ANEMIA ANEMIA SICKLE CELL Oleh Tim Dosen Farmasi











- Slides: 66

ANEMIA & ANEMIA SICKLE CELL Oleh: Tim Dosen Farmasi Klinik PSF FKUB

Anemia

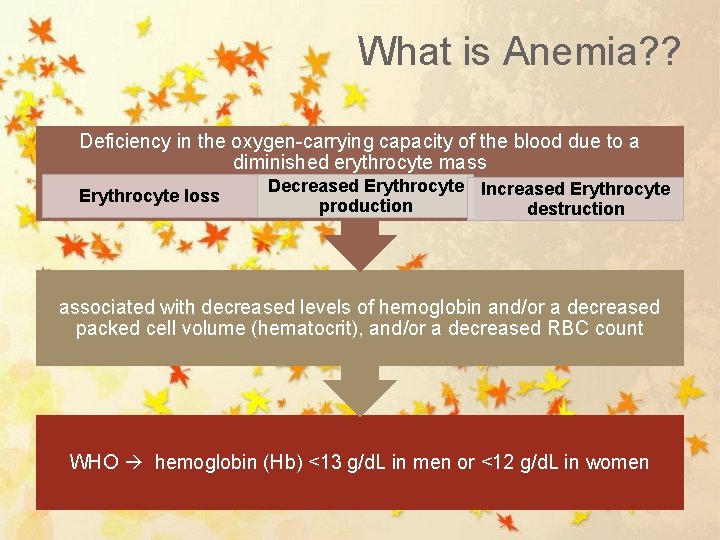
What is Anemia? ? Deficiency in the oxygen-carrying capacity of the blood due to a diminished erythrocyte mass Erythrocyte loss Decreased Erythrocyte Increased Erythrocyte production destruction associated with decreased levels of hemoglobin and/or a decreased packed cell volume (hematocrit), and/or a decreased RBC count WHO hemoglobin (Hb) <13 g/d. L in men or <12 g/d. L in women

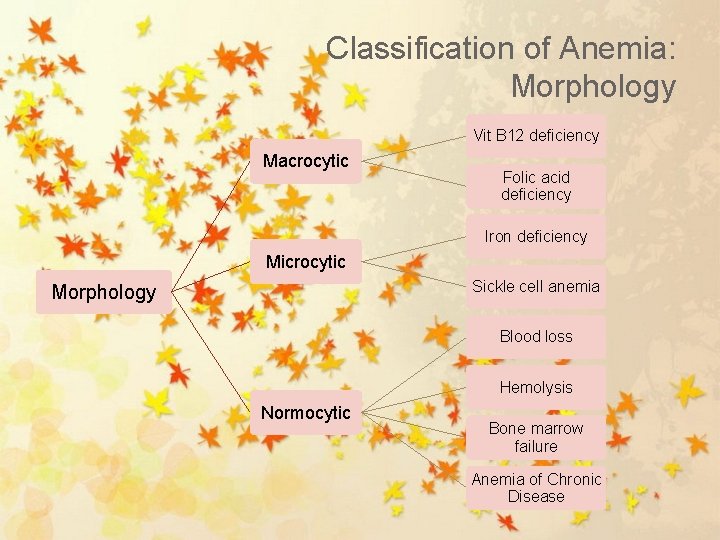
Classification of Anemia: Morphology Vit B 12 deficiency Macrocytic Folic acid deficiency Iron deficiency Microcytic Sickle cell anemia Morphology Blood loss Hemolysis Normocytic Bone marrow failure Anemia of Chronic Disease

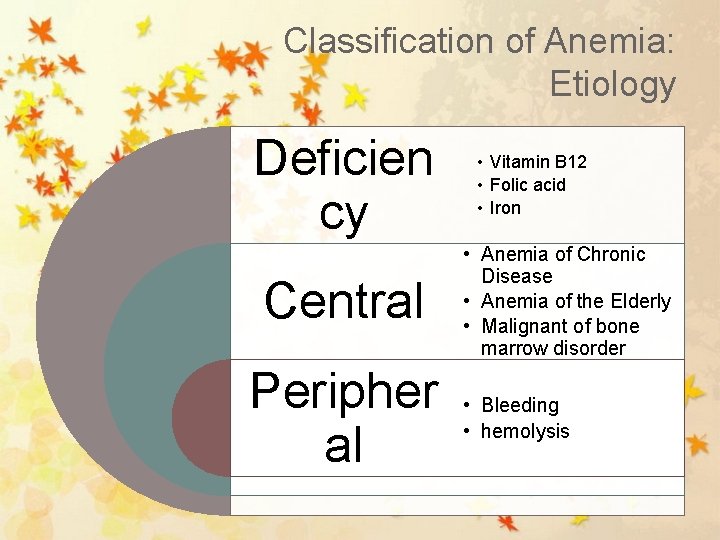
Classification of Anemia: Etiology Deficien cy Central Peripher al • Vitamin B 12 • Folic acid • Iron • Anemia of Chronic Disease • Anemia of the Elderly • Malignant of bone marrow disorder • Bleeding • hemolysis

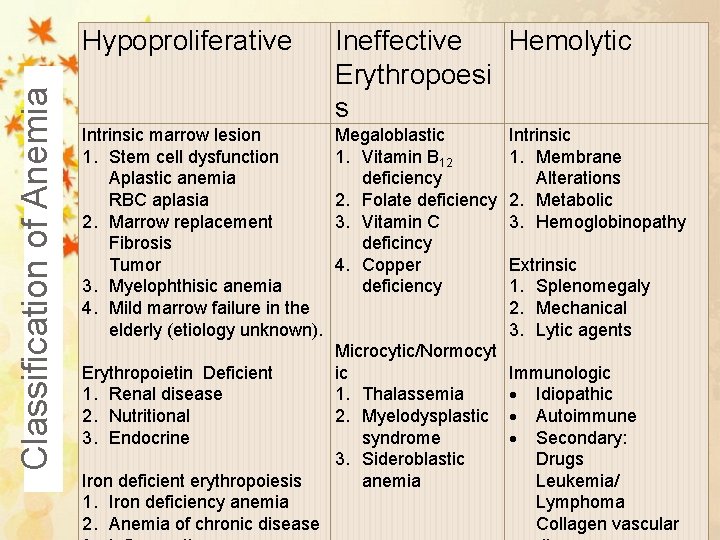
Classification of Anemia Hypoproliferative Ineffective Hemolytic Erythropoesi s Intrinsic marrow lesion 1. Stem cell dysfunction Aplastic anemia RBC aplasia 2. Marrow replacement Fibrosis Tumor 3. Myelophthisic anemia 4. Mild marrow failure in the elderly (etiology unknown). Erythropoietin Deficient 1. Renal disease 2. Nutritional 3. Endocrine Iron deficient erythropoiesis 1. Iron deficiency anemia 2. Anemia of chronic disease Megaloblastic 1. Vitamin B 12 deficiency 2. Folate deficiency 3. Vitamin C deficincy 4. Copper deficiency Microcytic/Normocyt ic 1. Thalassemia 2. Myelodysplastic syndrome 3. Sideroblastic anemia Intrinsic 1. Membrane Alterations 2. Metabolic 3. Hemoglobinopathy Extrinsic 1. Splenomegaly 2. Mechanical 3. Lytic agents Immunologic Idiopathic Autoimmune Secondary: Drugs Leukemia/ Lymphoma Collagen vascular

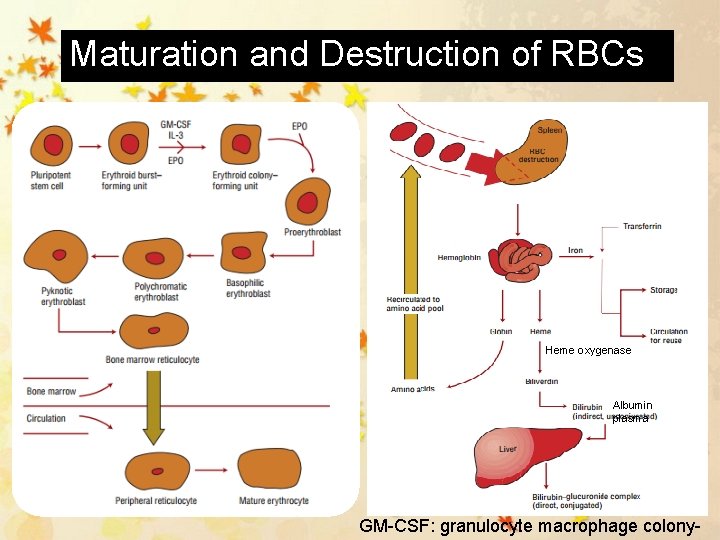
Maturation and Destruction of RBCs Heme oxygenase Albumin plasma GM-CSF: granulocyte macrophage colony-

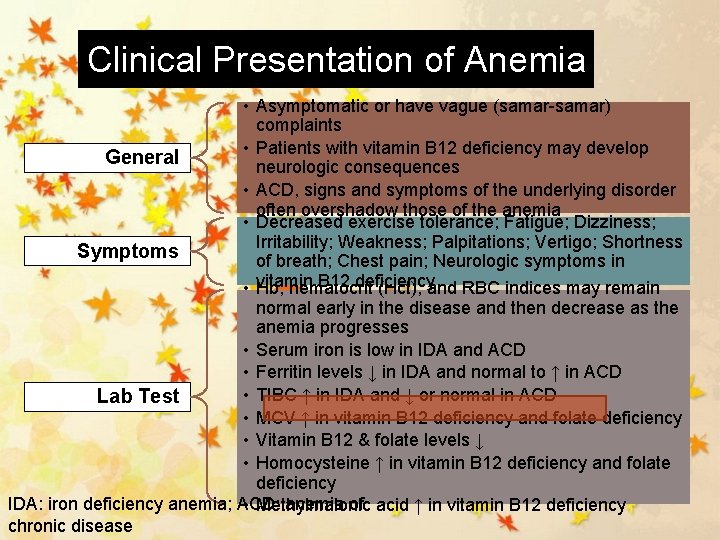
Clinical Presentation of Anemia • Asymptomatic or have vague (samar-samar) complaints • Patients with vitamin B 12 deficiency may develop General neurologic consequences • ACD, signs and symptoms of the underlying disorder often overshadow those of the anemia • Decreased exercise tolerance; Fatigue; Dizziness; Irritability; Weakness; Palpitations; Vertigo; Shortness Symptoms of breath; Chest pain; Neurologic symptoms in • vitamin B 12 deficiency Hb, hematocrit (Hct), and RBC indices may remain normal early in the disease and then decrease as the anemia progresses • Serum iron is low in IDA and ACD • Ferritin levels ↓ in IDA and normal to ↑ in ACD • TIBC ↑ in IDA and ↓ or normal in ACD Lab Test • MCV ↑ in vitamin B 12 deficiency and folate deficiency • Vitamin B 12 & folate levels ↓ • Homocysteine ↑ in vitamin B 12 deficiency and folate deficiency IDA: iron deficiency anemia; ACD: anemia of • Methylmalonic acid ↑ in vitamin B 12 deficiency chronic disease

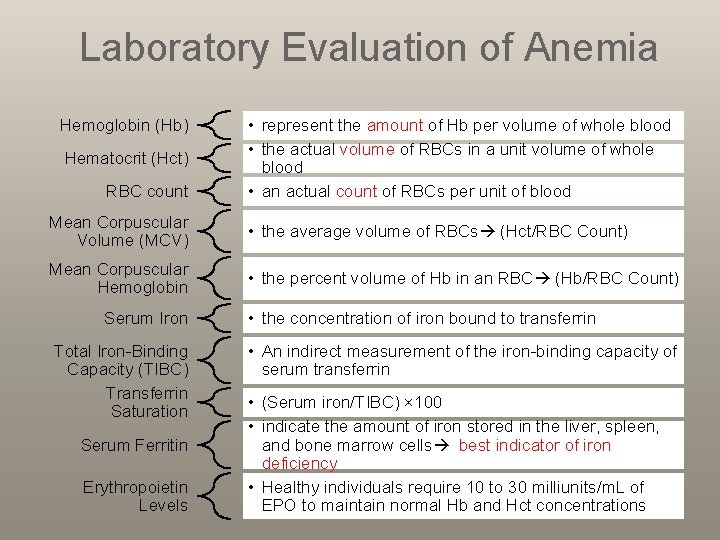
Laboratory Evaluation of Anemia Hemoglobin (Hb) Hematocrit (Hct) RBC count • represent the amount of Hb per volume of whole blood • the actual volume of RBCs in a unit volume of whole blood • an actual count of RBCs per unit of blood Mean Corpuscular Volume (MCV) • the average volume of RBCs (Hct/RBC Count) Mean Corpuscular Hemoglobin • the percent volume of Hb in an RBC (Hb/RBC Count) Serum Iron Total Iron-Binding Capacity (TIBC) Transferrin Saturation Serum Ferritin Erythropoietin Levels • the concentration of iron bound to transferrin • An indirect measurement of the iron-binding capacity of serum transferrin • (Serum iron/TIBC) × 100 • indicate the amount of iron stored in the liver, spleen, and bone marrow cells best indicator of iron deficiency • Healthy individuals require 10 to 30 milliunits/m. L of EPO to maintain normal Hb and Hct concentrations

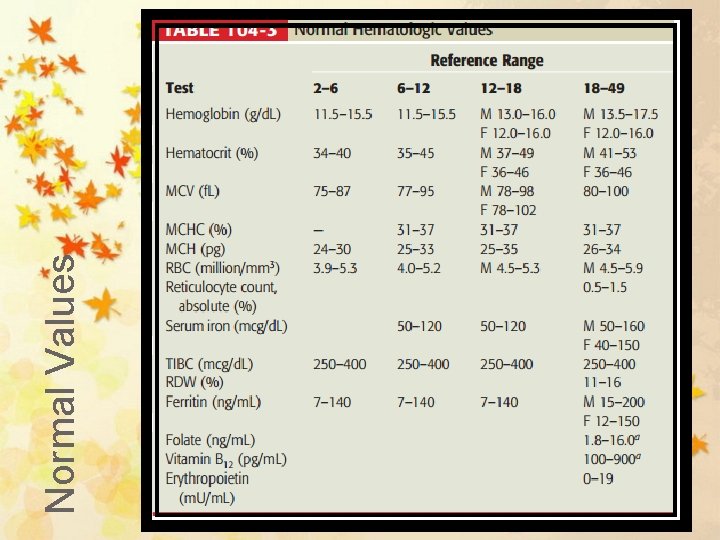
Normal Values

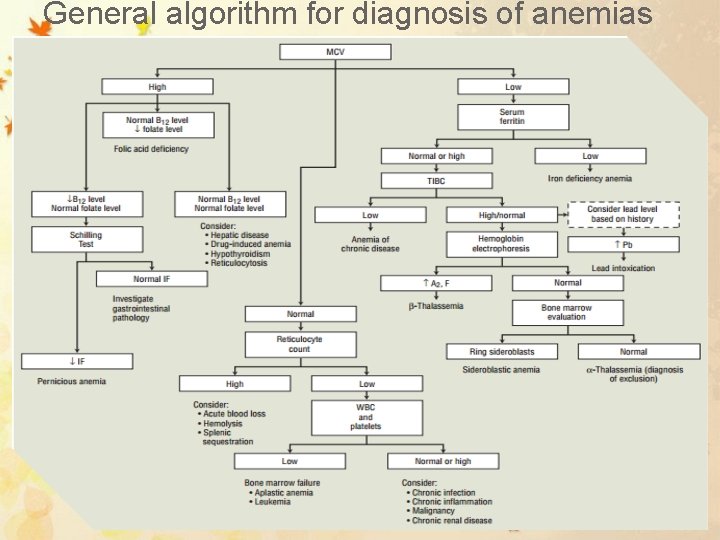
General algorithm for diagnosis of anemias

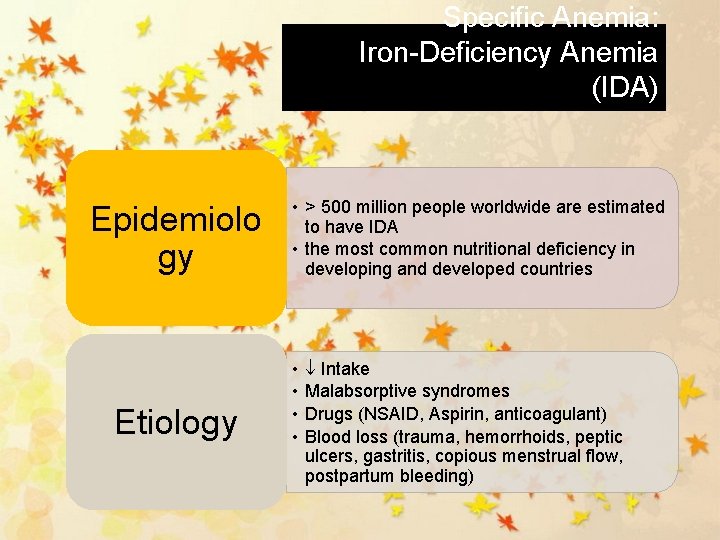
Specific Anemia: Iron-Deficiency Anemia (IDA) Epidemiolo gy Etiology • > 500 million people worldwide are estimated to have IDA • the most common nutritional deficiency in developing and developed countries • • Intake Malabsorptive syndromes Drugs (NSAID, Aspirin, anticoagulant) Blood loss (trauma, hemorrhoids, peptic ulcers, gastritis, copious menstrual flow, postpartum bleeding)

Iron-Deficiency Anemia (IDA) Without iron, cells lose Laboratory findings their capacity for electron transport and energy metabolism low serum iron and ferritin levels and high TIBC Iron a cofactor for oxidative metabolism, dopamine and DNA synthesis, and free radical function in neutrophils ferritin may not correlate with iron stores in the bone marrow because renal or hepatic disease, malignancies, infection, or inflammatory processes may increase ferritin values Transferrin saturation useful for assessing IDA

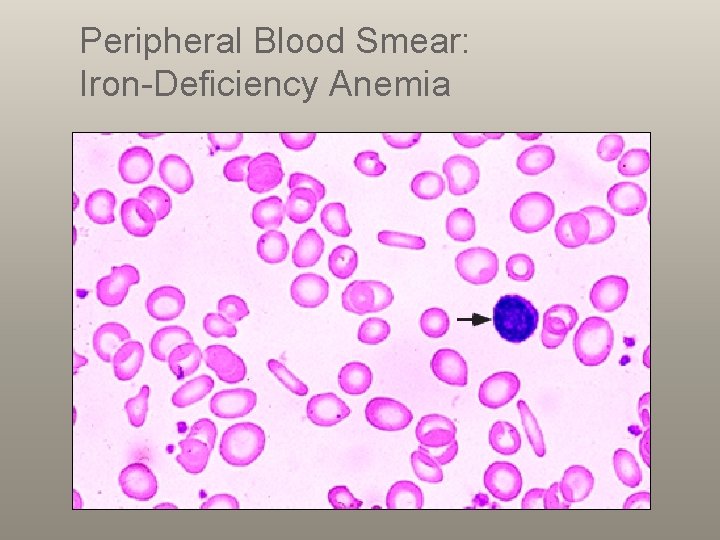
Peripheral Blood Smear: Iron-Deficiency Anemia

Treatment of IDA: Oral Iron Products

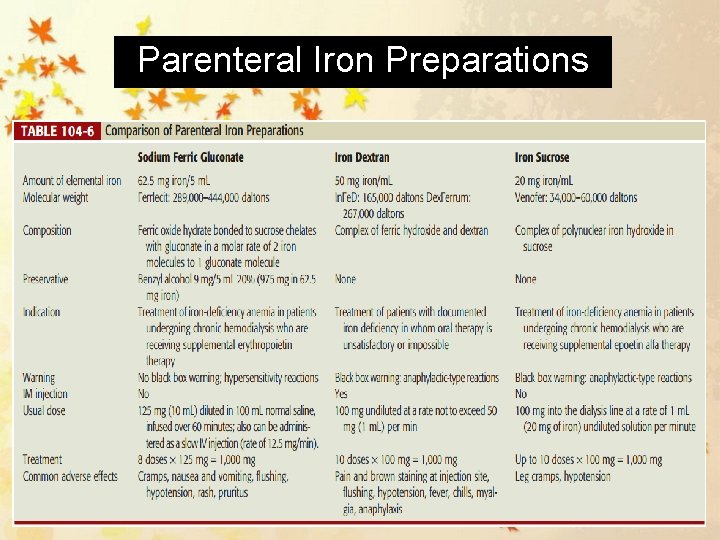
Parenteral Iron Preparations

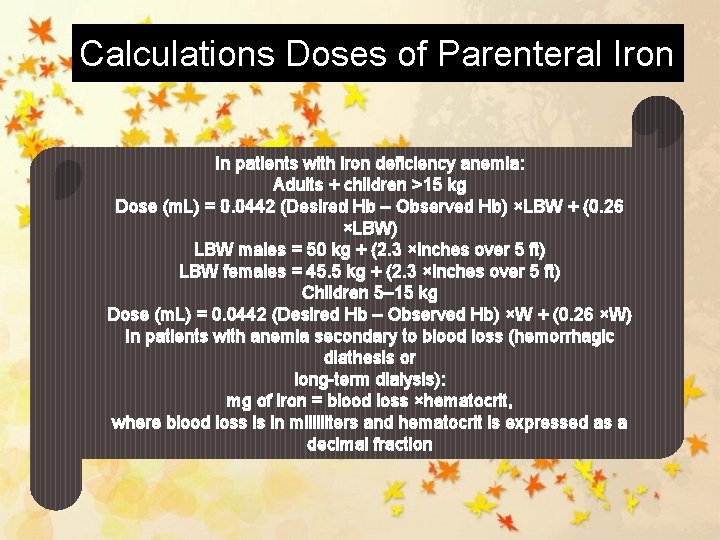
Calculations Doses of Parenteral Iron In patients with iron deficiency anemia: Adults + children >15 kg Dose (m. L) = 0. 0442 (Desired Hb – Observed Hb) ×LBW + (0. 26 ×LBW) LBW males = 50 kg + (2. 3 ×inches over 5 ft) LBW females = 45. 5 kg + (2. 3 ×inches over 5 ft) Children 5– 15 kg Dose (m. L) = 0. 0442 (Desired Hb – Observed Hb) ×W + (0. 26 ×W) In patients with anemia secondary to blood loss (hemorrhagic diathesis or long-term dialysis): mg of iron = blood loss ×hematocrit, where blood loss is in milliliters and hematocrit is expressed as a decimal fraction

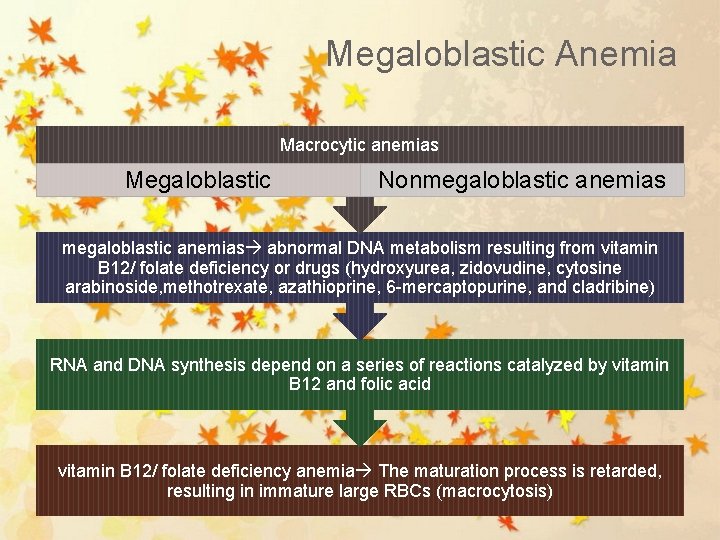
Megaloblastic Anemia Macrocytic anemias Megaloblastic Nonmegaloblastic anemias abnormal DNA metabolism resulting from vitamin B 12/ folate deficiency or drugs (hydroxyurea, zidovudine, cytosine arabinoside, methotrexate, azathioprine, 6 -mercaptopurine, and cladribine) RNA and DNA synthesis depend on a series of reactions catalyzed by vitamin B 12 and folic acid vitamin B 12/ folate deficiency anemia The maturation process is retarded, resulting in immature large RBCs (macrocytosis)

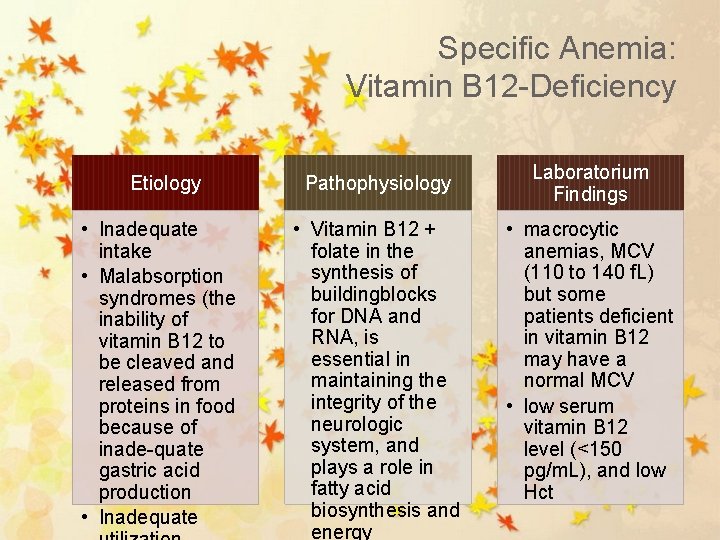
Specific Anemia: Vitamin B 12 -Deficiency Etiology Pathophysiology • Inadequate intake • Malabsorption syndromes (the inability of vitamin B 12 to be cleaved and released from proteins in food because of inade-quate gastric acid production • Inadequate • Vitamin B 12 + folate in the synthesis of buildingblocks for DNA and RNA, is essential in maintaining the integrity of the neurologic system, and plays a role in fatty acid biosynthesis and energy Laboratorium Findings • macrocytic anemias, MCV (110 to 140 f. L) but some patients deficient in vitamin B 12 may have a normal MCV • low serum vitamin B 12 level (<150 pg/m. L), and low Hct

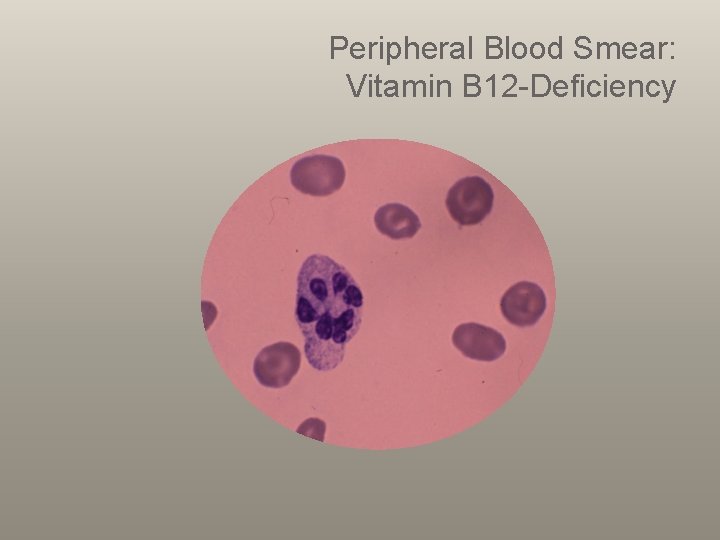
Peripheral Blood Smear: Vitamin B 12 -Deficiency

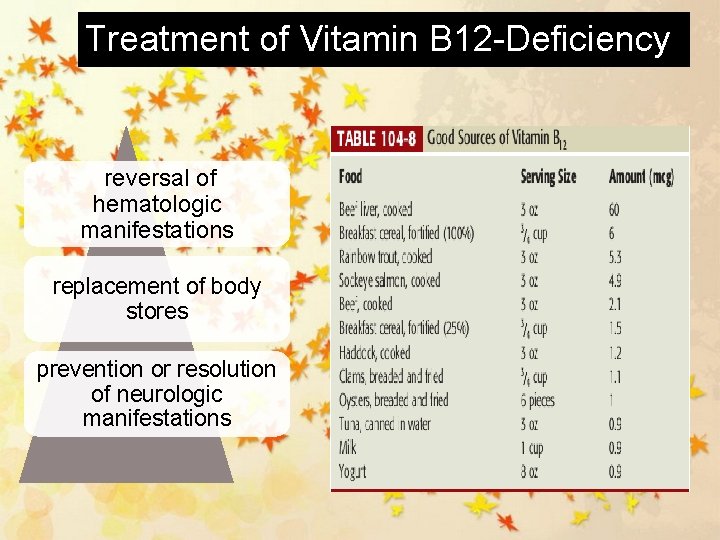
Treatment of Vitamin B 12 -Deficiency reversal of hematologic manifestations replacement of body stores prevention or resolution of neurologic manifestations

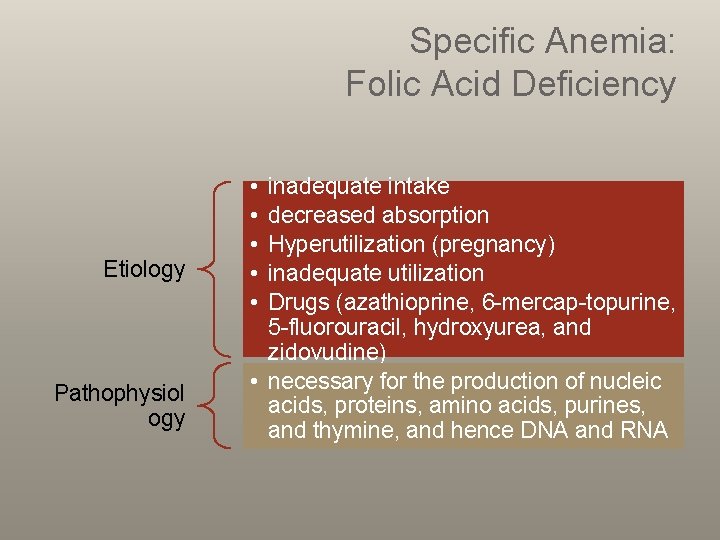
Specific Anemia: Folic Acid Deficiency Etiology Pathophysiol ogy • • • inadequate intake decreased absorption Hyperutilization (pregnancy) inadequate utilization Drugs (azathioprine, 6 -mercap-topurine, 5 -fluorouracil, hydroxyurea, and zidovudine) • necessary for the production of nucleic acids, proteins, amino acids, purines, and thymine, and hence DNA and RNA

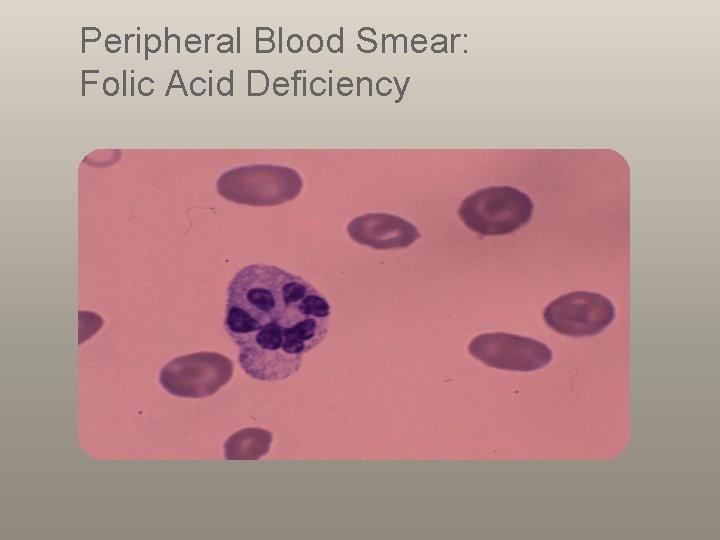
Peripheral Blood Smear: Folic Acid Deficiency

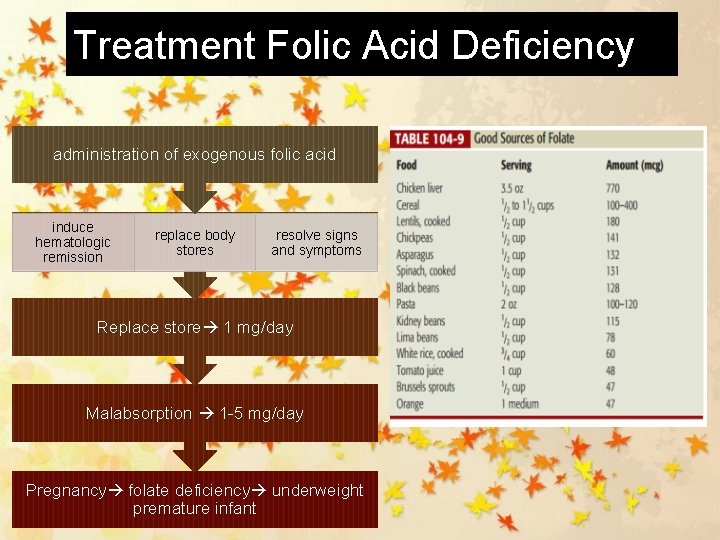
Treatment Folic Acid Deficiency administration of exogenous folic acid induce hematologic remission replace body stores resolve signs and symptoms Replace store 1 mg/day Malabsorption 1 -5 mg/day Pregnancy folate deficiency underweight premature infant

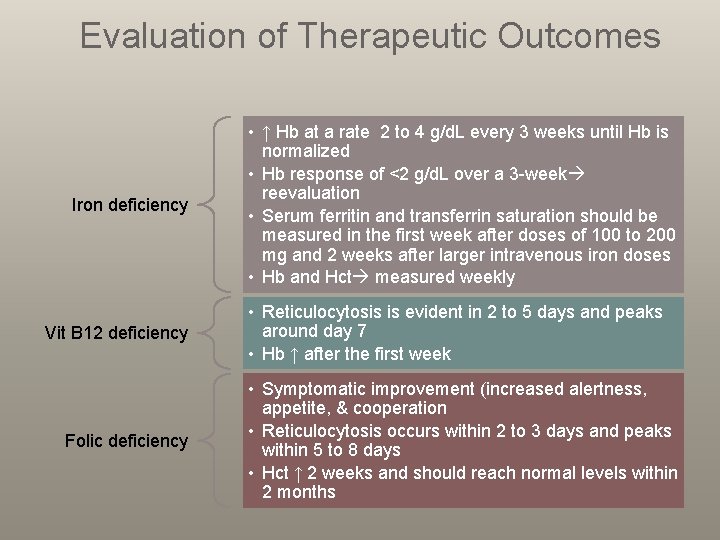
Evaluation of Therapeutic Outcomes Iron deficiency Vit B 12 deficiency Folic deficiency • ↑ Hb at a rate 2 to 4 g/d. L every 3 weeks until Hb is normalized • Hb response of <2 g/d. L over a 3 -week reevaluation • Serum ferritin and transferrin saturation should be measured in the first week after doses of 100 to 200 mg and 2 weeks after larger intravenous iron doses • Hb and Hct measured weekly • Reticulocytosis is evident in 2 to 5 days and peaks around day 7 • Hb ↑ after the first week • Symptomatic improvement (increased alertness, appetite, & cooperation • Reticulocytosis occurs within 2 to 3 days and peaks within 5 to 8 days • Hct ↑ 2 weeks and should reach normal levels within 2 months

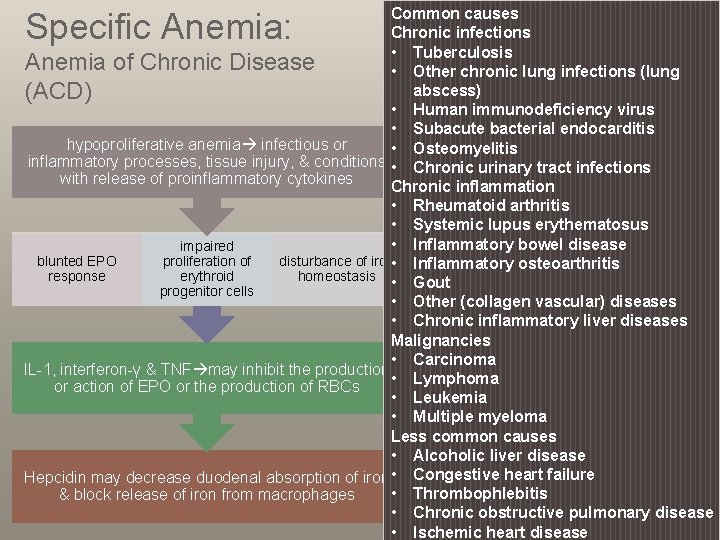
Common causes Chronic infections • Tuberculosis Anemia of Chronic Disease • Other chronic lung infections (lung abscess) (ACD) • Human immunodeficiency virus • Subacute bacterial endocarditis hypoproliferative anemia infectious or • Osteomyelitis inflammatory processes, tissue injury, & conditions • Chronic urinary tract infections with release of proinflammatory cytokines Chronic inflammation • Rheumatoid arthritis • Systemic lupus erythematosus • Inflammatory bowel disease impaired blunted EPO proliferation of disturbance of iron • Inflammatory osteoarthritis response erythroid homeostasis • Gout progenitor cells • Other (collagen vascular) diseases • Chronic inflammatory liver diseases Malignancies • Carcinoma IL-1, interferon-γ & TNF may inhibit the production • Lymphoma or action of EPO or the production of RBCs • Leukemia • Multiple myeloma Less common causes • Alcoholic liver disease Hepcidin may decrease duodenal absorption of iron • Congestive heart failure • Thrombophlebitis & block release of iron from macrophages • Chronic obstructive pulmonary disease • Ischemic heart disease Specific Anemia:

Treatment Anemia of Chronic Disease Iron • Effective only if iron deficiency is present • During inflammation, oral or parenteral iron therapy is ineffective Absorption ↓ because of downregulation of ferroportin and iron diversion mediated by cytokines • Parenteral iron may have a role for patients unresponsive to erythropoietic agents Transfussion • limited to situations (oxygen transport is inadequate) • The transfusion threshold varies from 8 to 10 g/d. L • bloodborne infections, development of autoantibodies, transfusion reactions & iron overload EPO • recombinant epoetin alfa & recombinant darbepoetin alfa • dosage of epoetin alfa is 50 to 100 IU/Kg. BB 3 x/week • ↑ 150 IU/Kg. BB per dose if no increase in Hb concentration occurs after 6 to 8 weeks

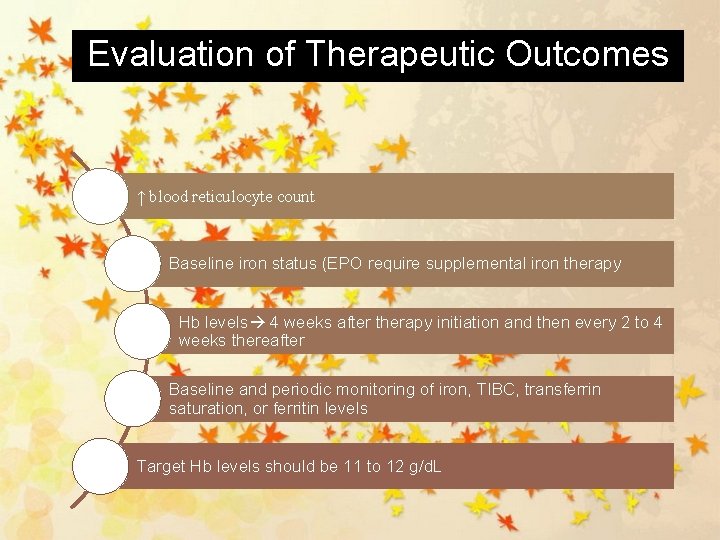
Evaluation of Therapeutic Outcomes ↑ blood reticulocyte count Baseline iron status (EPO require supplemental iron therapy Hb levels 4 weeks after therapy initiation and then every 2 to 4 weeks thereafter Baseline and periodic monitoring of iron, TIBC, transferrin saturation, or ferritin levels Target Hb levels should be 11 to 12 g/d. L

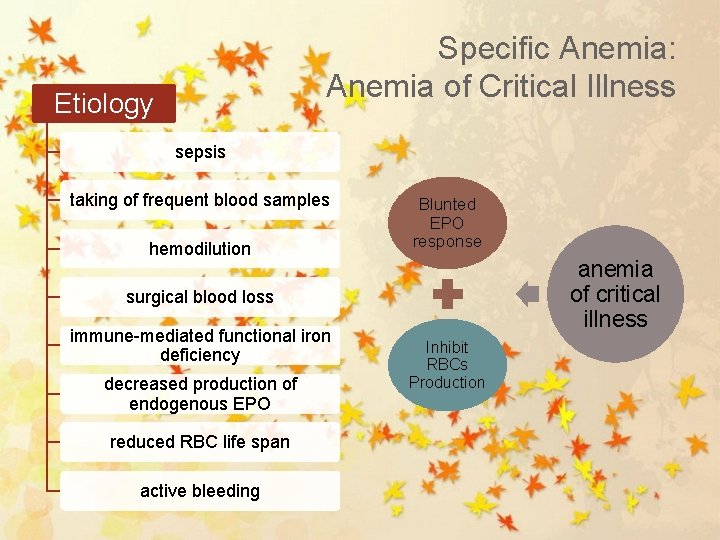
Specific Anemia: Anemia of Critical Illness Etiology sepsis taking of frequent blood samples hemodilution Blunted EPO response anemia of critical illness surgical blood loss immune-mediated functional iron deficiency decreased production of endogenous EPO reduced RBC life span active bleeding Inhibit RBCs Production

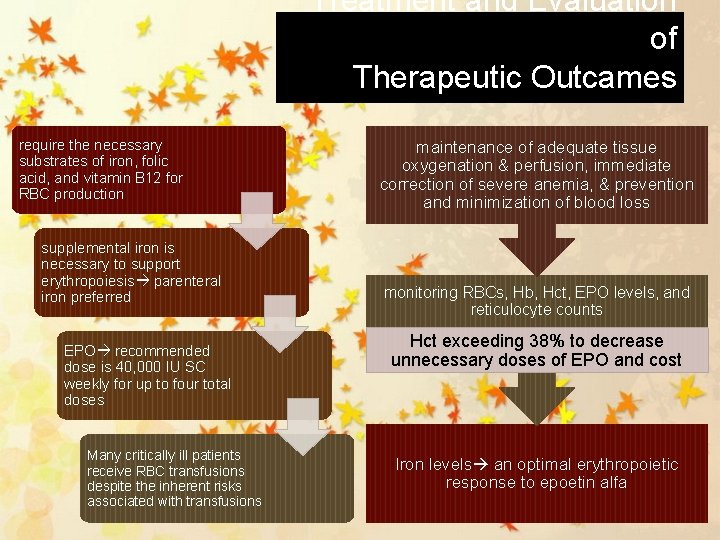
Treatment and Evaluation of Therapeutic Outcames require the necessary substrates of iron, folic acid, and vitamin B 12 for RBC production supplemental iron is necessary to support erythropoiesis parenteral iron preferred EPO recommended dose is 40, 000 IU SC weekly for up to four total doses Many critically ill patients receive RBC transfusions despite the inherent risks associated with transfusions maintenance of adequate tissue oxygenation & perfusion, immediate correction of severe anemia, & prevention and minimization of blood loss monitoring RBCs, Hb, Hct, EPO levels, and reticulocyte counts Hct exceeding 38% to decrease unnecessary doses of EPO and cost Iron levels an optimal erythropoietic response to epoetin alfa

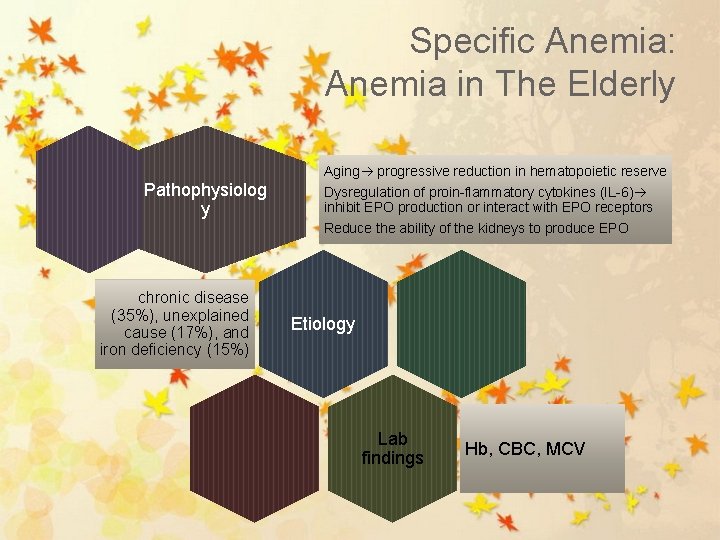
Specific Anemia: Anemia in The Elderly Aging progressive reduction in hematopoietic reserve Pathophysiolog y chronic disease (35%), unexplained cause (17%), and iron deficiency (15%) Dysregulation of proin-flammatory cytokines (IL-6) inhibit EPO production or interact with EPO receptors Reduce the ability of the kidneys to produce EPO Etiology Lab findings Hb, CBC, MCV

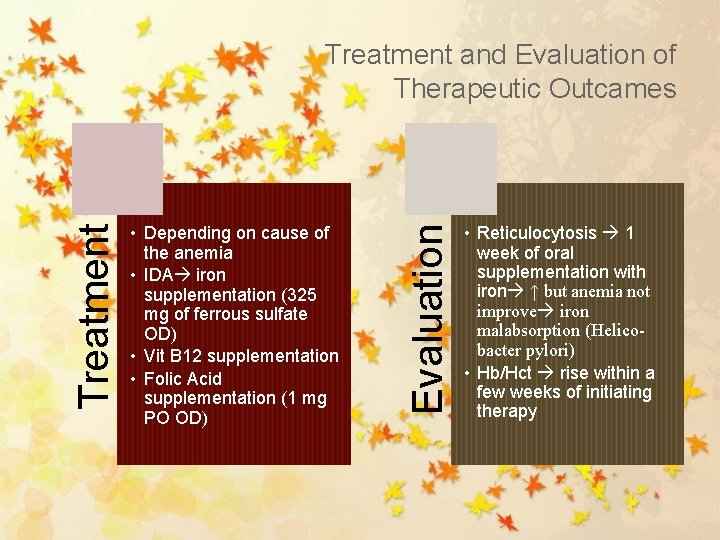
• Depending on cause of the anemia • IDA iron supplementation (325 mg of ferrous sulfate OD) • Vit B 12 supplementation • Folic Acid supplementation (1 mg PO OD) Evaluation Treatment and Evaluation of Therapeutic Outcames • Reticulocytosis 1 week of oral supplementation with iron ↑ but anemia not improve iron malabsorption (Helicobacter pylori) • Hb/Hct rise within a few weeks of initiating therapy

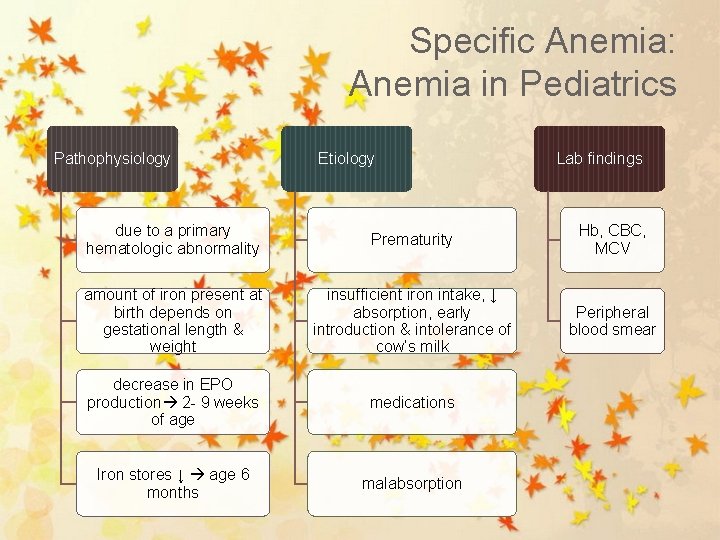
Specific Anemia: Anemia in Pediatrics Pathophysiology Etiology Lab findings due to a primary hematologic abnormality Prematurity Hb, CBC, MCV amount of iron present at birth depends on gestational length & weight insufficient iron intake, ↓ absorption, early introduction & intolerance of cow’s milk Peripheral blood smear decrease in EPO production 2 - 9 weeks of age medications Iron stores ↓ age 6 months malabsorption

Treatment and Evaluation of Therapeutic Outcames Treatment Anemia of prematurity RBCs transfusion; human milk need 2 mg/kg of iron supplementation daily 9 to 12 mo with a mild microcytic anemia Fe 2+ sulfate (3 mg/kg) 12 dd 1 4 weeks continued 2 -3 months macrocytic anemias in children folate supplementation (1 -3 mg OD) Evaluation Hb, Hct, and RBC 6 to 8 weeks after initiation of iron therapy Reticulocyte counts should be checked 4 to 6 weeks after birth Serum ferritin levels if not respond to therapy

Pharmacoeconomic of Anemia direct medical costs of anemia are unknown drug treatment must be weighed against the indirect costs associated with anemia The costs of laboratory tests must be considered frequency of blood transfusions impacts cost and therapeutic decision making in patients iron IV costly but has superior compared with oral preparations EPO is generally effective and safe, but it is expensive Vit B 12 and folate supplementatuon inexpensive

Sickle Cell Anemia

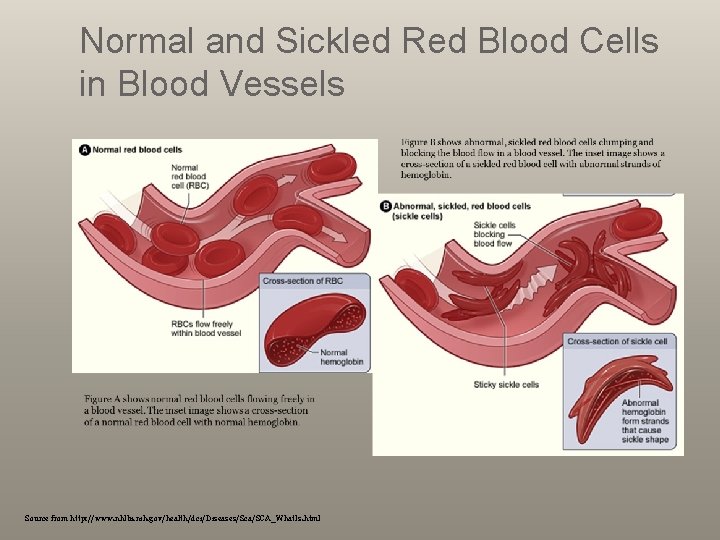
What is Sickle Cell Anemia? A serious condition red blood cells can become sickle-shaped Normal red blood cells (smooth & round) move easily through blood vessels to carry oxygen to all parts of the body. Sickle-shaped cells don’t move easily through blood stiff and sticky (lengket) and tend to form clumps and get stuck in blood vessels. The clumps ( gumpalan) of sickle cell block blood flow in the blood vessels Pain Infection Organ damage

Normal and Sickled Red Blood Cells in Blood Vessels Source from http: //www. nhlbi. nih. gov/health/dci/Diseases/Sca/SCA_What. Is. html

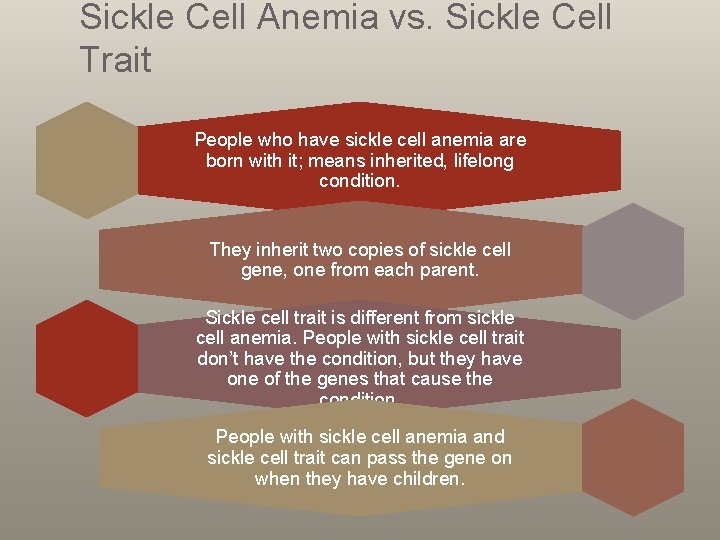
Sickle Cell Anemia vs. Sickle Cell Trait People who have sickle cell anemia are born with it; means inherited, lifelong condition. They inherit two copies of sickle cell gene, one from each parent. Sickle cell trait is different from sickle cell anemia. People with sickle cell trait don’t have the condition, but they have one of the genes that cause the condition. People with sickle cell anemia and sickle cell trait can pass the gene on when they have children.

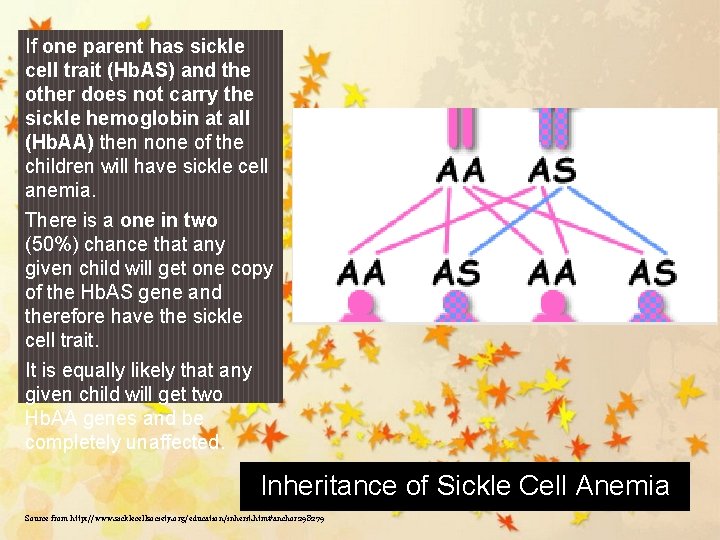
If one parent has sickle cell trait (Hb. AS) and the other does not carry the sickle hemoglobin at all (Hb. AA) then none of the children will have sickle cell anemia. There is a one in two (50%) chance that any given child will get one copy of the Hb. AS gene and therefore have the sickle cell trait. It is equally likely that any given child will get two Hb. AA genes and be completely unaffected. Inheritance of Sickle Cell Anemia Source from http: //www. sicklecellsociety. org/education/inherit. htm#anchor 298279

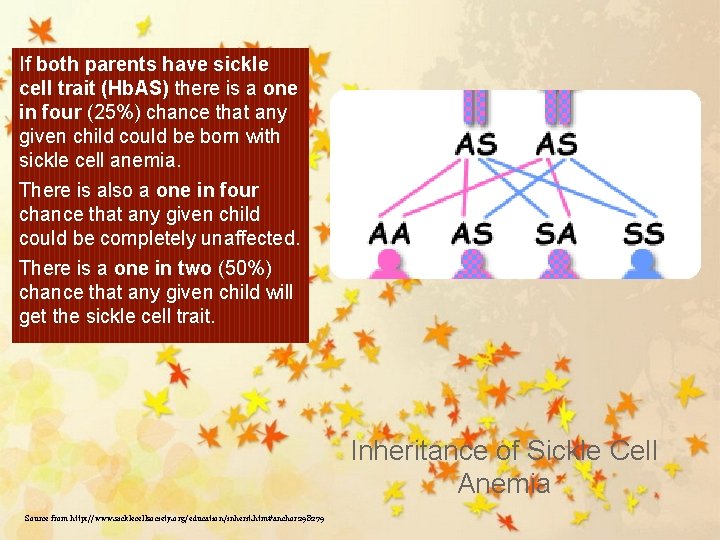
If both parents have sickle cell trait (Hb. AS) there is a one in four (25%) chance that any given child could be born with sickle cell anemia. There is also a one in four chance that any given child could be completely unaffected. There is a one in two (50%) chance that any given child will get the sickle cell trait. Inheritance of Sickle Cell Anemia Source from http: //www. sicklecellsociety. org/education/inherit. htm#anchor 298279

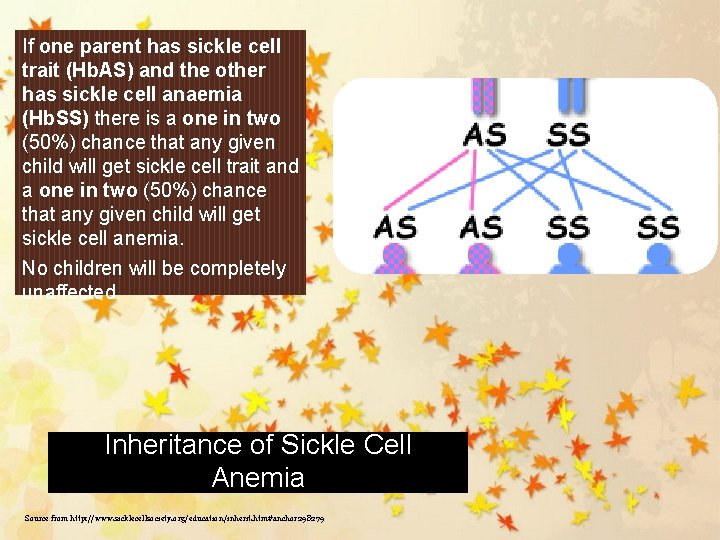
If one parent has sickle cell trait (Hb. AS) and the other has sickle cell anaemia (Hb. SS) there is a one in two (50%) chance that any given child will get sickle cell trait and a one in two (50%) chance that any given child will get sickle cell anemia. No children will be completely unaffected. Inheritance of Sickle Cell Anemia Source from http: //www. sicklecellsociety. org/education/inherit. htm#anchor 298279

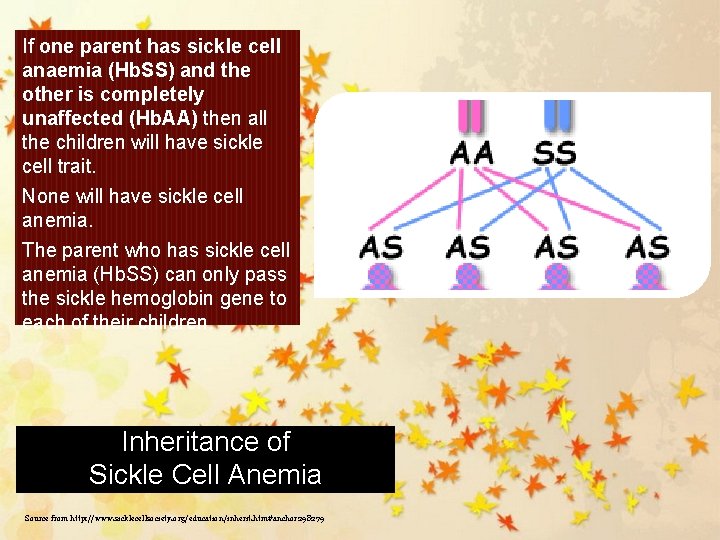
If one parent has sickle cell anaemia (Hb. SS) and the other is completely unaffected (Hb. AA) then all the children will have sickle cell trait. None will have sickle cell anemia. The parent who has sickle cell anemia (Hb. SS) can only pass the sickle hemoglobin gene to each of their children. Inheritance of Sickle Cell Anemia Source from http: //www. sicklecellsociety. org/education/inherit. htm#anchor 298279

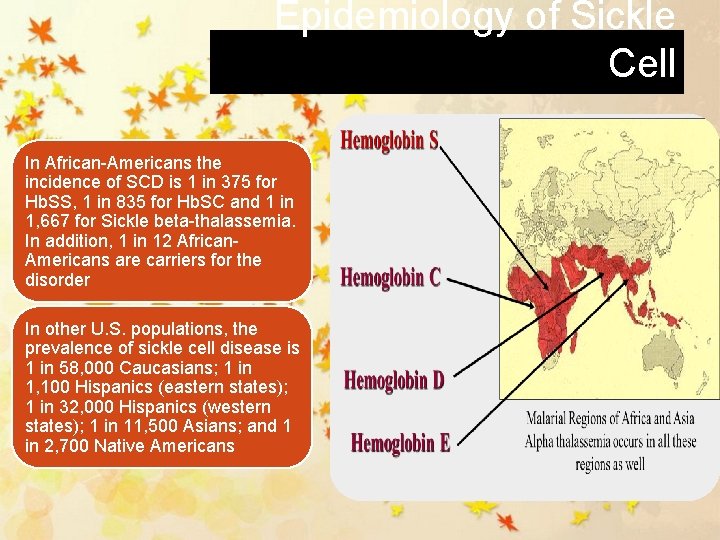
Epidemiology of Sickle Cell In African-Americans the incidence of SCD is 1 in 375 for Hb. SS, 1 in 835 for Hb. SC and 1 in 1, 667 for Sickle beta-thalassemia. In addition, 1 in 12 African. Americans are carriers for the disorder In other U. S. populations, the prevalence of sickle cell disease is 1 in 58, 000 Caucasians; 1 in 1, 100 Hispanics (eastern states); 1 in 32, 000 Hispanics (western states); 1 in 11, 500 Asians; and 1 in 2, 700 Native Americans

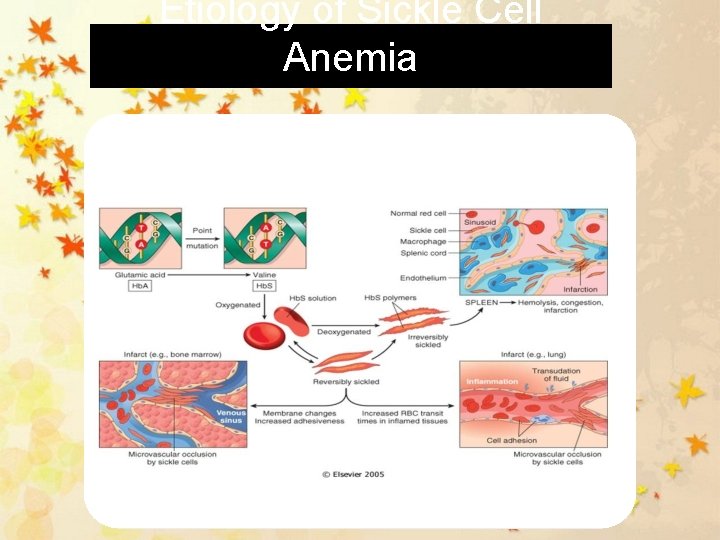
Etiology of Sickle Cell Anemia

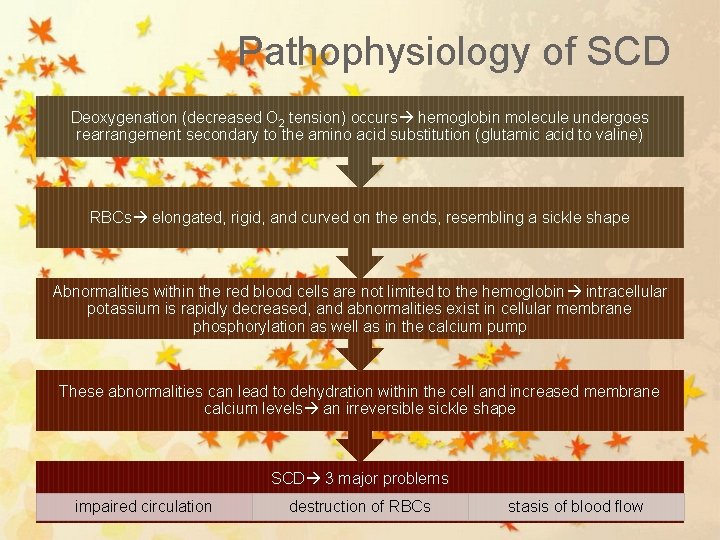
Pathophysiology of SCD Deoxygenation (decreased O 2 tension) occurs hemoglobin molecule undergoes rearrangement secondary to the amino acid substitution (glutamic acid to valine) RBCs elongated, rigid, and curved on the ends, resembling a sickle shape Abnormalities within the red blood cells are not limited to the hemoglobin intracellular potassium is rapidly decreased, and abnormalities exist in cellular membrane phosphorylation as well as in the calcium pump These abnormalities can lead to dehydration within the cell and increased membrane calcium levels an irreversible sickle shape SCD 3 major problems impaired circulation destruction of RBCs stasis of blood flow

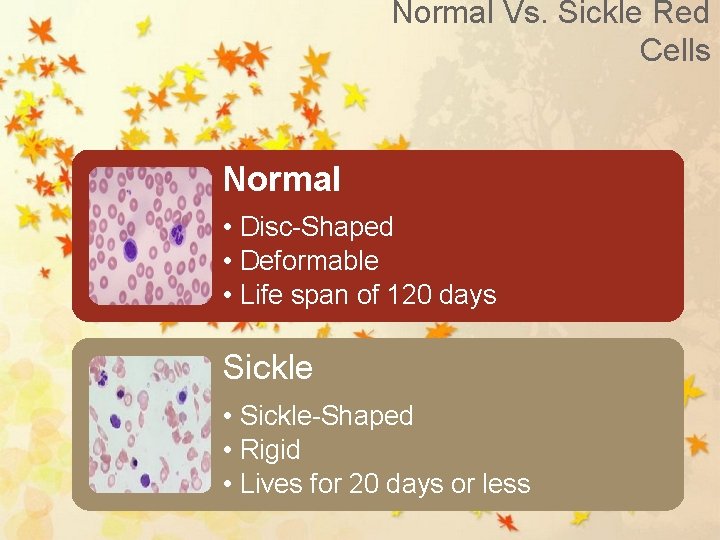
Normal Vs. Sickle Red Cells Normal • Disc-Shaped • Deformable • Life span of 120 days Sickle • Sickle-Shaped • Rigid • Lives for 20 days or less

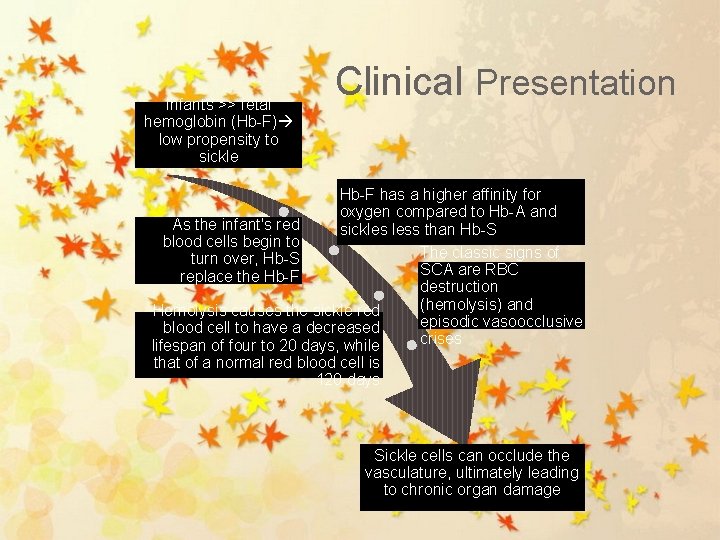
Infants >> fetal hemoglobin (Hb-F) low propensity to sickle Clinical Presentation Hb-F has a higher affinity for oxygen compared to Hb-A and As the infant's red sickles less than Hb-S blood cells begin to The classic signs of turn over, Hb-S SCA are RBC replace the Hb-F destruction (hemolysis) and Hemolysis causes the sickle red episodic vasoocclusive blood cell to have a decreased crises lifespan of four to 20 days, while that of a normal red blood cell is 120 days Sickle cells can occlude the vasculature, ultimately leading to chronic organ damage

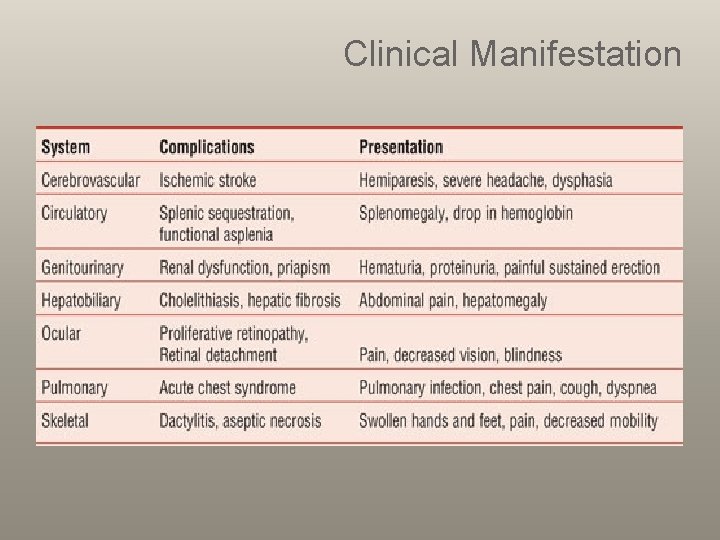
Clinical Manifestation

Clinical Manifestation Chronic Manifestations Acute Manifestations • Bacterial Sepsis or meningitis* • Recurrent vaso-occlusive pain (dactylitis, muscoskeletal or abdominal pain) • Splenic Sequestration* • Aplastic Crisis* • Acute Chest Syndrome* • Stroke* • Priapism • Hematuria, including papillary necrosis • • • • Anemia Jaundice Splenomegaly Functional asplenia Cardiomegaly and functional murmurs Hyposthenuria and enuresis Proteinemia Cholelithiasis Delayed growth and sexual maturation Restrictive lung disease* Pulmonary Hypertension* Avascular necrosis Proliferative retinopathy Leg ulcers Transfusional hemosiderosis* *Potential cause of mortality

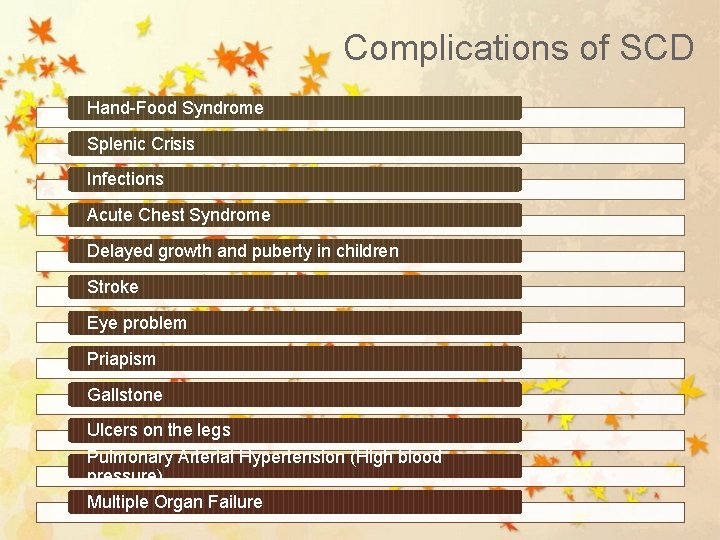
Complications of SCD Hand-Food Syndrome Splenic Crisis Infections Acute Chest Syndrome Delayed growth and puberty in children Stroke Eye problem Priapism Gallstone Ulcers on the legs Pulmonary Arterial Hypertension (High blood pressure) Multiple Organ Failure

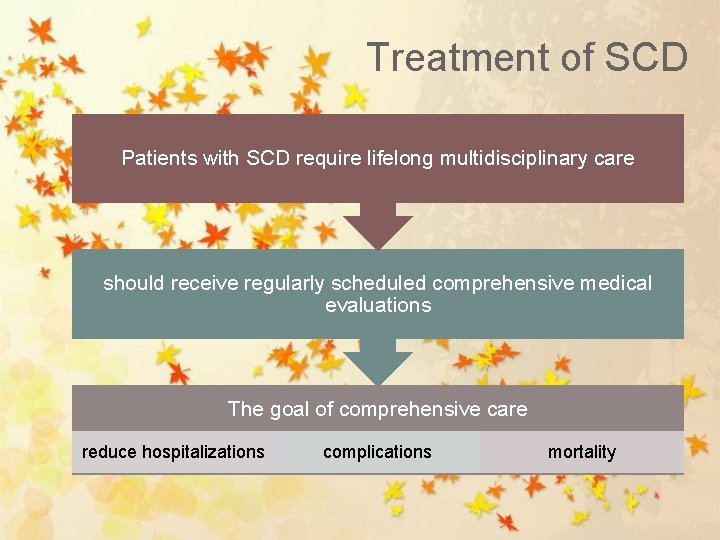
Treatment of SCD Patients with SCD require lifelong multidisciplinary care should receive regularly scheduled comprehensive medical evaluations The goal of comprehensive care reduce hospitalizations complications mortality

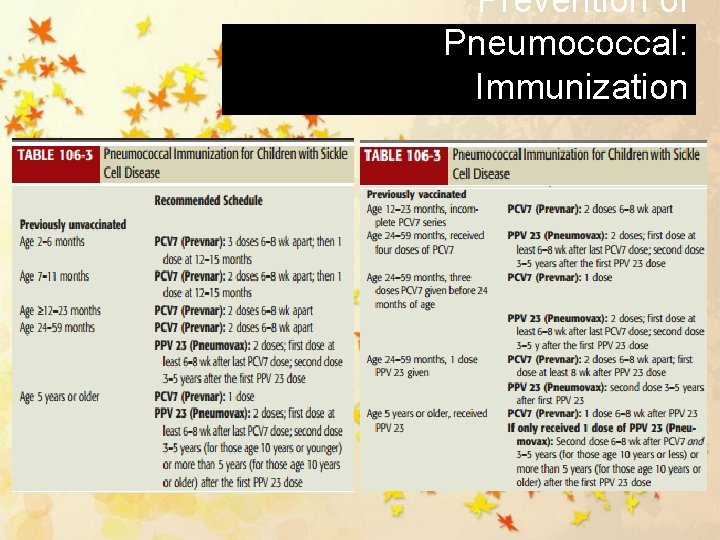
Prevention of Pneumococcal: Immunization

Prevention of Pneumococcal: Penicillin G benzathine

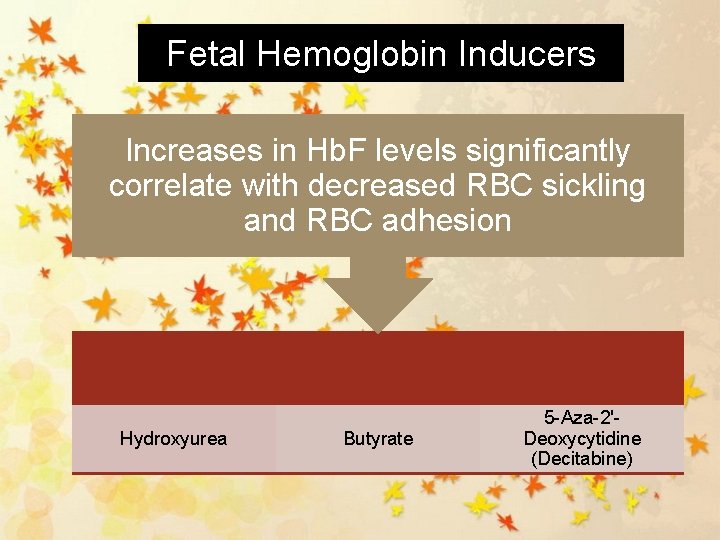
Fetal Hemoglobin Inducers Increases in Hb. F levels significantly correlate with decreased RBC sickling and RBC adhesion Hydroxyurea Butyrate 5 -Aza-2'Deoxycytidine (Decitabine)

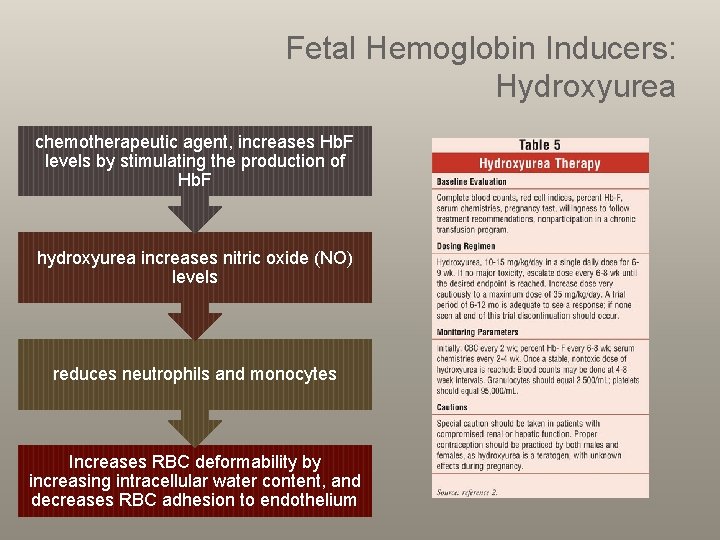
Fetal Hemoglobin Inducers: Hydroxyurea chemotherapeutic agent, increases Hb. F levels by stimulating the production of Hb. F hydroxyurea increases nitric oxide (NO) levels reduces neutrophils and monocytes Increases RBC deformability by increasing intracellular water content, and decreases RBC adhesion to endothelium

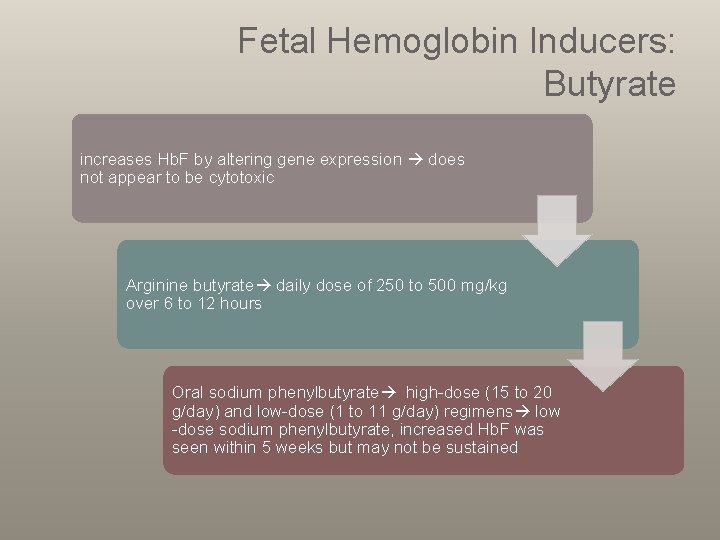
Fetal Hemoglobin Inducers: Butyrate increases Hb. F by altering gene expression does not appear to be cytotoxic Arginine butyrate daily dose of 250 to 500 mg/kg over 6 to 12 hours Oral sodium phenylbutyrate high-dose (15 to 20 g/day) and low-dose (1 to 11 g/day) regimens low -dose sodium phenylbutyrate, increased Hb. F was seen within 5 weeks but may not be sustained

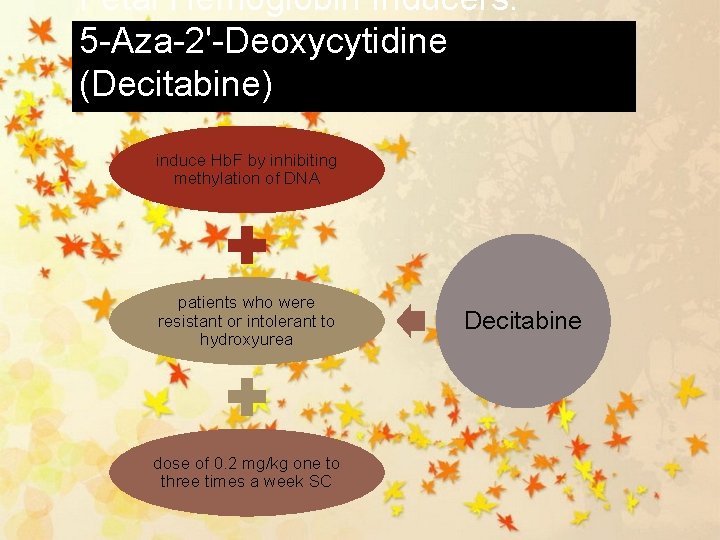
Fetal Hemoglobin Inducers: 5 -Aza-2'-Deoxycytidine (Decitabine) induce Hb. F by inhibiting methylation of DNA patients who were resistant or intolerant to hydroxyurea dose of 0. 2 mg/kg one to three times a week SC Decitabine

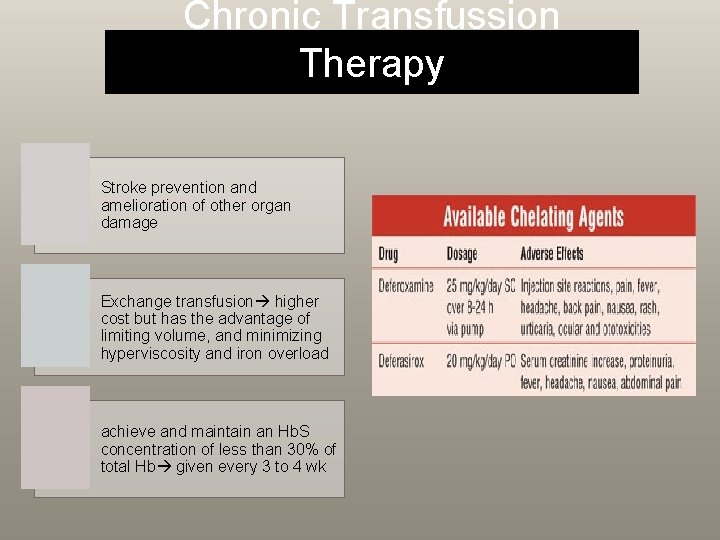
Chronic Transfussion Therapy Stroke prevention and amelioration of other organ damage Exchange transfusion higher cost but has the advantage of limiting volume, and minimizing hyperviscosity and iron overload achieve and maintain an Hb. S concentration of less than 30% of total Hb given every 3 to 4 wk

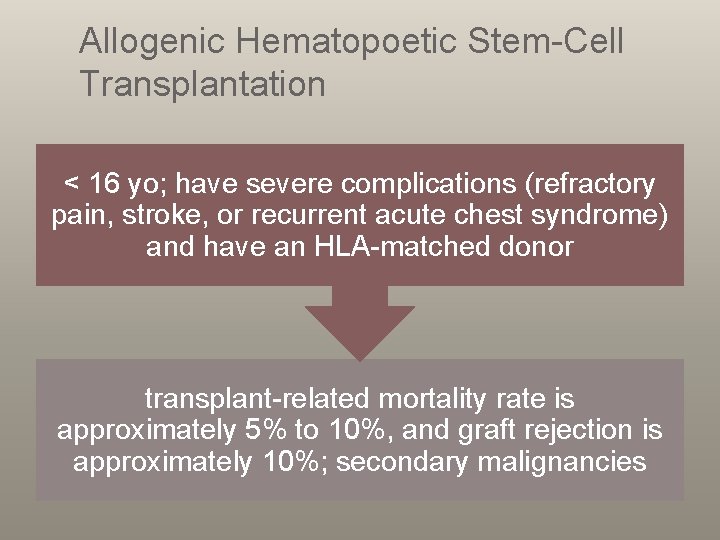
Allogenic Hematopoetic Stem-Cell Transplantation < 16 yo; have severe complications (refractory pain, stroke, or recurrent acute chest syndrome) and have an HLA-matched donor transplant-related mortality rate is approximately 5% to 10%, and graft rejection is approximately 10%; secondary malignancies

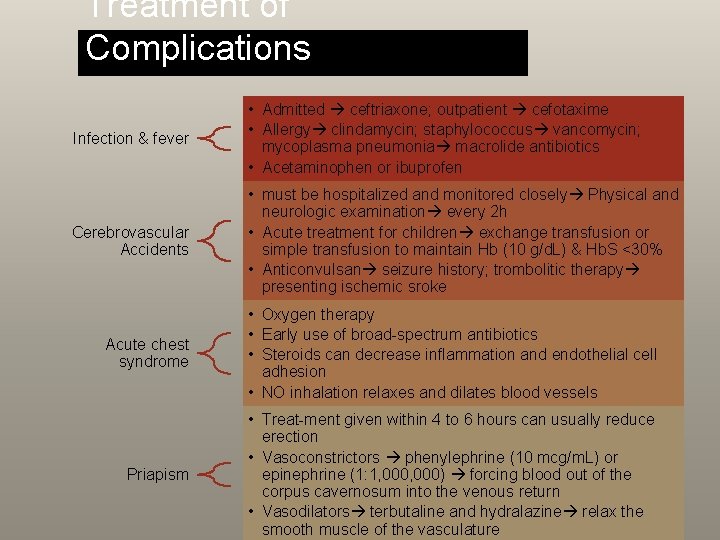
Treatment of Complications Infection & fever • Admitted ceftriaxone; outpatient cefotaxime • Allergy clindamycin; staphylococcus vancomycin; mycoplasma pneumonia macrolide antibiotics • Acetaminophen or ibuprofen Cerebrovascular Accidents • must be hospitalized and monitored closely Physical and neurologic examination every 2 h • Acute treatment for children exchange transfusion or simple transfusion to maintain Hb (10 g/d. L) & Hb. S <30% • Anticonvulsan seizure history; trombolitic therapy presenting ischemic sroke Acute chest syndrome • Oxygen therapy • Early use of broad-spectrum antibiotics • Steroids can decrease inflammation and endothelial cell adhesion • NO inhalation relaxes and dilates blood vessels Priapism • Treat-ment given within 4 to 6 hours can usually reduce erection • Vasoconstrictors phenylephrine (10 mcg/m. L) or epinephrine (1: 1, 000) forcing blood out of the corpus cavernosum into the venous return • Vasodilators terbutaline and hydralazine relax the smooth muscle of the vasculature

Management of Crisis

Analgesic Regimens

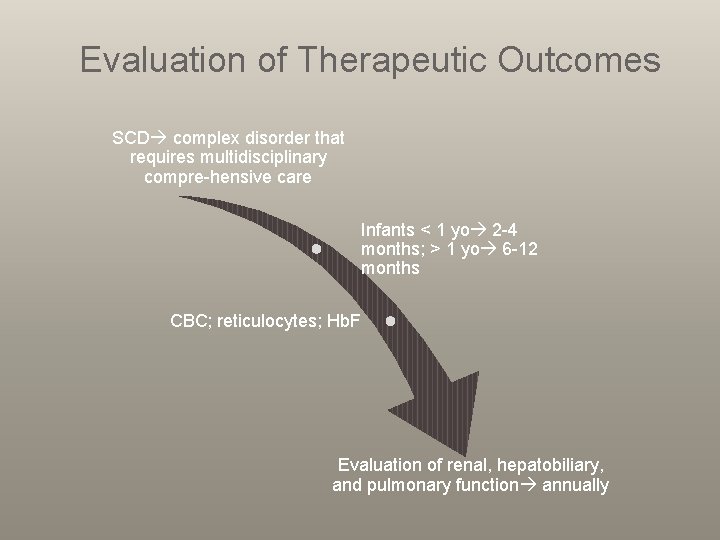
Evaluation of Therapeutic Outcomes SCD complex disorder that requires multidisciplinary compre-hensive care Infants < 1 yo 2 -4 months; > 1 yo 6 -12 months CBC; reticulocytes; Hb. F Evaluation of renal, hepatobiliary, and pulmonary function annually

Pharmacoeconomic of SCD newborn screening, cost of managing acute and chronic complications, and the economic impact of new treatment modalities chronic transfusions further increase cost Allogeneic HSCT can potentially cure the disease, and if successful can reduce long-term costs, but it requires a high up-front cost The new therapies, although expensive, might reduce visits to emergency departments and inpatient hospitalizations, improving the cost-effectiveness of those therapies over a patient’s lifetime

Question ……. Selesai juga…