Anesthesia Machine VAPORIZERS Vaporizers Convert liquid anesthetic into





















- Slides: 24

Anesthesia Machine VAPORIZERS

Vaporizers • Convert liquid anesthetic into a volatile inhalation agent • Based on laws of physics • You must memorize the chemical properties of the volatile agents

Basic Design • Gas enters vaporizer • Flow is split – Majority is bypassed – Some enters vaporizing chamber • Saturated gas leaves chamber • Diluted by bypass gas • Delivered to patient

Applied Physics • Vapor pressure – Dalton’s law – Based on characteristics of agent – Varies with temperature

Applied Physics (con’t) • Boiling point – Vapor pressure equals atmospheric pressure • Latent heat of vaporization – Heat required to change liquid into a vapor – Comes from liquid and environment

Types of Vaporizers • Historic – Copper kettle – Vernitrol • Modern – Ohmeda Tec 4 – Drager Vapor 19. 1

Characteristics of Drager and Ohmeda • • • Variable bypass Flow over Temperature compensated Agent specific Out of circuit

Copper Kettle and Vernitrol • • • Measured flow Bubble through Non temperature compensated Multiple agent Out of circuit

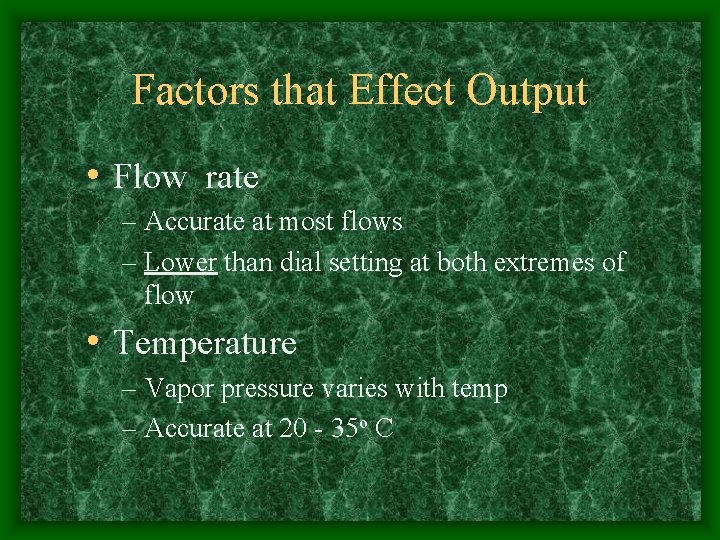
Factors that Effect Output • Flow rate – Accurate at most flows – Lower than dial setting at both extremes of flow • Temperature – Vapor pressure varies with temp – Accurate at 20 - 35 o C

Factors Effecting Output (con’t #1) • Intermittent back pressure – Retrograde flow – Higher than dial setting • especially at low flows and high ventilator pressures • Carrier gas composition – N 2 O causes transient drop

Vaporizer Interlock System • • Only 1 vaporizer can be turned on Gas enters only the “on” vaporizer Leak of trace gas is minimized Vaporizers are locked into the circuit

What is the concentration of an anesthetic gas in the vaporizing chamber?

Vapor pressure X 100 atmospheric pressure

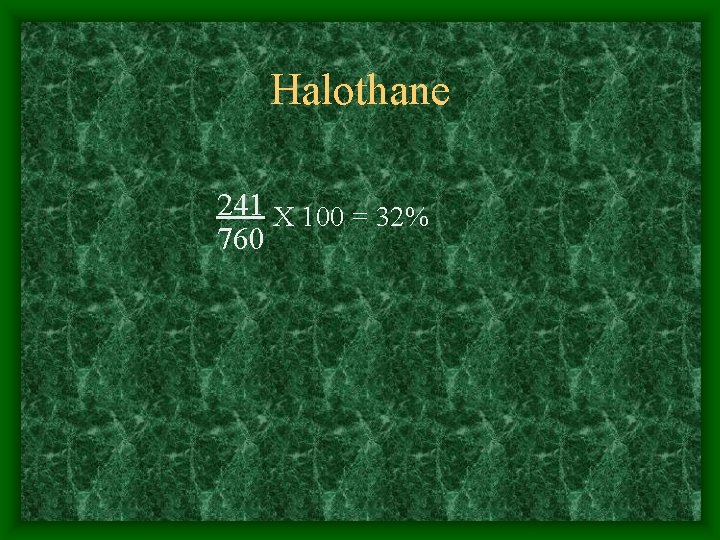
Vapor Pressures: Isoflurane - 238 Enflurane - 175 Halothane - 241

Isoflurane 238 X 100 = 31% 760

Enflurane 175 X 100 = 23% 760

Halothane 241 X 100 = 32% 760

Desflurane • Requires special vaporizer – Vapor pressure 664 – Pressurized, heated chamber • 1550 mm / Hg prevents boiling

Liquid to Vapor CC’s of Gas per ml of Liquid • Isoflurane -- 206 • Enflurane -- 210 • Halothane -- 240

How long will your anesthetic gas last?

Vaporizer problem # 1 • You have 15 cc’s of forane liquid and are giving 1% at 3 l / m. How long will it last?

Vaporizer problem # 2 You have: Forane 15 m. L X 206 cc / ml = 3090 cc You use: 1% X 3000 = 30 cc / min Therefore: 3090 cc 30 cc / min = 103 minutes

Vaporizer Hazards • • • Misfilling Tipping Dual vaporizers on Leaks Free standing vaporizers

Misfilling • Vaporizers are calibrated according to the vapor pressure of the agent • If you fill with an agent with a higher v. p. -overdose • If you fill with an agent with a lower v. p. -underdose