Analytical Chemistry Neutral Titration Introduction n n Neutral










- Slides: 21

Analytical Chemistry Neutral Titration

Introduction n n Neutral titrations are considered the most volumetric analysis titrations practiced since they feature high accuracy and precision along side being quick and easy to practice. Hence, it has been used to estimate a number of organic and inorganic substances that feature acidic or basic qualities. These titrations produce water and salt. The salt might be of a neutral effect. Therefore, the eq. point is situated in a neutral medium. It also might be of an acidic effect as a result of a weak base titration such as titrating ammonia with NH 4 Cl which forms ammonium, that contains an acid which leaves the eq. point in the acidic portion. The eq. point can also be situated in an alkaline medium like, the titration of a weak acid such as acetic acid with sodium hydroxide, then sodium acetates are formed which provide acetates of a base effect.

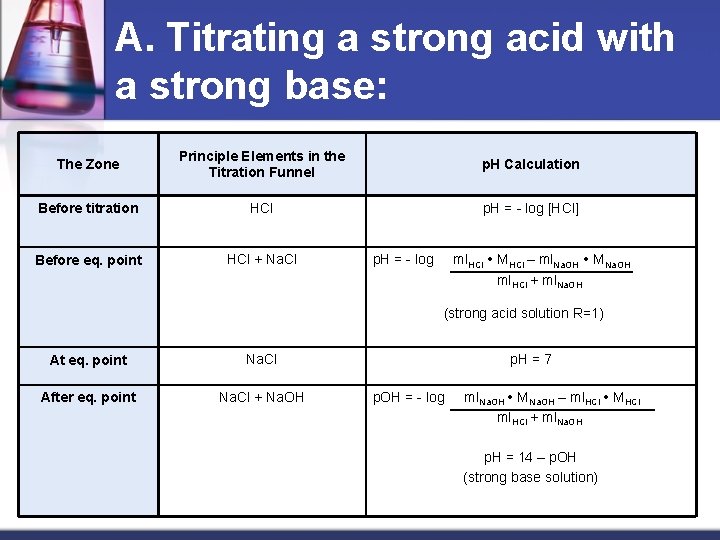
1. Titrating a strong acid with a strong base and vice versa A. Titrating a strong acid with a strong base: For e. g. titrating HCl with sodium hydroxide. Such titration characterizes by the enormous changes in the p. H units around the eq. point. HCl + Na. OH Na. Cl + H 2 O H+ + OHH 2 O this reaction is almost complete: Keq = [H 2 O] = 1 x 10 -14 Kw 1 x 10 -14

A. Titrating a strong acid with a strong base: The Zone Principle Elements in the Titration Funnel p. H Calculation Before titration HCl p. H = - log [HCl] Before eq. point HCl + Na. Cl p. H = - log ml. HCl MHCl – ml. Na. OH MNa. OH ml. HCl + ml. Na. OH (strong acid solution R=1) At eq. point Na. Cl After eq. point Na. Cl + Na. OH p. H = 7 p. OH = - log ml. Na. OH MNa. OH – ml. HCl MHCl ml. HCl + ml. Na. OH p. H = 14 – p. OH (strong base solution)


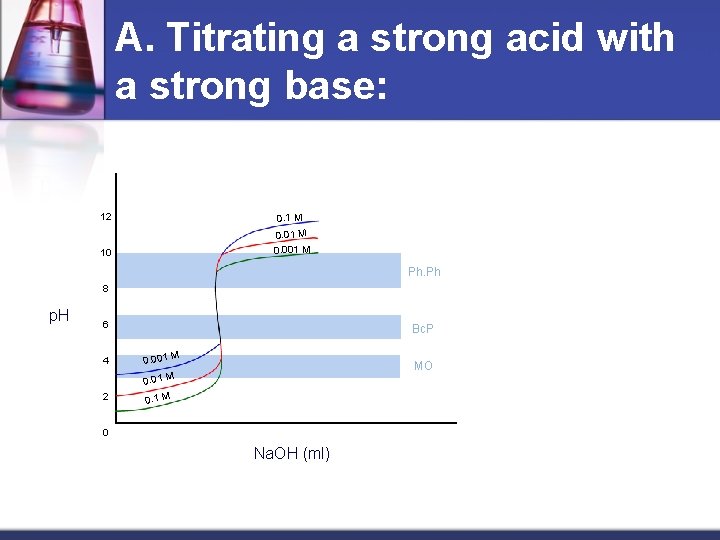
A. Titrating a strong acid with a strong base: 12 0. 1 M 10 0. 01 M 0. 001 M Ph. Ph 8 p. H 6 4 Bc. P 0. 001 M MO 0. 01 M 2 0. 1 M 0 Na. OH (ml)

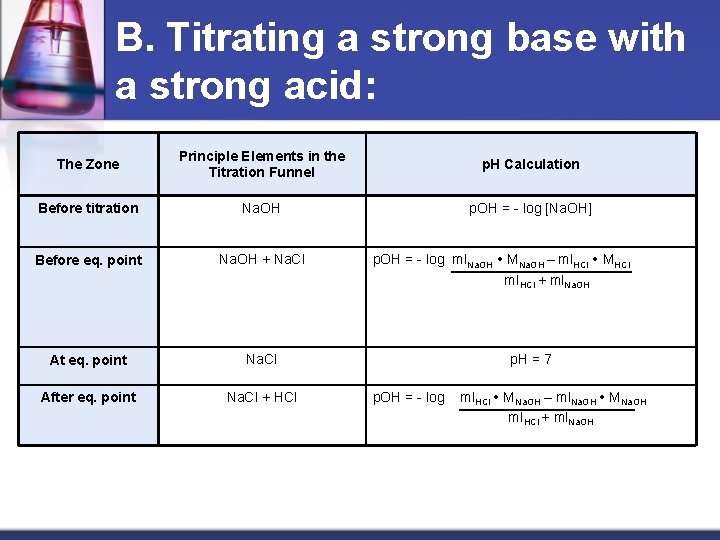
B. Titrating a strong base with a strong acid: The Zone Principle Elements in the Titration Funnel p. H Calculation Before titration Na. OH p. OH = - log [Na. OH] Before eq. point Na. OH + Na. Cl At eq. point Na. Cl After eq. point Na. Cl + HCl p. OH = - log ml. Na. OH MNa. OH – ml. HCl MHCl ml. HCl + ml. Na. OH p. H = 7 p. OH = - log ml. HCl MNa. OH – ml. Na. OH MNa. OH ml. HCl + ml. Na. OH

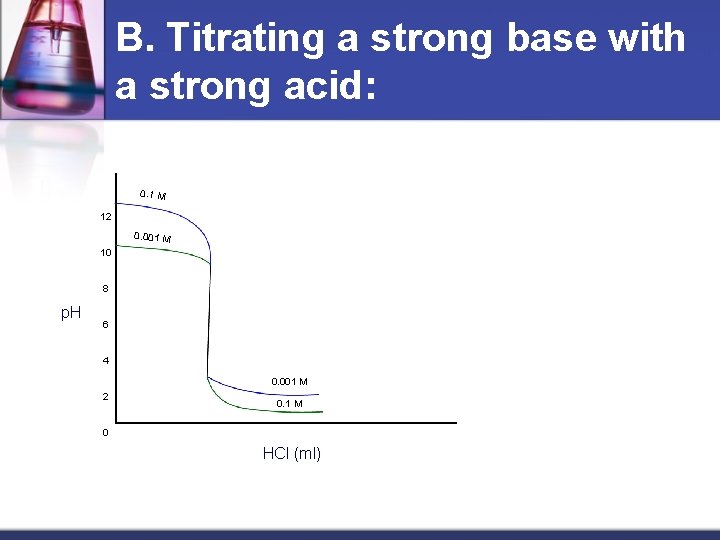
B. Titrating a strong base with a strong acid: 0. 1 M 12 0. 001 M 10 8 p. H 6 4 0. 001 M 2 0. 1 M 0 HCl (ml)

2. Titrating a weak acid with a strong base and vice versa: A. Titrating a weak acid with a strong base: Here, we obtain eq. point when the weak acid HA converts to Na. A where the anion A works as a base: A - + H 2 O OH- + HA Therefore, the eq. point will take place in the basic portion. For e. g. titrating 100 ml of the acid HA with the concentration of 0. 1 molar and the contuent dissociation Ka = 10 -5, with sodium hydroxide concentration 0. 1 molar. 1. p. H before addition Before eq. point At eq. point After eq. point 2. 3. 4.

A. Titrating a weak acid with a strong base 1. p. H before addition: We can determine the hydrogen ion concentration of the weak acid solution as following: [H+] = Ka Ca = 10 -5 x 0. 1 = 10 -3 M p. H = 3. 00 2. Before eq. point: This means that we are coming across a solution that contains a weak acid and its conjugate, so this solution is a buffer solution. If the amount of the added base was 10 ml that means that the produced salt: [Na. A] = 10 x 0. 1 = 9 x 10 -3 M 100 + 10 and the remaining acid concentration: [HA] = 100 x 0. 1 – 10 x 0. 1 = 0. 08 M 100 + 10 [H+] = Ka x Ca Cs -5 = 10 x 0. 8 = 8. 9 x 10 -5 M 9 x 10 -3 p. H = 4. 05

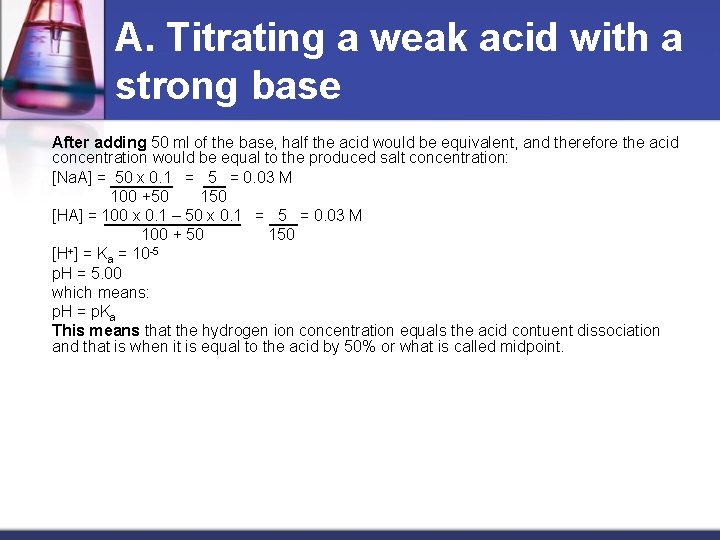
A. Titrating a weak acid with a strong base After adding 50 ml of the base, half the acid would be equivalent, and therefore the acid concentration would be equal to the produced salt concentration: [Na. A] = 50 x 0. 1 = 5 = 0. 03 M 100 +50 150 [HA] = 100 x 0. 1 – 50 x 0. 1 = 5 = 0. 03 M 100 + 50 150 + -5 [H ] = Ka = 10 p. H = 5. 00 which means: p. H = p. Ka This means that the hydrogen ion concentration equals the acid contuent dissociation and that is when it is equal to the acid by 50% or what is called midpoint.

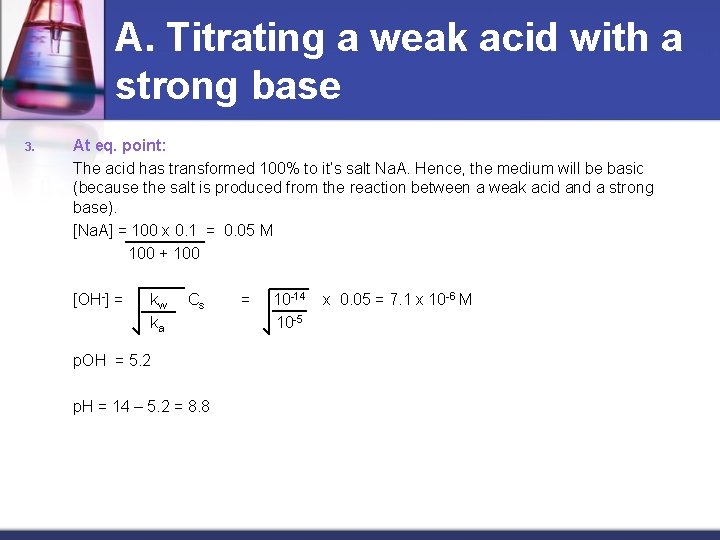
A. Titrating a weak acid with a strong base 3. At eq. point: The acid has transformed 100% to it’s salt Na. A. Hence, the medium will be basic (because the salt is produced from the reaction between a weak acid and a strong base). [Na. A] = 100 x 0. 1 = 0. 05 M 100 + 100 [OH-] = kw ka Cs p. OH = 5. 2 p. H = 14 – 5. 2 = 8. 8 = 10 -14 10 -5 x 0. 05 = 7. 1 x 10 -6 M

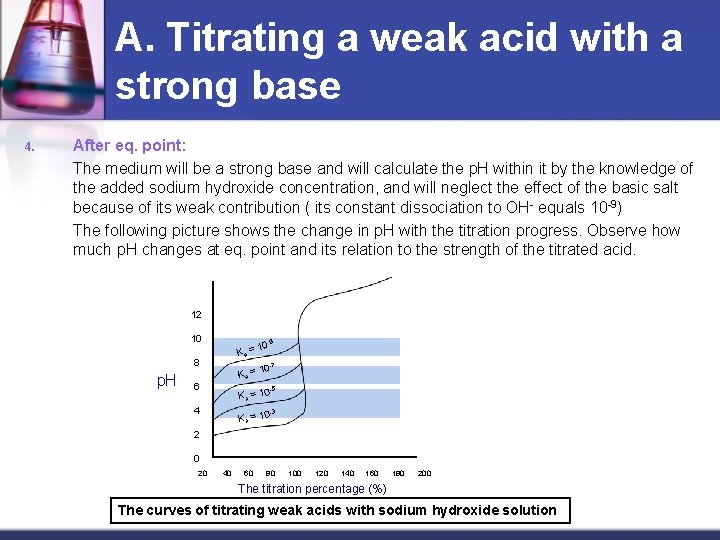
A. Titrating a weak acid with a strong base 4. After eq. point: The medium will be a strong base and will calculate the p. H within it by the knowledge of the added sodium hydroxide concentration, and will neglect the effect of the basic salt because of its weak contribution ( its constant dissociation to OH- equals 10 -9) The following picture shows the change in p. H with the titration progress. Observe how much p. H changes at eq. point and its relation to the strength of the titrated acid. 12 10 Ka = 8 p. H -9 10 0 -7 Ka = 1 6 -5 K a = 10 4 -3 Ka = 10 2 0 20 40 60 80 100 120 140 160 180 200 The titration percentage (%) The curves of titrating weak acids with sodium hydroxide solution

Example 1 n n Titrating Na. OH with CH 3 COOH Na. OH + CH 3 COOH CH 3 COONa + H 2 O Before titration: weak acid solution p. H = -log Ka Ca Before eq. point: A solution containing (CH 3 COONa + CH 3 COOH) and it is a buffer solution p. H = p. Ka + log Cs Ca Cs = ml. Na. OH MNa. OH (R= 1) ml. Na. OH ml. CH 3 COOH Ca = ml. CH 3 COOH MCH 3 COOH - ml. Na. OH MNa. OH ml. Na. OH + ml. CH 3 COOH At eq. point: A solution that contains (Na. OH + CH 3 COONa) neglects the effect of the salt which is a weak base and is calculated as a Na. OH solution: p. OH = -log ml. Na. OH MNa. OH - ml. CH 3 COOH MCH 3 COOH ml. Na. OH ml. CH 3 COOH

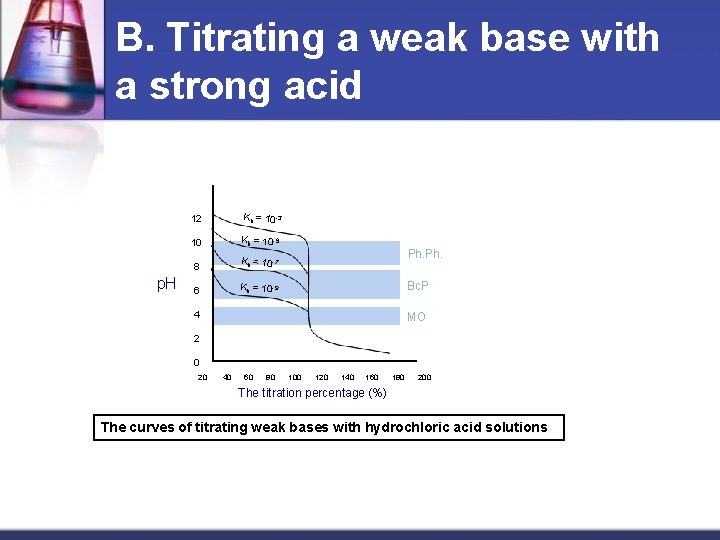
B. Titrating a weak base with a strong acid n This is the inverse curve of titrating a weak acid with a strong base. In this curve, the eq. point is in the acidic portion because the salt BHX is formed. It gives the kation BH + which is of an acidic quality. BH+ + H 2 O B + H 3 O + hence, the solution will obtain an acidic feature at eq. point. n The following picture shows such curves. It also shows the effect of the weak base constant dissociation value at the clarity of the end point (considering the number of p. H units that change meanwhile) where the equilibrium constant of the reaction equals: keq = kb kw To achieve the value 10 -6 required in the equilibrium constant, kb must not be less than 108. Accordingly, weak bases with dissociated constants less than 10 -8 can not be titrated. n

B. Titrating a weak base with a strong acid p. H 12 Kb = 10 -3 10 Kb = 10 -5 8 Kb = 10 -7 6 Kb = 10 -9 Ph. Bc. P 4 MO 2 0 20 40 60 80 100 120 140 160 180 200 The titration percentage (%) The curves of titrating weak bases with hydrochloric acid solutions

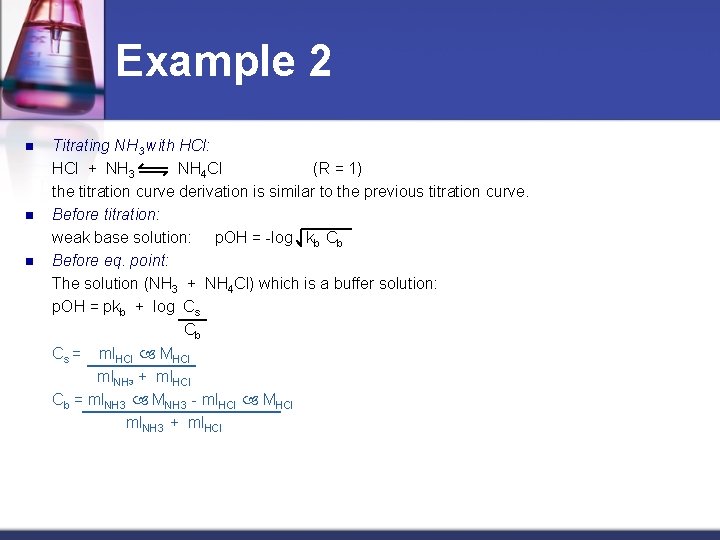
Example 2 n n n Titrating NH 3 with HCl: HCl + NH 3 NH 4 Cl (R = 1) the titration curve derivation is similar to the previous titration curve. Before titration: weak base solution: p. OH = -log kb Cb Before eq. point: The solution (NH 3 + NH 4 Cl) which is a buffer solution: p. OH = pkb + log Cs Cb Cs = ml. HCl MHCl ml. NH 3 + ml. HCl Cb = ml. NH 3 MNH 3 - ml. HCl MHCl ml. NH 3 + ml. HCl

Example 2 n n At eq. point: Salt solution NH 4 Cl (weak acid) p. H = - log kw Cs kb Cs = ml. HCl MHCl = ml. NH 3 MNH 3 ml. HCl + ml. NH 3 + ml. HCl After eq. point: Solution (HCl + NH 4 Cl) is calculated based on the fact that it is a strong acid solution and neglects the effect of the salt NH 4 Cl. p. H = -log ml. HCl MHCl - ml. NH 3 MNH 3 ml. HCl + ml. NH 3

Example 2 n n As we referred to before, when titrating a weak base with a strong acid, the p. OH at midtitration (at the titration of 50% of the base) is equal to pkb of the titrated base: kb = [OH-] [NH+4] [NH 3] pkb = p. OH In conclusion, we realize the great importance of the titration curves which give us an idea about the reaction. It also helps in choosing the best evidence to recognize the end point.

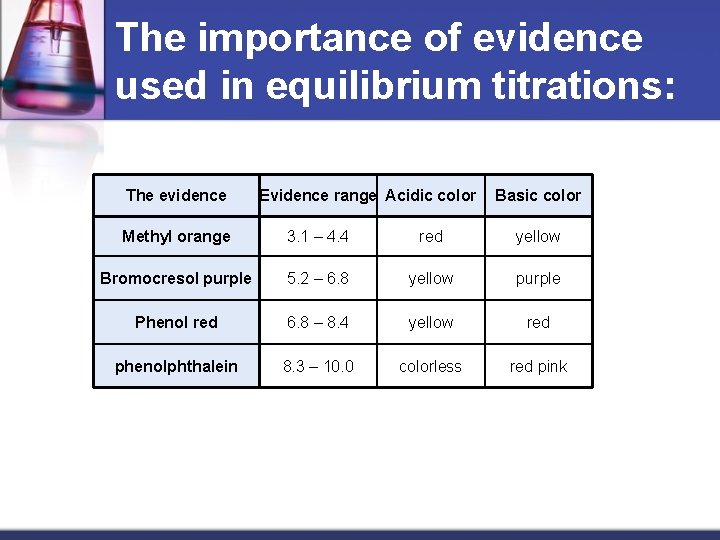
The importance of evidence used in equilibrium titrations: The evidence Evidence range Acidic color Basic color Methyl orange 3. 1 – 4. 4 red yellow Bromocresol purple 5. 2 – 6. 8 yellow purple Phenol red 6. 8 – 8. 4 yellow red phenolphthalein 8. 3 – 10. 0 colorless red pink

The importance of evidence used in equilibrium titrations: Methyl orange evidence Color: basic yellow and acidic red Phenolphthalein Color: basic pink and the acidic medium is colorless
 Introduction to analytical chemistry
Introduction to analytical chemistry Mechanical entrapment coprecipitation
Mechanical entrapment coprecipitation Titration vs back titration
Titration vs back titration Titration vs back titration
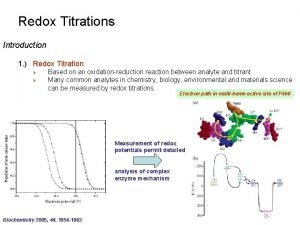
Titration vs back titration Redox titration
Redox titration Redox titration definition
Redox titration definition Analytical chemistry definition
Analytical chemistry definition Errors in chemistry
Errors in chemistry Jelaskan kegunaan statistika dalam analisis kimia
Jelaskan kegunaan statistika dalam analisis kimia Chemistry apparatus
Chemistry apparatus Calibration verification and linearity
Calibration verification and linearity Gaussian curve
Gaussian curve Redox reaction in alkaline medium
Redox reaction in alkaline medium Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry definition
Analytical chemistry definition Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry chapters
Analytical chemistry chapters Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry Titration mechanism
Titration mechanism