Announcements 1 Write down one thing you like






- Slides: 10

Announcements 1. Write down one thing you like about this course. 2. Write down one thing that could be changed to make the course better. 3. Write down one thing you as the student can change to make the course better. 4. Write down any concerns related to TA’s, labs, or discussions.

Key Terms • Limiting reactant • Excess reactant • Balanced equation • Mole ratio

Discussion #1 What is true about a limiting reactant? 1. It is always the reactant with the smallest coefficient. 2. It is always the reactant that starts with the smallest number of moles. 3. It is always the reactant that runs out first. 4. It is always the reactant that is remaining after the reaction. 5. It is always the reactant that has the largest coefficient.

Discussion #2 When comparing what you’re given to a balanced chemical equation, you must be in units of 1) moles 2) grams 3) molarity 4) Any unit can be directly related to a balanced chemical equation.

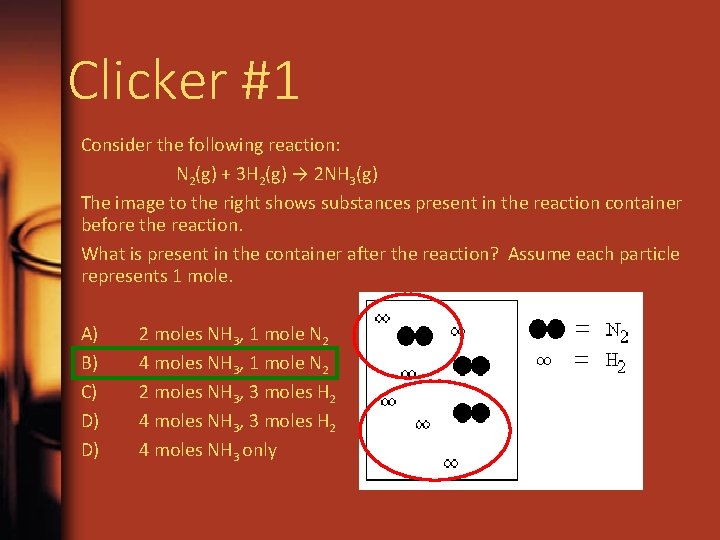
Clicker #1 Consider the following reaction: N 2(g) + 3 H 2(g) → 2 NH 3(g) The image to the right shows substances present in the reaction container before the reaction. What is present in the container after the reaction? Assume each particle represents 1 mole. A) B) C) D) D) 2 moles NH 3, 1 mole N 2 4 moles NH 3, 1 mole N 2 2 moles NH 3, 3 moles H 2 4 moles NH 3 only

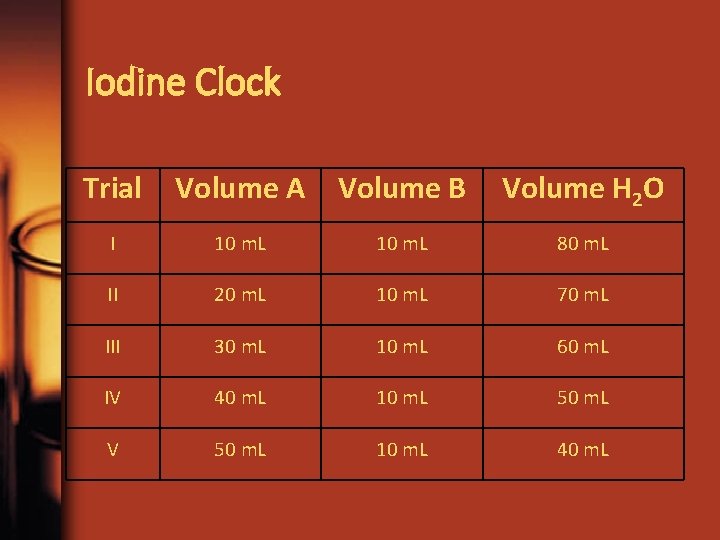
Iodine Clock Trial Volume A Volume B Volume H 2 O I 10 m. L 80 m. L II 20 m. L 10 m. L 70 m. L III 30 m. L 10 m. L 60 m. L IV 40 m. L 10 m. L 50 m. L V 50 m. L 10 m. L 40 m. L

Example #1 Assume you have 0. 025 mol Mg and 0. 100 mol HCl in the balloon over flask 1. 1. Identify the limiting reactant and use it to determine the number of moles of H 2 produced. 2. Determine the number of moles of excess reactant leftover.

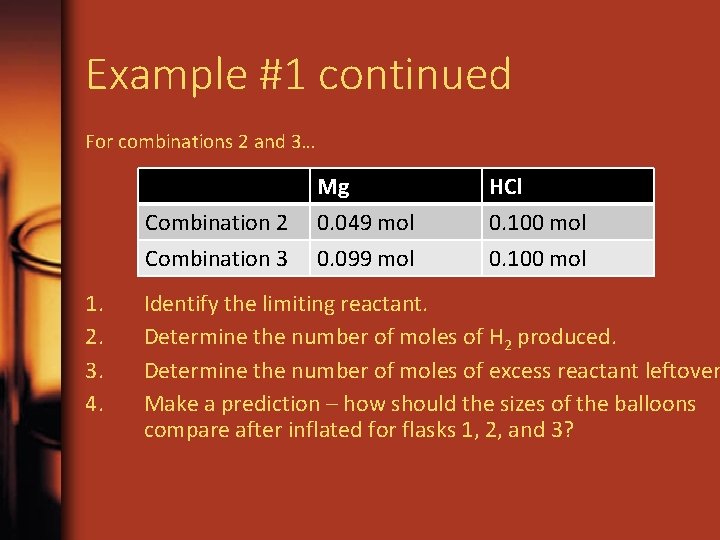
Example #1 continued For combinations 2 and 3… Combination 2 Combination 3 1. 2. 3. 4. Mg 0. 049 mol 0. 099 mol HCl 0. 100 mol Identify the limiting reactant. Determine the number of moles of H 2 produced. Determine the number of moles of excess reactant leftover Make a prediction – how should the sizes of the balloons compare after inflated for flasks 1, 2, and 3?

Example #2 Assume you have 5. 00 g of Mg and 10. 0 g of CO 2 available. You allow them to react to form solid magnesium oxide and carbon. 1. Determine the masses of both products produced. 2. Determine the mass of excess reactant. 3. Prove that mass has been conserved.

Clicker #2 What should be the total mass present after the reaction is complete? A) B) C) D) E) 1. 24 g 5. 00 g 9. 54 g 10. 0 g 15. 0 g