Anatomy I Unit 3 Basic Biochemistry What is
Anatomy I - Unit 3: Basic Biochemistry
What is Biochemistry? ¡ ¡ Biochemistry is the study of the chemical interactions of living things. Biochemists study the structures and physical properties of biological molecules. l Often are involved in the manufacture of new drugs and medical treatments

Elements in Living Organisms ¡ The most common elements found in living organisms include: l Carbon (C) l Oxygen (O) l Nitrogen (N) l Hydrogen (H) l Phosphorus (P) l Sulfur (S)
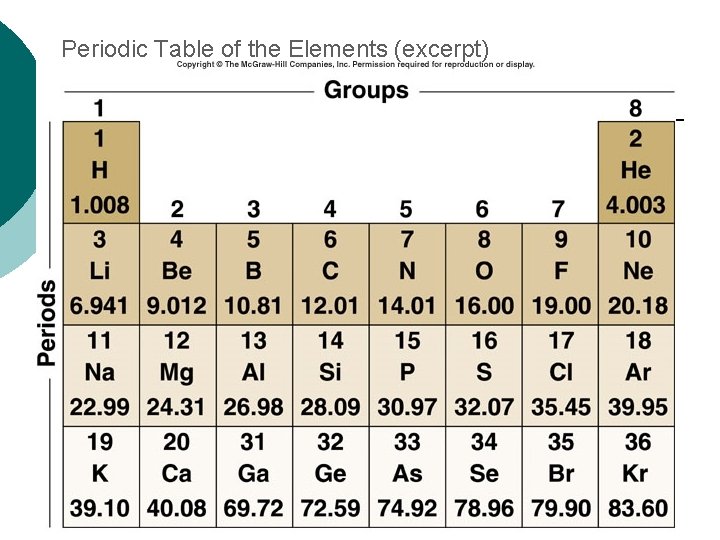
Periodic Table of the Elements (excerpt)
Biochemistry: where chemistry and biology meet head-on ¡ Living things require millions of chemical reactions within the body, just to survive. ¡ Metabolism = all the chemical reactions occurring in the body. ¡ Organic molecules: l l usually associated with living things. always contain CARBON. are “large” molecules, with many atoms always have covalent bonds (share electrons)
Macromolecules of Cells ¡ Macro = large ¡ 4 types of macromolecules in cellular biology 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic Acids

Macromolecule #1: Carbohydrates ¡ Sugars and groups of sugars ¡ Purposes: energy and structure ¡ Includes three types: l l l Monosaccharide (1 sugar – quick energy) Disaccharide (2 sugars – short storage) Polysaccharide (many sugars – energy long storage & form structures)

Macromolecule #1: Carbohydrates ¡ Polysaccharide Examples: l Glycogen—glucose polymer stored for future energy needs. Found in liver, muscle and sperm, etc. l Cellulose—glucose polymer used to form fibers for plant structures. Humans can’t digest (fiber). Most abundant organic molecule. l Chitin—glucose polymer for exoskeletons of some crustaceans & insects.
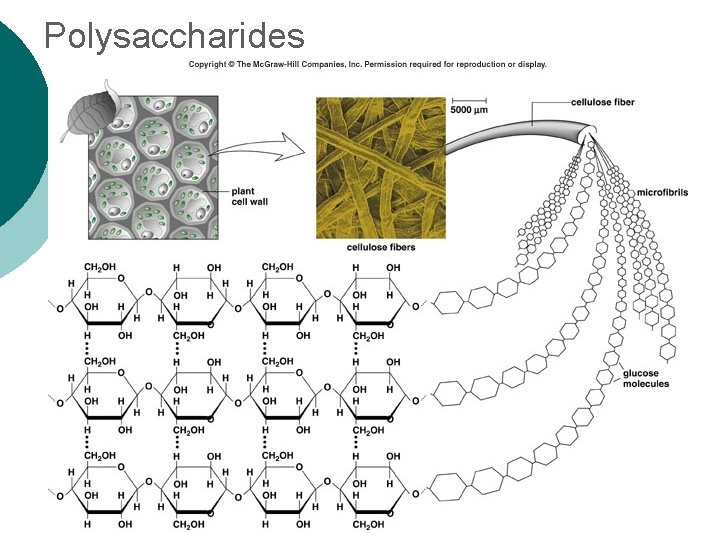
Polysaccharides
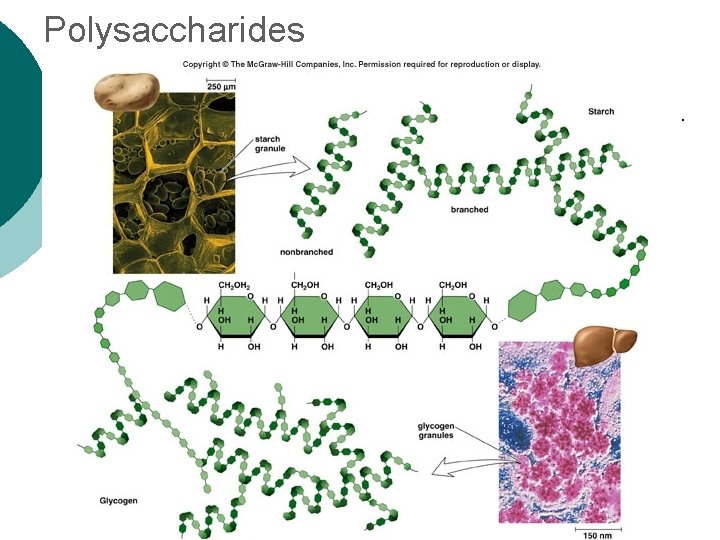
Polysaccharides

Macromolecule #2: Lipids Insoluble in water (think oil & water) 4 types: ¡ l 1 -triglycerides (fats & oils) ¡ l l 2 -phospholipids (primary component of cell membrane) 3 -steroids (cell signaling) ¡ l (long-term energy storage, insulation) cholesterol molecules modified to form sex hormones. (e. g. testosterone, estrogen, etc. ) 4 -waxes (protection, prevents water loss) ¡ Used mainly by plants, but also bees, some furry animals and humans.
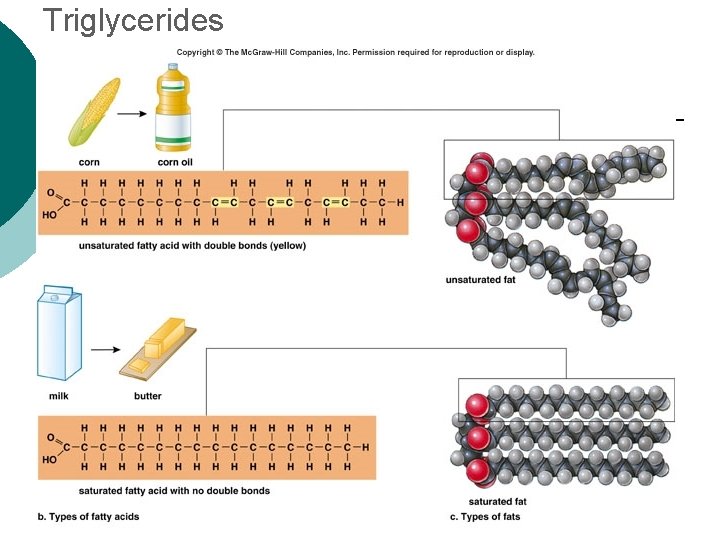
Triglycerides
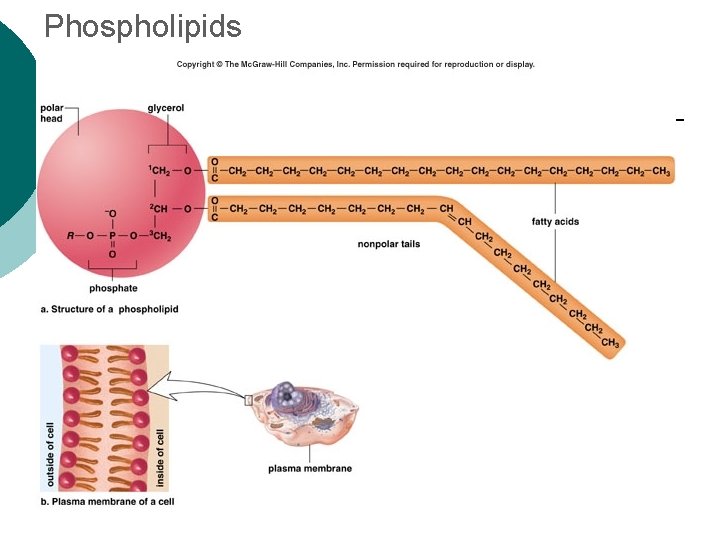
Phospholipids
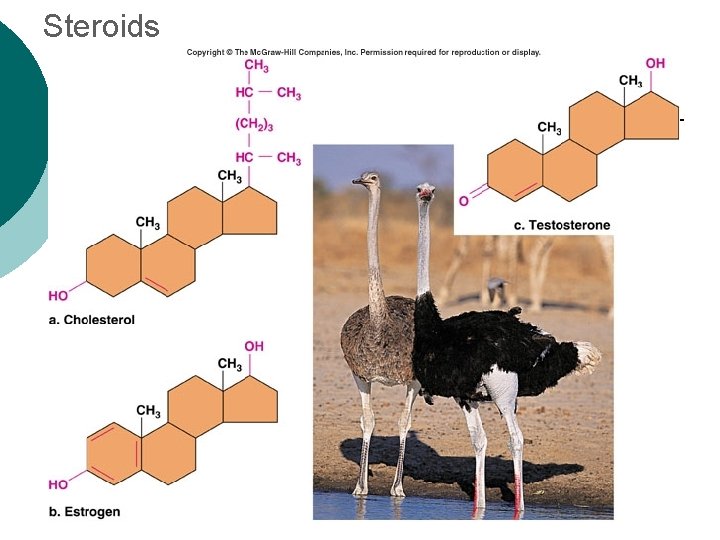
Steroids
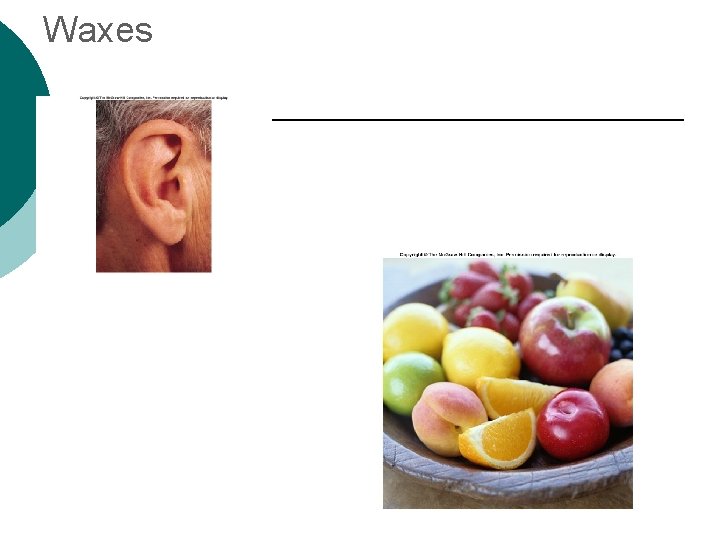
Waxes
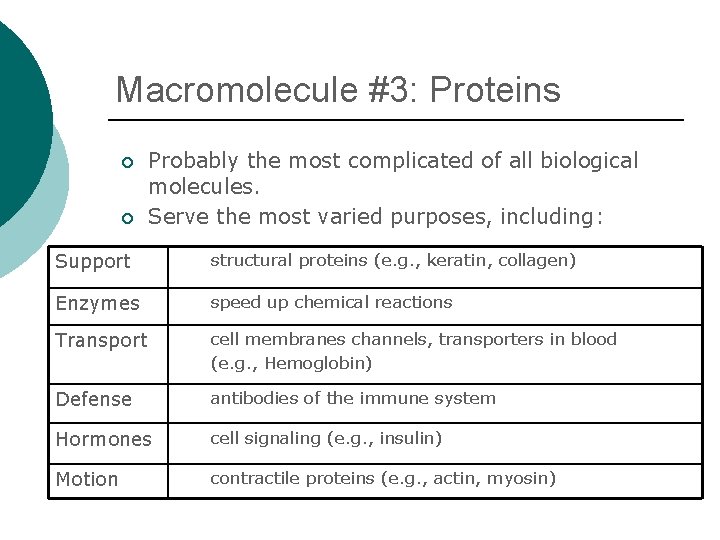
Macromolecule #3: Proteins ¡ ¡ Probably the most complicated of all biological molecules. Serve the most varied purposes, including: Support structural proteins (e. g. , keratin, collagen) Enzymes speed up chemical reactions Transport cell membranes channels, transporters in blood (e. g. , Hemoglobin) Defense antibodies of the immune system Hormones cell signaling (e. g. , insulin) Motion contractile proteins (e. g. , actin, myosin)
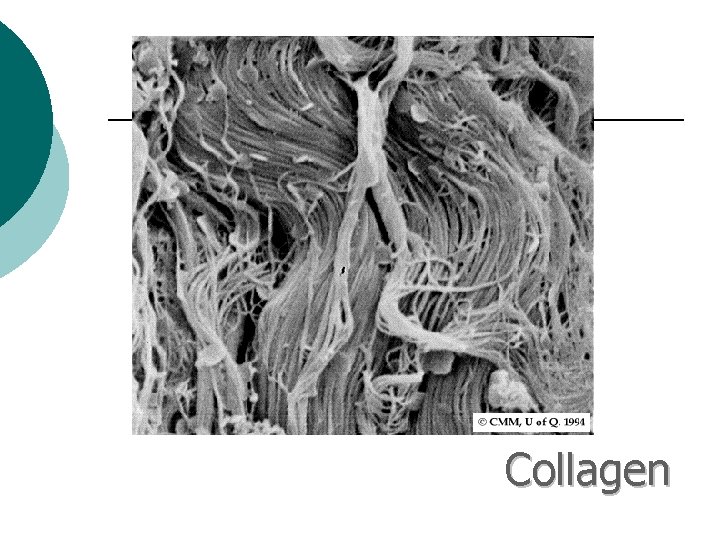
Collagen
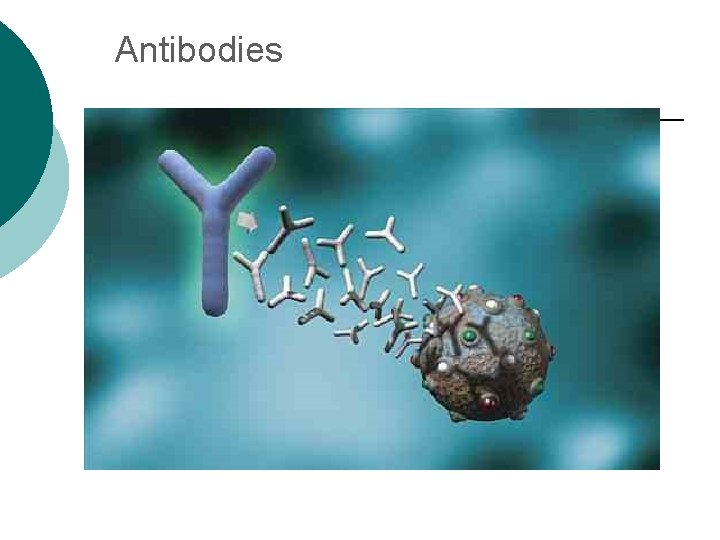
Antibodies
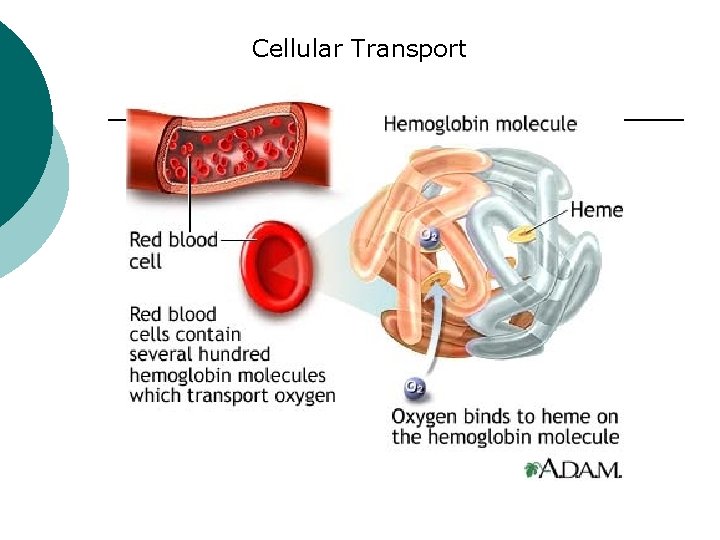
Cellular Transport
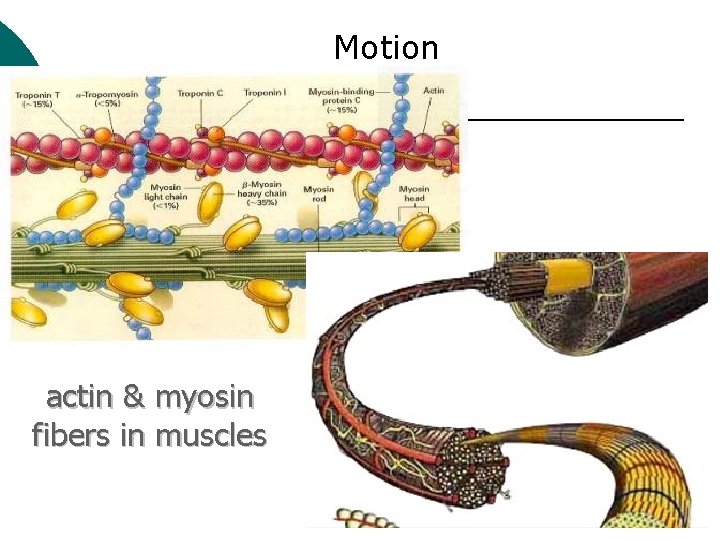
Motion actin & myosin fibers in muscles
Macromolecule #3: Proteins The building blocks of proteins are AMINO ACIDS. There are only 20 types of Amino Acids. ¡ There are millions of different proteins, and they are all built from different combinations of the 20 amino acids. ¡ Amino acids join together to form peptides, polypeptides, and polypeptide chains. ¡
Macromolecule #4: Nucleic Acids ¡ Nucleotides: building blocks of nucleic acids. l ¡ Each nucleotide contains ¡ (a) phosphate molecule, ¡ (b) nitrogenous base, and ¡ (c) 5 -carbon sugar Several types of nucleic acids, including: l l l DNA: deoxyribonucleic acid ¡ Genetic material, double stranded helix RNA: ribonucleic acid ¡ Genetic material, single stranded ATP: adenosine triphosphate ¡ High energy compound
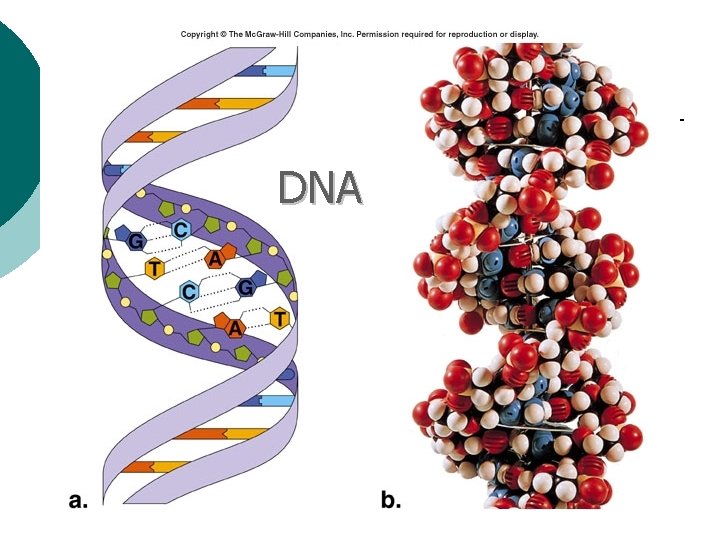
DNA
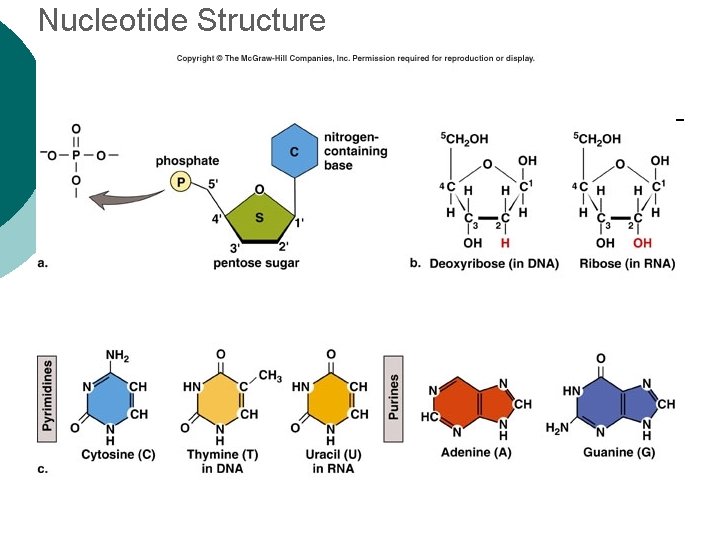
Nucleotide Structure
THE BIG PICTURE Chemistry is essential for life…
- Slides: 25