ANNOUNCEMENTS Final Exam What to Bring Format Friday

ANNOUNCEMENTS Final Exam: What to Bring: Format: Friday, May 14 th at 8: 00 am Calculator No. 2 Pencils Two pages of notes Multiple choice (scantron) Review Session: Wednesday, May 12 th at 10: 00 am IRC 3
![Determine the p. H of a weak acid solution from [acid] What is the Determine the p. H of a weak acid solution from [acid] What is the](http://slidetodoc.com/presentation_image_h2/a2e31a7a8e8aefaef63d552e78a6efa0/image-2.jpg)
Determine the p. H of a weak acid solution from [acid] What is the p. H of a 0. 15 M solution of HF?

Predicting the p. H of a weak acid: HCN What is the p. H of a 0. 23 M solution of HCN? Ka= 4. 0 x 10 -10
![Determine the p. H of a strong base solution from [base] What is the Determine the p. H of a strong base solution from [base] What is the](http://slidetodoc.com/presentation_image_h2/a2e31a7a8e8aefaef63d552e78a6efa0/image-4.jpg)
Determine the p. H of a strong base solution from [base] What is the p. H of a 0. 15 M solution of Ca(OH)2?
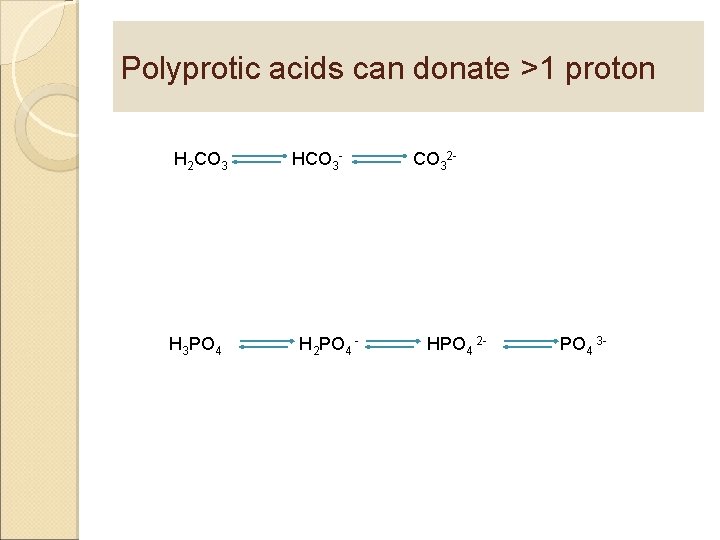
Polyprotic acids can donate >1 proton H 2 CO 3 H 3 PO 4 HCO 3 - H 2 PO 4 - CO 32 - HPO 4 2 - PO 4 3 -
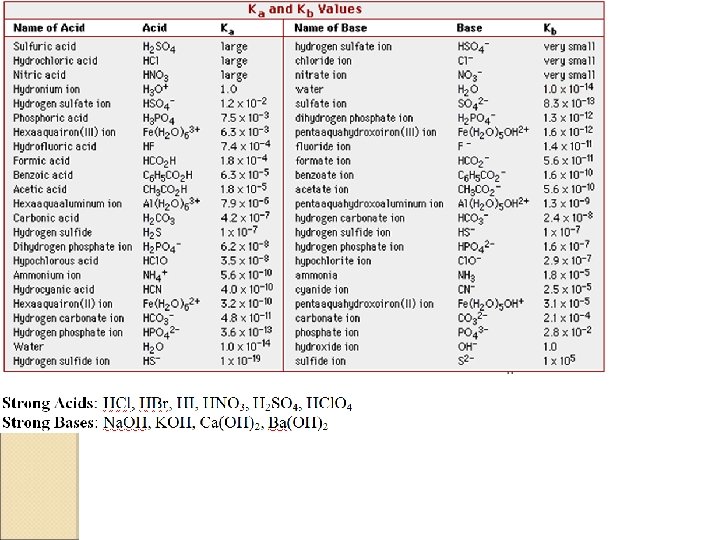

How to determine whether a salt will be acidic or basic Most acids are neutral Most bases are anions Salts ◦ Look at cation and anion ◦ Determine whether ions are acid/base/neutral NH 4+ = only acidic cation Anions that are conjugates of strong A/B = neutral ◦ Ions determine acidity/basicity of compound
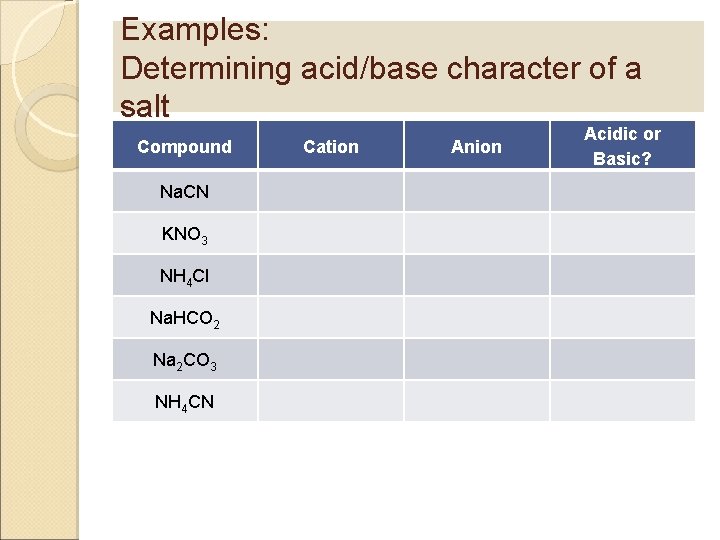
Examples: Determining acid/base character of a salt Compound Na. CN KNO 3 NH 4 Cl Na. HCO 2 Na 2 CO 3 NH 4 CN Cation Anion Acidic or Basic?
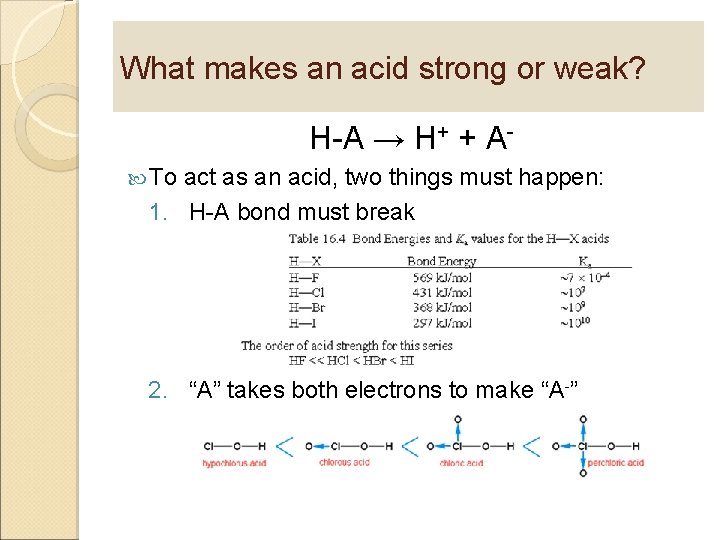
What makes an acid strong or weak? H-A → H+ + A To act as an acid, two things must happen: 1. H-A bond must break 2. “A” takes both electrons to make “A-”

Acid strength is related to bond strength and electronegativity As bond strength increases, acid strength decreases As electronegativity increases, acid strength increases
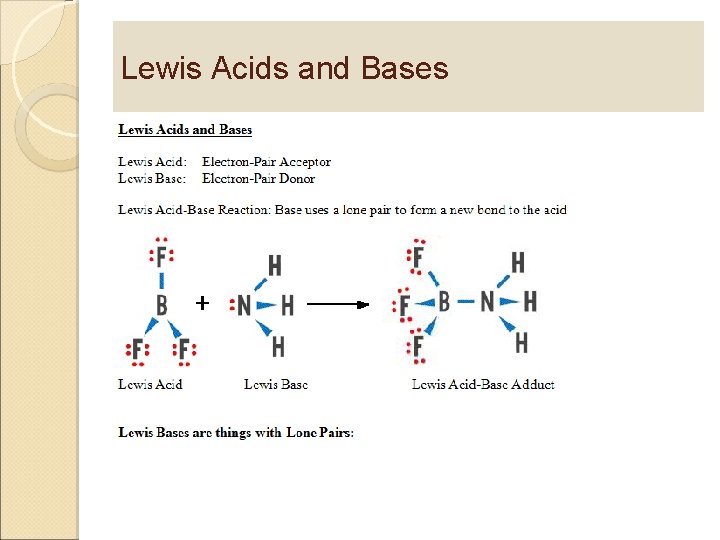
Lewis Acids and Bases
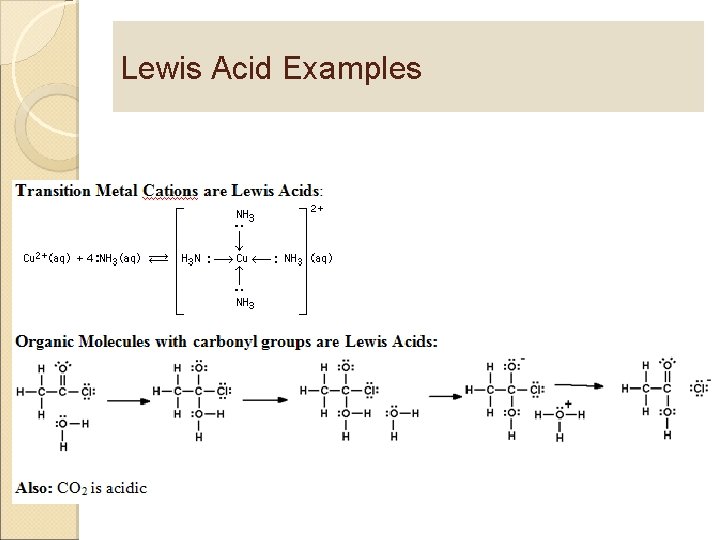
Lewis Acid Examples
- Slides: 13