Analytical methods forensic alcohol analysis Sample and sampling
























- Slides: 47

Analytical methods forensic alcohol analysis

Sample and sampling forensic alcohol analysis

Vitreous humor � Fluid that occupies the space between the lens and the retina of the eye. � It is colourless, transparent and gel-like, consisting of 99% water with small amounts of salts and mucoprotein. � Vitreous humor is in a protected position behind the lens of the eye. � Because of this protected position, it is isolated from putrefactive processes, from charring and from trauma.

Vitreous humor � The time required for ethanol to enter the blood stream and penetrate eyes seems to be short. � VH is useful for analysis of alcohol because: 1 - It has watery nature 2 - It is remote from the gut and less prone to contamination by spread of bacteria (important in decomposition and severe trauma) 3 - Ethanol and many abused drugs are stable in VH during prolonged period of storage at 4˚C

Vitreous humor � Vitreous humor can be obtained intact even if a corpse has been extensively burned or damaged. � Blood is very susceptible to postmortem changes. � Vitreous fluid is less susceptible to these effects, particularly because it is likely to be free from microorganisms.

Blood � Peripheral blood (femoral vein) concentration have been shown to be more reliable for toxicological analysis than the conventional heart blood. � Sodium fluoride protects blood from postmortem changes such as bacterial production of ethanol or other alcohols. � It also helps to protect other labile drugs such as cocaine, nitrazepam and clonazepam from degradation.

Blood � Many species of bacteria, yeast, and fungi have the ability to produce ethanol and other volatile organic compounds in postmortem specimens. � The potential for postmortem ethanol formation complicates the interpretation of ethanol-positive results. � The prevention of ethanol formation at all steps following specimen collection is a priority. � Sodium fluoride is the most commonly used preservative for postmortem specimens.

Fluoride and enolase activity � The fluoride ion is seemingly effective in inhibiting the activity of several kinds of enzymes, such as enolase a component in the glycolytic pathway. � This enzyme is important for the action of yeasts, fungi and many micro-organisms responsible for fermentation.

Blood preservation � Ethanol formation was virtually eliminated when specimens were mixed with 1 -2% W/V sodium fluoride (Na. F). � There are published reports concluding that sodium fluoride may be ineffective for the prevention of ethanol formation in blood samples containing sufficiently high concentrations of Candida Albicans.

Assay Methodologies � Gas chromatography � Enzymatic � Chemical � Breath oxidation reaction alcohol analysis

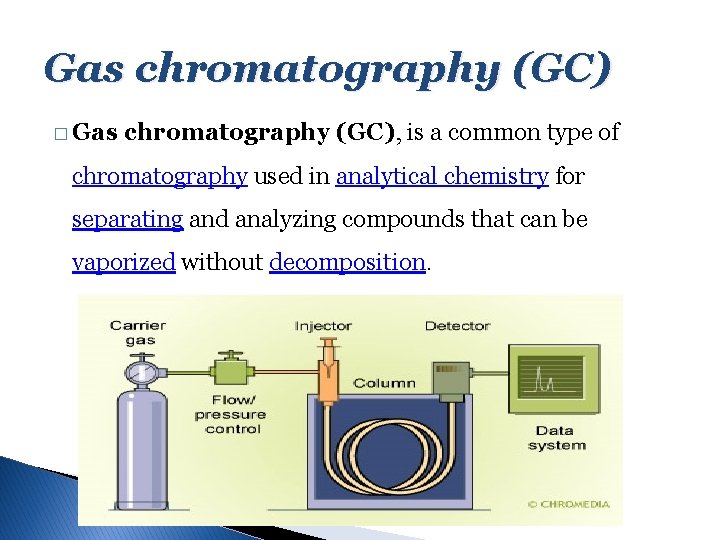
Gas chromatography (GC) � Gas chromatography (GC), is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition.

Gas Chromatography � Advantages: � Specificity for ethanol, methanol and other types of alcohol identification and quantitation. � Enhanced with the use of multiple columns or varying chromatographic conditions.

Gas Chromatography � Disadvantages: � Requires specialized instrumentation (gas chromatograph) � Requires � Analysis highly trained technical staff slower than enzymatic assay

A gas chromatograph with a headspace sampler

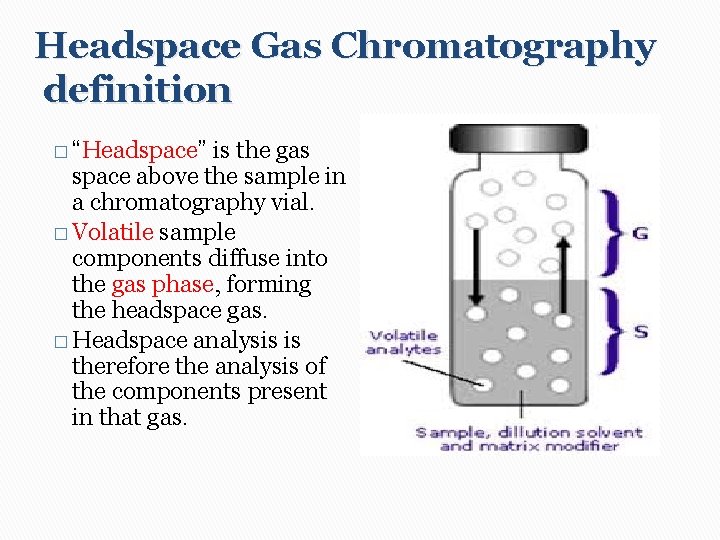
Headspace Gas Chromatography definition � “Headspace” is the gas space above the sample in a chromatography vial. � Volatile sample components diffuse into the gas phase, forming the headspace gas. � Headspace analysis is therefore the analysis of the components present in that gas.

Headspace suitability � Headspace gas chromatography is most suited to the analysis of the very light volatiles in samples that can be efficiently partitioned into the headspace gas volume from the liquid or solid matrix sample. � Complex sample matrices, which may be difficult to analyse directly or would otherwise require sample extraction or preparation, are ideal candidates for headspace since they can be placed directly in a vial with little or no preparation.

HSGC in forensic toxicology � The technique of static headspace gas chromatography has great acceptance in the forensic field, especially for the determination of alcohols in biological samples, so most forensic laboratories in the world have this equipment and perform this analysis on a routine basis.

Enzymatic Oxidation Assay � Most of the commercial kits use alcohol dehydrogenase (ADH): � C 2 H 5 OH � The ADH + NAD+<=====>CH 3 CHO+NADH+H+ reaction is monitored following the absorbance of NADH at 340 nm or that of a color product at a higher (visible) wavelength formed by reacting NADH with a dye.

Enzymatic Oxidation Assay � Advantages: � Rapid, � This easy to use kits are widely available allows the smallest of clinical laboratories to perform stat quantitative alcohol test � Disadvantages: � Not specific for ethanol. Other alcohols can interfere at high concentrations � Will miss methanol and isopropanol overdose

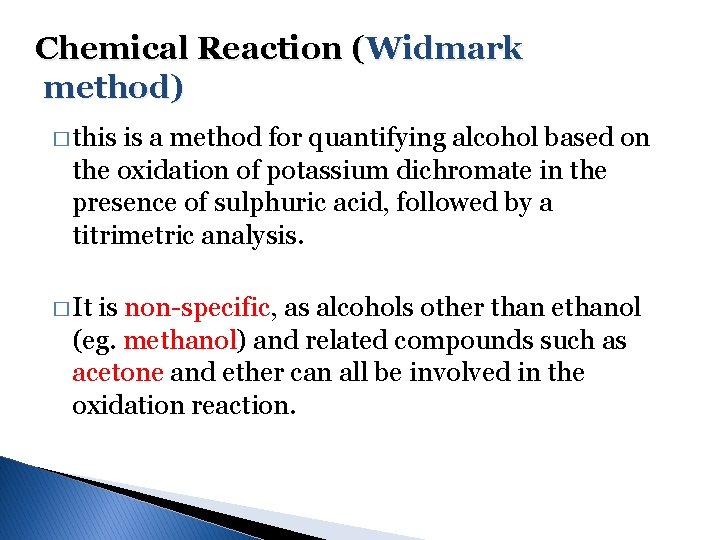
Chemical Reaction (Widmark method) � this is a method for quantifying alcohol based on the oxidation of potassium dichromate in the presence of sulphuric acid, followed by a titrimetric analysis. � It is non-specific, as alcohols other than ethanol (eg. methanol) and related compounds such as acetone and ether can all be involved in the oxidation reaction.

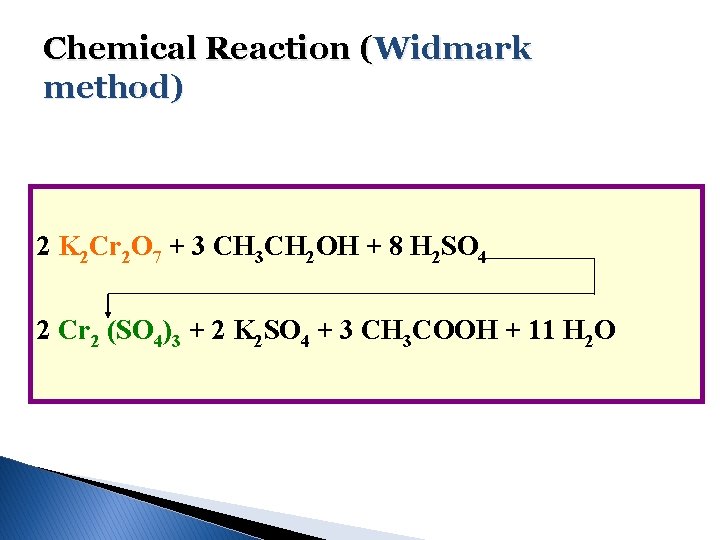
Chemical Reaction (Widmark method) 2 K 2 Cr 2 O 7 + 3 CH 3 CH 2 OH + 8 H 2 SO 4 2 Cr 2 (SO 4)3 + 2 K 2 SO 4 + 3 CH 3 COOH + 11 H 2 O

Potassium dichromate conversion to Chromium sulfate

Breath alcohol analyser


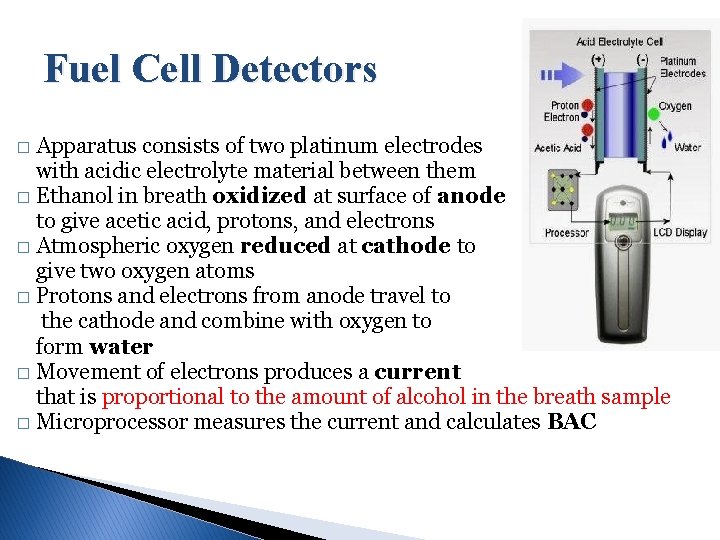
Fuel Cell Detectors Apparatus consists of two platinum electrodes with acidic electrolyte material between them � Ethanol in breath oxidized at surface of anode to give acetic acid, protons, and electrons � Atmospheric oxygen reduced at cathode to give two oxygen atoms � Protons and electrons from anode travel to the cathode and combine with oxygen to form water � Movement of electrons produces a current that is proportional to the amount of alcohol in the breath sample � Microprocessor measures the current and calculates BAC �

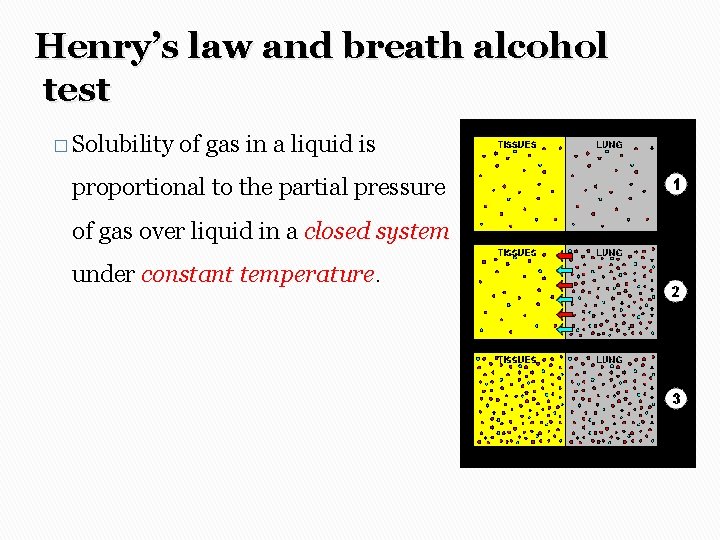
Henry’s law and breath alcohol test � Solubility of gas in a liquid is proportional to the partial pressure of gas over liquid in a closed system under constant temperature.

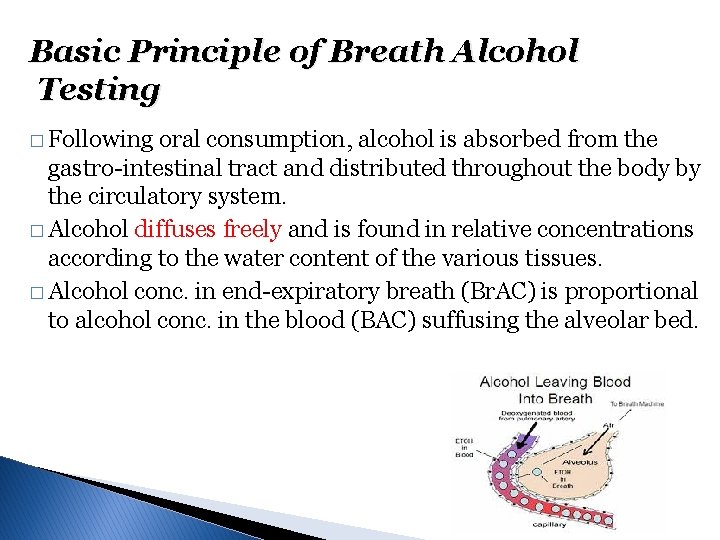
Basic Principle of Breath Alcohol Testing � Following oral consumption, alcohol is absorbed from the gastro-intestinal tract and distributed throughout the body by the circulatory system. � Alcohol diffuses freely and is found in relative concentrations according to the water content of the various tissues. � Alcohol conc. in end-expiratory breath (Br. AC) is proportional to alcohol conc. in the blood (BAC) suffusing the alveolar bed.

Breath Alcohol Concentration (Br. AC) Measurement � Advantages: • Breath collection is noninvasive • Collection does not require phlebotomist; can be performed by many more people • Instrument designed for portability and easy breath collection; onsite testing • Collection and test can be done simultaneously with immediate result

Interfering compounds � Dieters and diabetics may have acetone levels hundreds or even thousands of times higher than those in others. � Acetone is one of the many substances that can be falsely identified as ethyl alcohol by some breath machines. � However, fuel cell based systems are nonresponsive to substances like acetone.

Issues in Breath Testing Mouth alcohol effect � Residual alcohol, temporarily trapped in the mouth may result in an elevated breath alcohol concentration � Sources ◦ ◦ of mouth alcohol Recent ingestion of alcohol Regurgitation or vomiting Asthma inhalers Breath sprays and mouthwashes

Mouth alcohol To help guard against mouth-alcohol contamination, observation the test subject carefully for at least 15– 20 minutes before administering the test is necessary

“Fool” breath analyis � There a number of substances or techniques that can supposedly "fool" a breath analysis results. � Some methods such as breath mints, onions, denture cream, mouthwash (mouthwashes may contains 27% alcohol), pennies and batteries; proved ineffective. � Using items such as breath mints, onions, denture cream and mouthwash to cover the smell of alcohol may fool a person, but, since they will not actually reduce a person's BAC, there will be no effect on a breath analysis results regardless of the quantity used.

How to report laboratory results � Laboratory Report Should Contain the Following Information: � Patient’s name or identification number � Specimen number � Date and time of specimen collection and receipt in laboratory � Specimen type � Alcohol concentration

Other laboratory measurements associated with ethanol ingestion � Ethyl Glucuronide (EG) � Fatty Acid Ethyl Ester (FAEE) � Gamma Glutaryl Transferase (GGT)

Interpretation of alcohol analysis results

Blood ethanol in acute alcohol poisoning � The following basic information is required in evaluation of results: 1 - Site and method of collection of blood sample 2 - Time after death and state of body when sample was collected

Blood ethanol in acute alcohol poisoning 3 - Conditions of storage of sample, preservative used, and time elapsed before analysis 4 - Method used for analysis 5 - Administration of IV solutions (manitol) 6 - Concomitant use of other drugs

Analytical Considerations � Neo-formation of ethanol during storage can occur if there is a source of microbial contamination and a suitable substrate for fermentation (e. g. glucose). � More likely to occur in postmortem cases but can also occur in samples taken from living subjects. � Sodium fluoride will prevent the neo-formation of ethanol during storage.

Ethanol production by microorganisms � Micro-organisms can use a number of different substrates to produce alcohol, the main one being glucose but others include glycogen, glycols, pyruvate, lactate, amino acids, ribose. � The specific pathway, by-products and endproducts of the process vary according to the substrates available and the enzymes present in the micro-organisms.

Ethanol production by microorganisms � Organisms capable of producing alcohol in deceased bodies include Candida albicans (yeast), Clostridium sp. , Escherichia coli, Streptococcus faecalis, Lactobacillus sp. and Proteus vulgaris. � Many of these organisms are present within the bowel during life. � Other species that are present on the skin or in soil may enter the body after death, particularly when the skin has been breached, as in the case of traumatised bodies.

Alcohol Concentrations in Different Specimen Types � Alcohol is distributed throughout the body in proportion to the water content of the body fluid. � Plasma and serum alcohol concentrations are higher than whole blood by 12 -18%. � Saliva alcohol concentration is higher than whole blood by 7%. � Urine alcohol concentration may be 30% higher than whole blood. � The laboratory report must indicate the specimen type.

VAC & BAC � If the VAC is negative in the face of a positive BAC, endogenous alcohol formation is a strong possibility. BAC = conversion factor x VAC � This ‘conversion factor’ has been variously calculated as being between 0. 85 and 0. 95.

BAC & UAC � If BAC is positive and UAC is negative, there are two possibilities: 1 - Alcohol measured in the blood has been produced post-mortem. 2 - When ethanol has only recently been ingested and has not yet had time to be filtered into the urine and collect in the bladder.

Absorptive phase � In the absorptive phase, BAC may be higher than VAC and UAC.

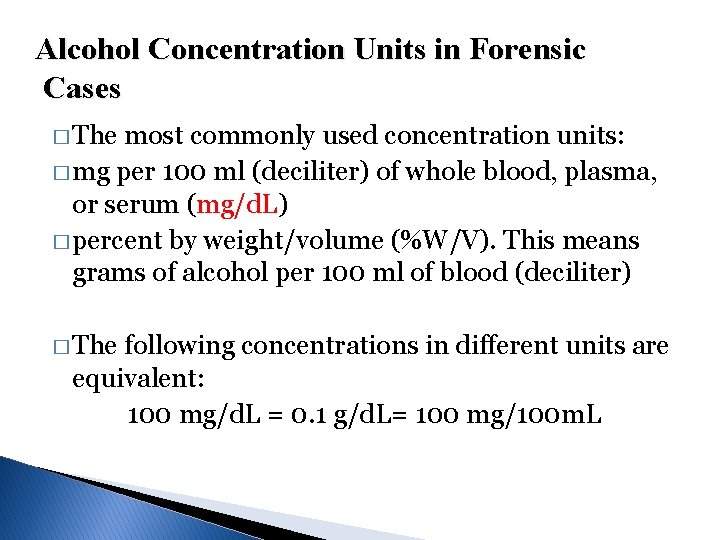
Alcohol Concentration Units in Forensic Cases � The most commonly used concentration units: � mg per 100 ml (deciliter) of whole blood, plasma, or serum (mg/d. L) � percent by weight/volume (%W/V). This means grams of alcohol per 100 ml of blood (deciliter) � The following concentrations in different units are equivalent: 100 mg/d. L = 0. 1 g/d. L= 100 mg/100 m. L

Analytical Considerations Ethanol losses during storage can occur by three mechanisms: 1. Diffusion from improperly sealed containers 2. Metabolism of ethanol by microorganisms 3. Oxidation of ethanol acetaldehyde

Concluding remarks � Toxicological analysis constitutes an essential element in all investigations of unnatural and sudden deaths and in this connection alcohol intoxication and drunkenness play an important role. � Methods for quantitative and qualitative analysis of ethanol in body fluids are the same regardless of whether specimens are taken from living or dead.