressures of the two gases. Follow up: Explain based on kinetic molecular theory – why does CO 2 have a higher partial pressure than N 2 O 4?

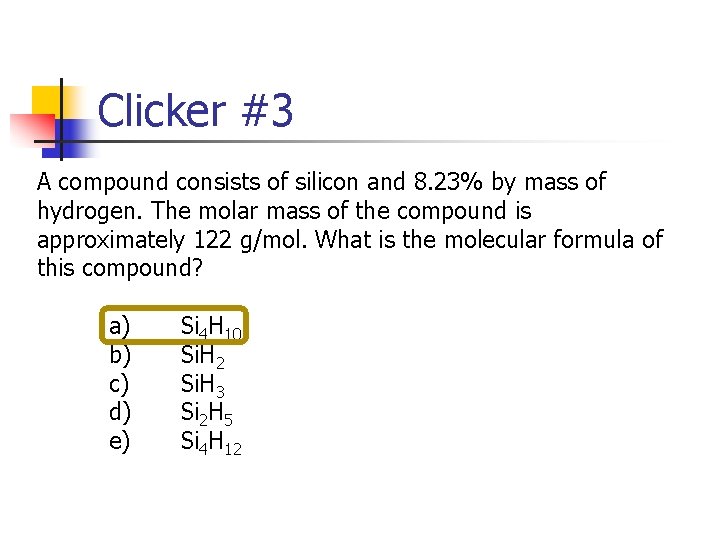
Clicker #3 A compound consists of silicon and 8. 23% by mass of hydrogen. The molar mass of the compound is approximately 122 g/mol. What is the molecular formula of this compound? a) Si 4 H 10 b) Si. H 2 c) Si. H 3 d) Si 2 H 5 e) Si 4 H 12

Clicker #4 If you have equal mole samples of each of the following compounds, which compound contains the greatest number of oxygen atoms? a) sodium sulfate b) dinitrogen pentoxide c) iron(III) phosphate d) barium oxide e) potassium acetate

Clicker #5 A member of the alkaline earth metal family whose most stable ion contains 36 electrons forms a compound with bromine. What is the correct formula for this compound? a) Ca. Br 2 b) Ba. Br. O 3 c) Kr. Br d) Rb. Br e) Sr. Br 2

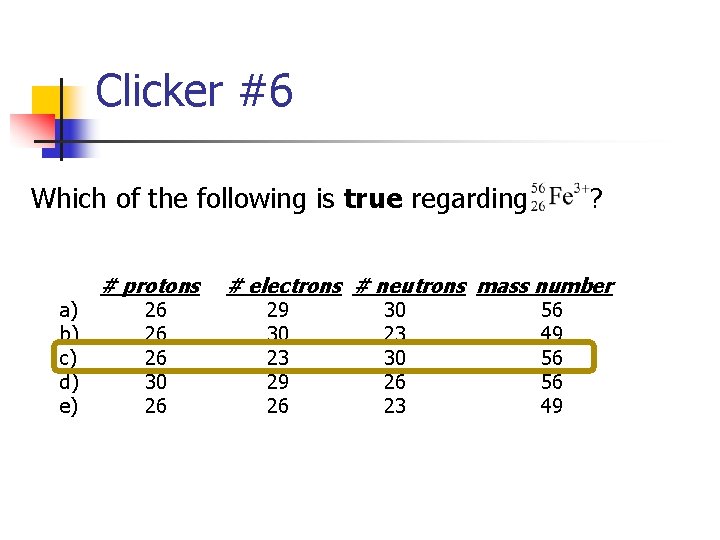
Clicker #6 Which of the following is true regarding ? a) b) c) d) e) # protons 26 26 26 30 26 # electrons # neutrons mass number 29 30 23 29 26 30 23 30 26 23 56 49 56 56 49

Clicker #7 Which sample contains the greatest number of atoms? a) 10. 0 g Na b) 10. 0 g Fe c) 10. 0 g Ag d) 10. 0 g Cu e) 10. 0 g Ba

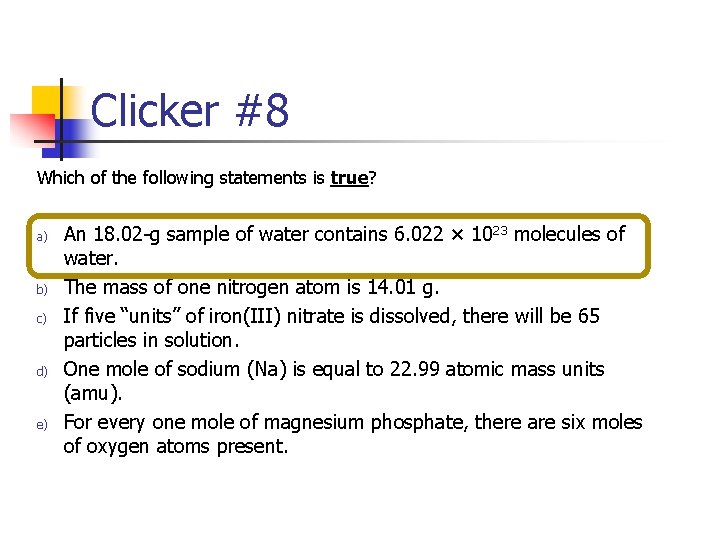
Clicker #8 Which of the following statements is true? a) b) c) d) e) An 18. 02 -g sample of water contains 6. 022 × 1023 molecules of water. The mass of one nitrogen atom is 14. 01 g. If five “units” of iron(III) nitrate is dissolved, there will be 65 particles in solution. One mole of sodium (Na) is equal to 22. 99 atomic mass units (amu). For every one mole of magnesium phosphate, there are six moles of oxygen atoms present.

Clicker #9 A gas sample containing 2. 50 mol at 25°C exerts a pressure of 1. 00 atm. Some gas is added to the same container and the temperature is increased to 50. °C. If the pressure increases to 2. 00 atm, how many moles of gas were added to the container? Assume a constant-volume container. a) 1. 25 mol b) 2. 11 mol c) 2. 50 mol d) 4. 61 mol e) 5. 00 mol

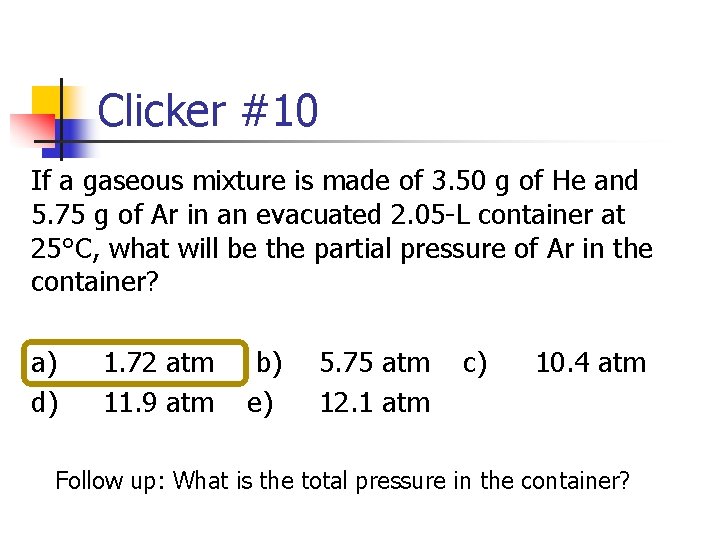
Clicker #10 If a gaseous mixture is made of 3. 50 g of He and 5. 75 g of Ar in an evacuated 2. 05 -L container at 25°C, what will be the partial pressure of Ar in the container? a) d) 1. 72 atm 11. 9 atm b) e) 5. 75 atm 12. 1 atm c) 10. 4 atm Follow up: What is the total pressure in the container?

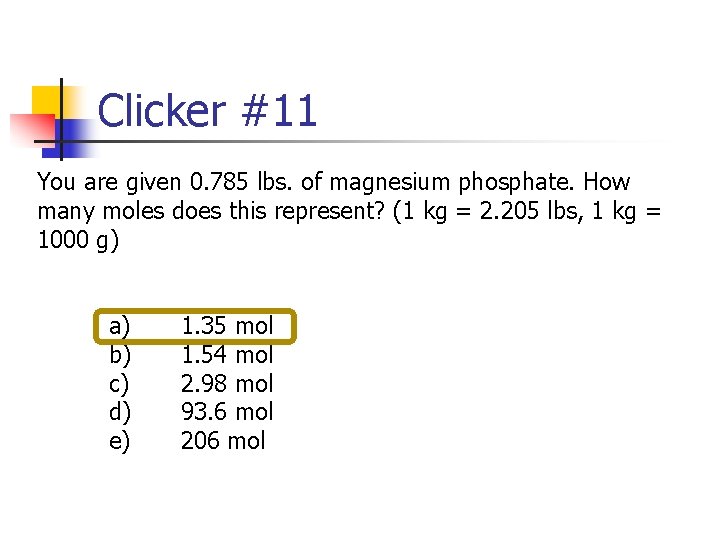
Clicker #11 You are given 0. 785 lbs. of magnesium phosphate. How many moles does this represent? (1 kg = 2. 205 lbs, 1 kg = 1000 g) a) b) c) d) e) 1. 35 mol 1. 54 mol 2. 98 mol 93. 6 mol 206 mol

Clicker #12 A flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon is at sea level, where the temperature is 25 o. C and the barometric pressure is 0. 947 atm. The balloon then rises to an altitude of 6000 ft, where the pressure is 0. 796 atm and the temperature is 15 o. C. What is the change in the volume of the balloon as it ascends from sea level to 6000 ft? a) 128 L b) 373 L c) 610. L d) 983 L e) none of these?
 Kayl announcements
Kayl announcements R/announcements!
R/announcements! General announcements
General announcements David ritthaler
David ritthaler Fahrenheit 451 burning bright summary
Fahrenheit 451 burning bright summary Pvu market cap
Pvu market cap Range of melody
Range of melody Comic relief in romeo and juliet
Comic relief in romeo and juliet What's in it for you
What's in it for you Phases of the moon northern hemisphere
Phases of the moon northern hemisphere Eat present simple passive
Eat present simple passive How thin and sharp is the moon tonight
How thin and sharp is the moon tonight Had better sentences
Had better sentences Pablo neruda tonight i can write
Pablo neruda tonight i can write Li.comked
Li.comked If you died tonight would you go to heaven
If you died tonight would you go to heaven