Anemia in CKD KDOQI guideline 2006 2012 5











- Slides: 29

Anemia in CKD - KDOQI guideline 2006 - 2012. 5. 9 R 4 우성애

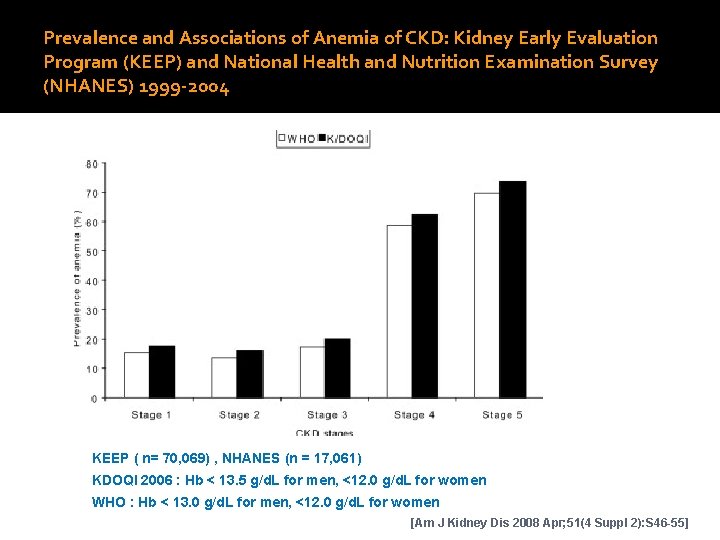
Prevalence and Associations of Anemia of CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999 -2004 KEEP ( n= 70, 069) , NHANES (n = 17, 061) KDOQI 2006 : Hb < 13. 5 g/d. L for men, <12. 0 g/d. L for women WHO : Hb < 13. 0 g/d. L for men, <12. 0 g/d. L for women [Am J Kidney Dis 2008 Apr; 51(4 Suppl 2): S 46 -55]

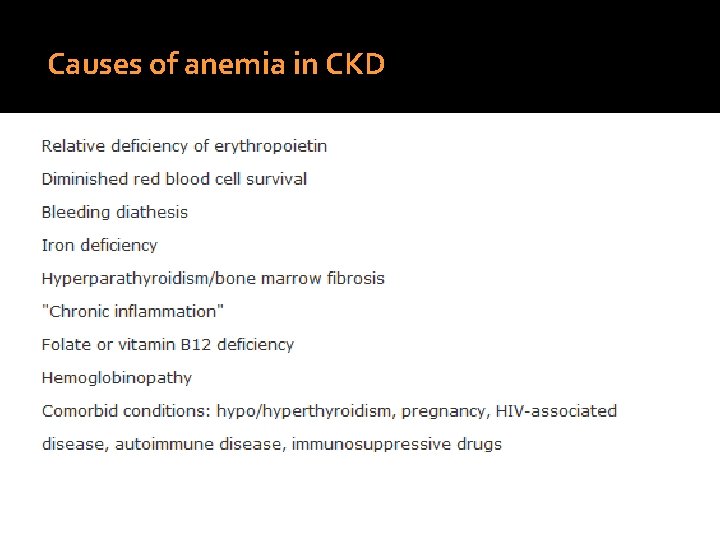
Causes of anemia in CKD

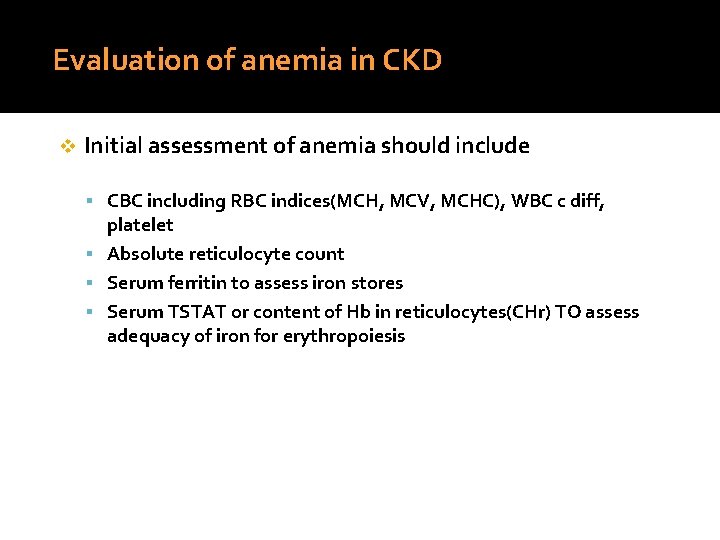
Evaluation of anemia in CKD v Initial assessment of anemia should include CBC including RBC indices(MCH, MCV, MCHC), WBC c diff, platelet Absolute reticulocyte count Serum ferritin to assess iron stores Serum TSTAT or content of Hb in reticulocytes(CHr) TO assess adequacy of iron for erythropoiesis

Using ESAs(erythropoiesis stimulating agents) v to start ESA therapy, following issues must be considered, Choosing an ESA(epoetin alfa, epoetin beta, darbepoetin) Initial ESA dose Route and frequency of ESA administration Planning a Hb level monitoring schedule Predicting a desired rate of increase in Hb level Anticipating ESA dose adjustments for Hb results

Using ESAs(erythropoiesis stimulating agents) l ESA dosing - The initial ESA dose and ESA dose adjustments should be determined by the patient’s Hb level, the target Hb level, the observed rate of increase in Hb level, and clinical circumstances l Initial dose - Epoetin 50~100 IU/kg three times weekly - Darbepoetin alfa 0. 45 mcg/kg once weekly l Hb monitoring at least monthly l The objective of initial ESA therapy; increase in Hb levels of 1 -2 g/d. L per month l The minimum interval between ESA dose adjustments is 2 wks (the effect of most dose changes will not be seen within a shorter interval) (25% titration)

Using ESAs(erythropoiesis stimulating agents) l ESA doses should be decreased, but not necessarily held, when a downward adjustment of Hb level is needed. ( withholding ESA doses may lead to a delayed decrease in Hb levls to less than target range) l ESA doses not be withheld routinely for Hb levels greater than target range, hospitalization, poorly controlled HTN, or vascular access occlusion ( A/E ; HTN, headache, influenza-like symptom) l Route l PD-CKD/ND-CKD : SC administration is the only routinely feasible route of administration l HD-CKD : either SC or IV administration is possible, but the risk for PRCA is relatively high with SC administration → FDA recommend the IV route

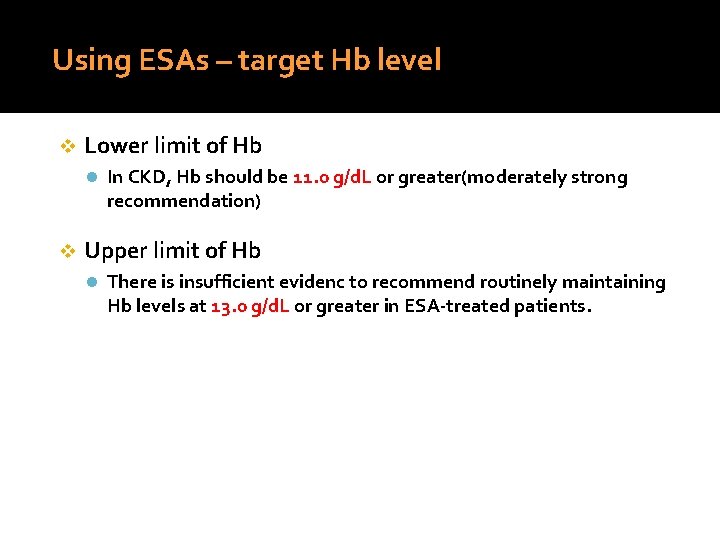
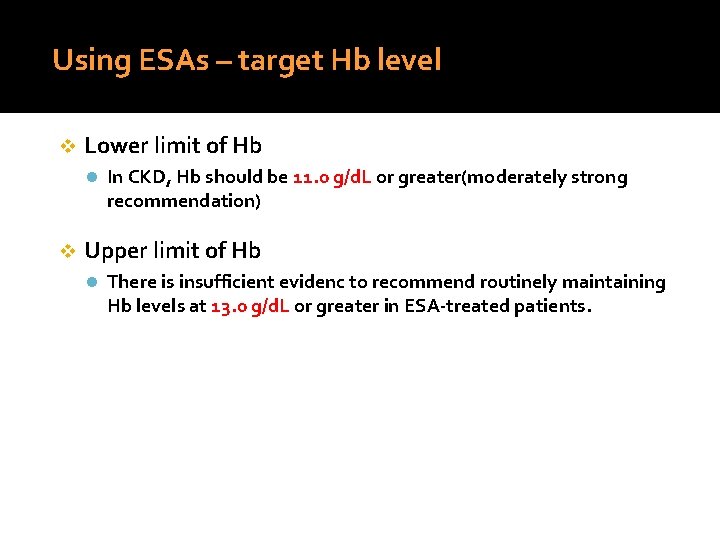
Using ESAs – target Hb level v Lower limit of Hb l In CKD, Hb should be 11. 0 g/d. L or greater(moderately strong recommendation) v Upper limit of Hb l There is insufficient evidenc to recommend routinely maintaining Hb levels at 13. 0 g/d. L or greater in ESA-treated patients.

3 RCTs Designed to Address Whether Anemia Correction in CKD May Improve CV Morbidity and Mortality � CREATE (Cardiovascular risk Reduction by Early Anemia Treatment with Epoetin beta) - Completed Determine the impact of early vs late anemia correction on mortality and cardiovascular morbidity in patients with CKD � CHOIR (Correction of Hemoglobin and Outcomes In Renal insufficiency) – Terminated Early Determine the impact of degree of anemia correction on mortality and cardiovascular morbidity in patients with CKD � TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) Enrolling Determine the impact of anemia therapy (yes/no) on mortality and cardiovascular morbidity in patients with CKD and type 2 diabetes

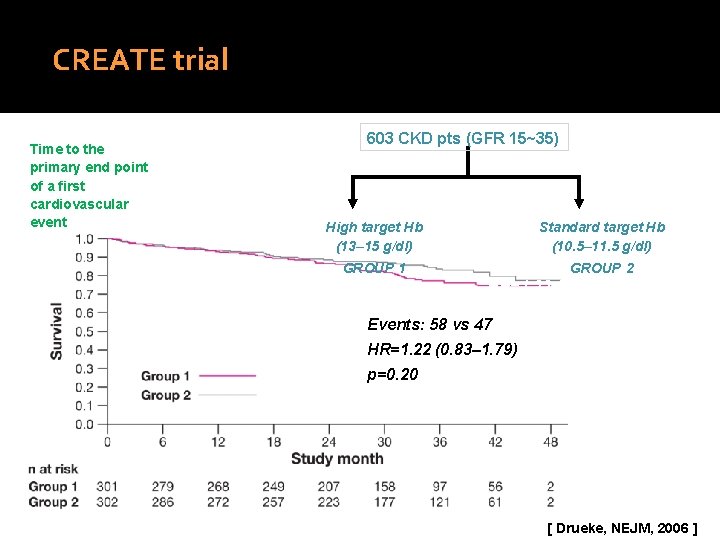
CREATE trial Time to the primary end point of a first cardiovascular event 603 CKD pts (GFR 15~35) High target Hb (13– 15 g/dl) GROUP 1 Standard target Hb (10. 5– 11. 5 g/dl) Events: 58 GROUP vs 47 2 HR=1. 22 (0. 83– 1. 79) Log rank test p=0. 20 Events: 58 vs 47 HR=1. 22 (0. 83– 1. 79) p=0. 20 [ Drueke, NEJM, 2006 ]

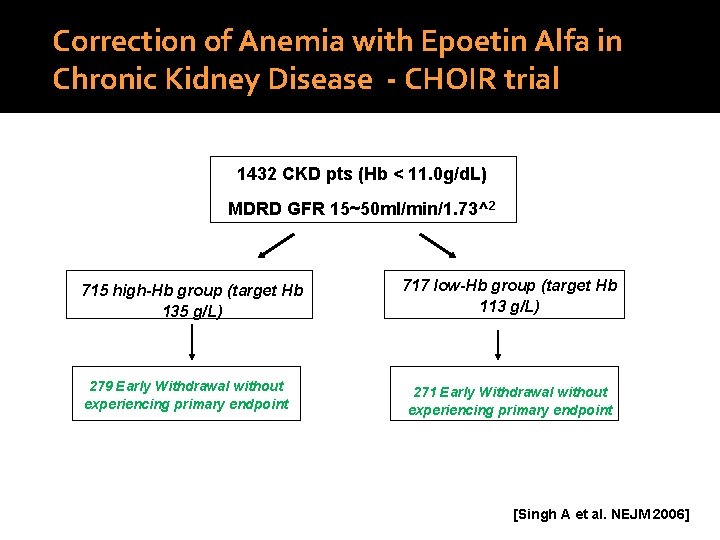
Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease - CHOIR trial 1432 CKD pts (Hb < 11. 0 g/d. L) MDRD GFR 15~50 ml/min/1. 73^2 715 high-Hb group (target Hb 135 g/L) 279 Early Withdrawal without experiencing primary endpoint 717 low-Hb group (target Hb 113 g/L) 271 Early Withdrawal without experiencing primary endpoint [Singh A et al. NEJM 2006]

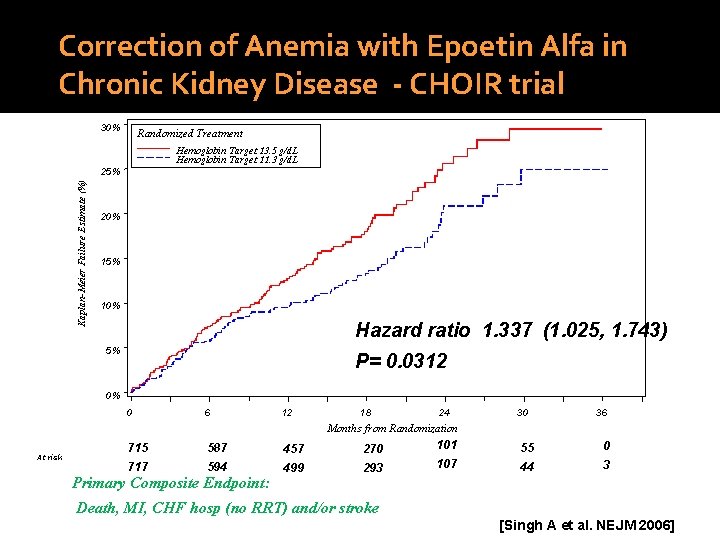
Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease - CHOIR trial 30% Randomized Treatment Hemoglobin Target 13. 5 g/d. L Hemoglobin Target 11. 3 g/d. L Kaplan-Meier Failure Estimate (%) 25% 20% 15% 10% Hazard ratio 1. 337 (1. 025, 1. 743) 5% P= 0. 0312 0% 0 6 12 18 24 30 36 Months from Randomization At risk 715 587 457 270 101 55 0 717 594 499 293 107 44 3 Primary Composite Endpoint: Death, MI, CHF hosp (no RRT) and/or stroke [Singh A et al. NEJM 2006]

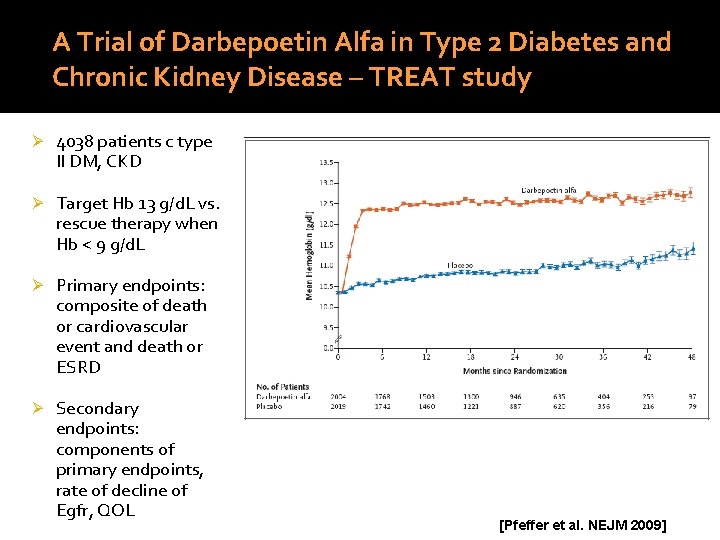
A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease – TREAT study Ø 4038 patients c type II DM, CKD Ø Target Hb 13 g/d. L vs. rescue therapy when Hb < 9 g/d. L Ø Primary endpoints: composite of death or cardiovascular event and death or ESRD Ø Secondary endpoints: components of primary endpoints, rate of decline of Egfr, QOL [Pfeffer et al. NEJM 2009]

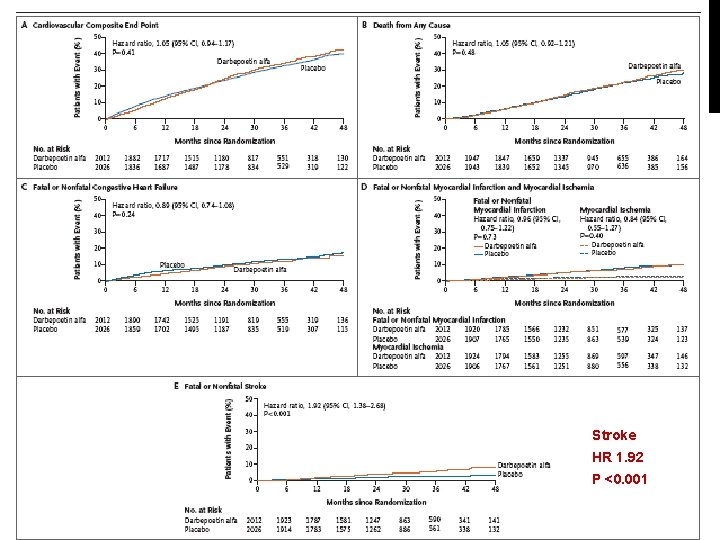
Stroke HR 1. 92 P <0. 001

Using ESAs – target Hb level v Lower limit of Hb l In CKD, Hb should be 11. 0 g/d. L or greater(moderately strong recommendation) v Upper limit of Hb l There is insufficient evidenc to recommend routinely maintaining Hb levels at 13. 0 g/d. L or greater in ESA-treated patients.

Using iron agents v Target of iron therapy l HD – CKD Serum ferritin > 200 ng/m. L AND TSAT > 20% l PD – CKD and ND-CKD Serum ferritin > 100 ng/m. L AND TSAT > 20%

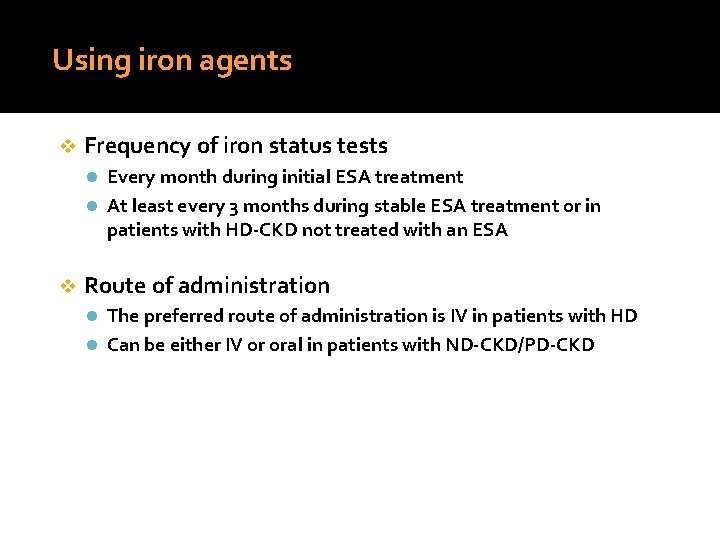
Using iron agents v Frequency of iron status tests l Every month during initial ESA treatment l At least every 3 months during stable ESA treatment or in patients with HD-CKD not treated with an ESA v Route of administration l The preferred route of administration is IV in patients with HD l Can be either IV or oral in patients with ND-CKD/PD-CKD

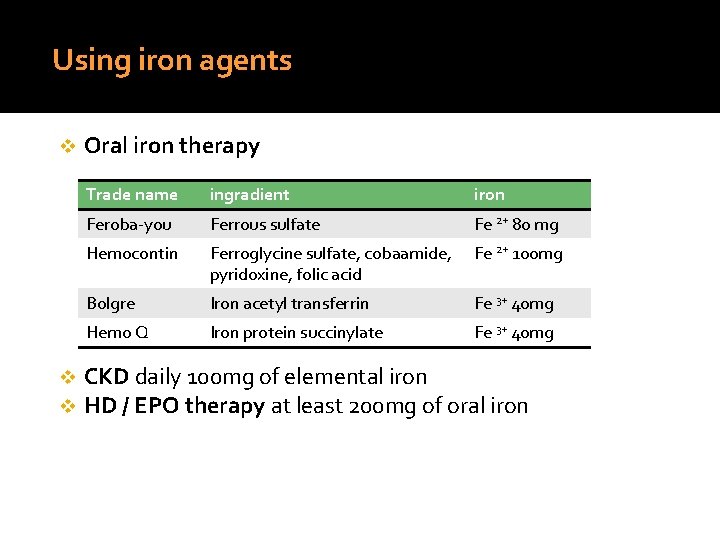
Using iron agents v v v Oral iron therapy Trade name ingradient iron Feroba-you Ferrous sulfate Fe 2+ 80 mg Hemocontin Ferroglycine sulfate, cobaamide, pyridoxine, folic acid Fe 2+ 100 mg Bolgre Iron acetyl transferrin Fe 3+ 40 mg Hemo Q Iron protein succinylate Fe 3+ 40 mg CKD daily 100 mg of elemental iron HD / EPO therapy at least 200 mg of oral iron

Using iron agents v IV iron therapy l Iron sucrose ( Venoferrum ®) l Iron gluconate ( Ferrlecit ®) l Iron dextran ( Cosmofer ®) l IV dosing in HD : 1000 mg dose every 8 -10 consecutive HD

Using iron agents v Adverse reactions of IV iron l Immune-mediated (anaphylaxis) ü iron dextran > ferric gluconate/ iron sucrose (0. 6 -0. 7% of lifethreatening reactions) ü requires stopping the agent l Labile or free-iron reactions ü Iron agent releases bioactive partially unbound iron into circulation resulting in oxidative stress and hypotension (hypotension, cramping, diarrhea etc. ) ü More frequent with non-dextran forms of iron ü Require a decrease in dose and/or rate of infusion but don’t necessitate stopping the agent

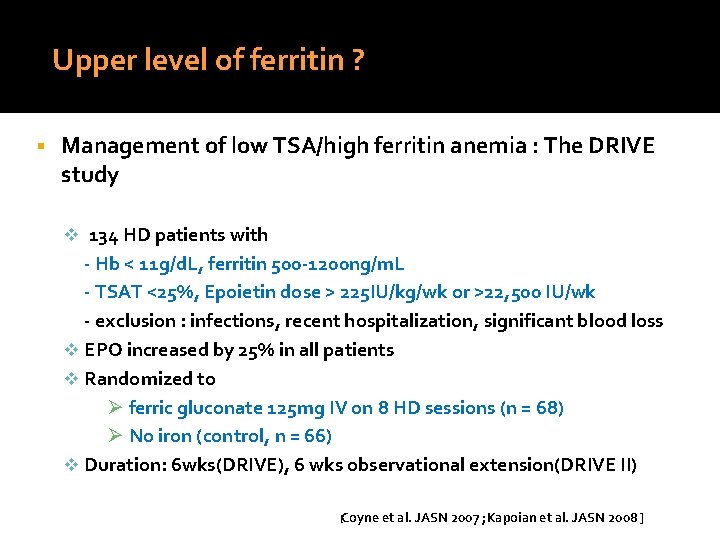
Upper level of ferritin ? Management of low TSA/high ferritin anemia : The DRIVE study v 134 HD patients with - Hb < 11 g/d. L, ferritin 500 -1200 ng/m. L - TSAT <25%, Epoietin dose > 225 IU/kg/wk or >22, 500 IU/wk - exclusion : infections, recent hospitalization, significant blood loss v EPO increased by 25% in all patients v Randomized to Ø ferric gluconate 125 mg IV on 8 HD sessions (n = 68) Ø No iron (control, n = 66) v Duration: 6 wks(DRIVE), 6 wks observational extension(DRIVE II) [Coyne et al. JASN 2007 ; Kapoian et al. JASN 2008 ]

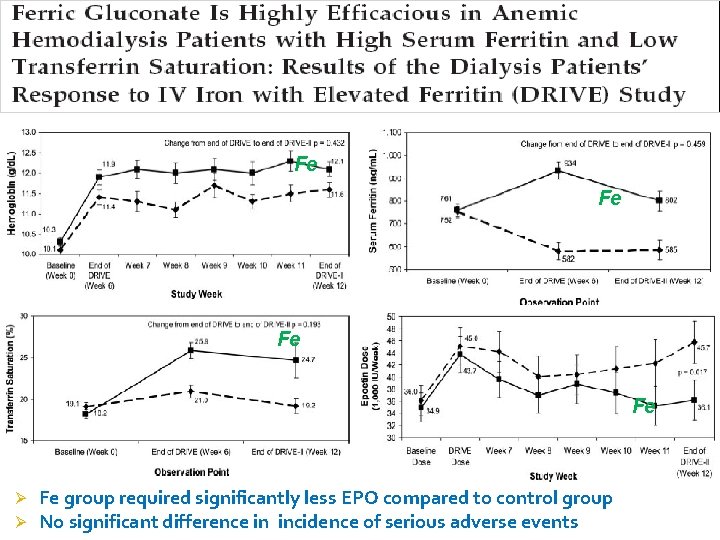
Fe Fe Ø Ø Fe group required significantly less EPO compared to control group No significant difference in incidence of serious adverse events

Using iron agents – Upper level of ferritin v However, long-term safety of IV iron remains untested. potentia concerns: • risk of bacterial infections • Free radical generation from free iron causing oxidant mediated tissue injury • Parenchymal cell iron deposition with organ dysfunction v There is insufficient evidence to recommend routine administration of IV iron if serum ferritin level is greater than 500 ng/m. L.

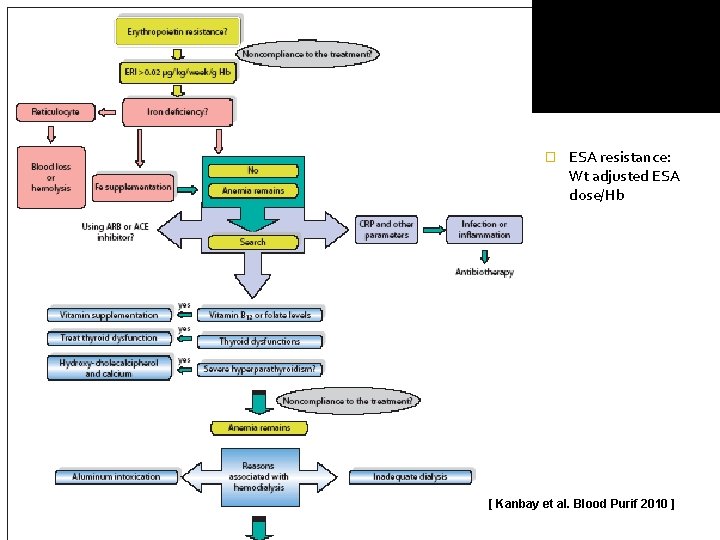
Evaluating and correcting persistent railure to reach or maintain intended Hb A. B. Hyporesponse to ESA and iron therapy Evaluation for Ab- mediated PRCA(pure red cell aplasia)

Hyporesponsiveness to ESAs a significant increase in the ESA dose requirement to maintain a certain Hb level or a significant decrease in Hb level at a constant ESA dose Ø a failure to increase the Hb level > 11 g/d. L despite an ESA dose equivalent to epoetin > 500 IU/kg/week Ø v resistance to ESAs is associated with an increased risk of death or cardiovascular events in CKD

Hyporesponsiveness to ESAs v m/c factors - v Persistent iron deficiency Frequent hospitalization Hospitalization for infection Temporary catheter insertion Permanent catheter insertion Hypoalbuminemia Elevated CRP level Preexisting hematologic disorders { thalassemia, Hb H, Hb S, Multiple myeloma etc}

� ESA resistance: Wt adjusted ESA dose/Hb [ Kanbay et al. Blood Purif 2010 ]

Antibody mediated PRCA v Evaluation for Ab- mediated PRCA(pure red cell aplasia) should be undertaken when a patient receiving ESA therapy for more than 4 weeks develops each of the following: Ø Ø Ø v Sudden rapid decrease in Hb level at the rate of 0. 5 to 1. 0 g/d. L/ wk, or requirement of red blood cell transfusions at the rate of approximately 1 to 2 per week, AND { sudden development of severe transfusion-dependent anemia } Normal platelet and WBC count, AND { nonerythroid marrow is unaffected } Absolute reticulocyte count less than 10, 000/mcl Reported in subcutaneous administration,

Antibody mediated PRCA Ø Neutralizing Ig. G Ab to the protein component of exogenous recombinant EPO are found that cross-react with endogenous erythropoietin Ø Anemia characterized by a very low reticulocyte count and the virtual absence of erythroid precursors in the BM Ø Management ü ü ü Transfusion for symptomatic anemia Discontinue the administration of any ESA product !! (avoid any alternative EPO preparation, including darbepoietin alfa) - anti-EPO Ab crossreact not only with the endogenous hor, but also c all recombinant EPO molecules, including darbepoietin alfa - rechallenge c EPO may incite the formation of allergic skin and systemic reaction(anaphylaxis) Immunosuppressive therapy, should probably be provided in most cases