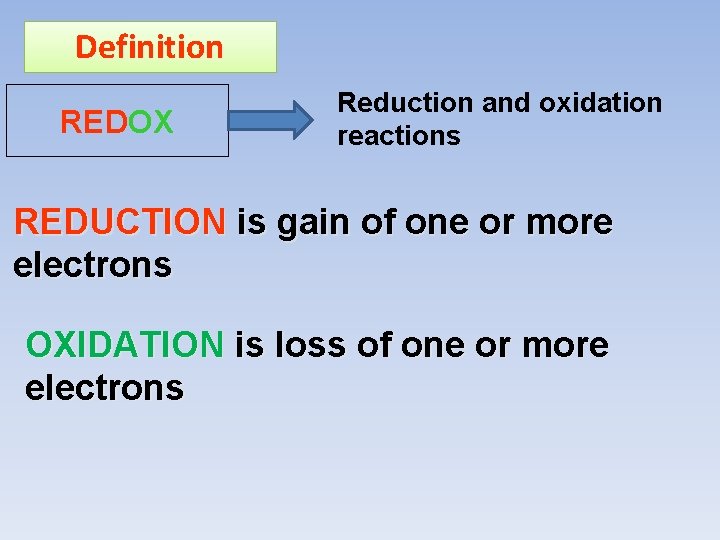
Analytical Chemistry II Redox Reactions Definition REDOX Reduction



- Slides: 12

Analytical Chemistry II Redox Reactions

Definition REDOX Reduction and oxidation reactions REDUCTION is gain of one or more electrons OXIDATION is loss of one or more electrons

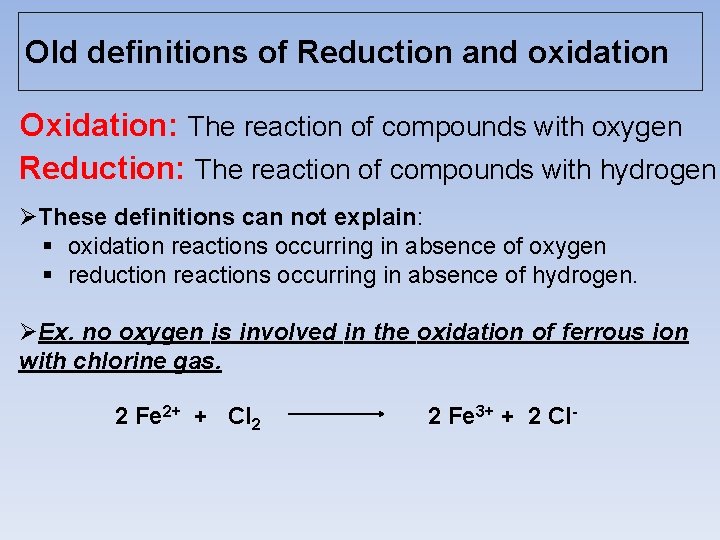
Old definitions of Reduction and oxidation Oxidation: The reaction of compounds with oxygen Reduction: The reaction of compounds with hydrogen ØThese definitions can not explain: § oxidation reactions occurring in absence of oxygen § reduction reactions occurring in absence of hydrogen. ØEx. no oxygen is involved in the oxidation of ferrous ion with chlorine gas. 2 Fe 2+ + Cl 2 2 Fe 3+ + 2 Cl-

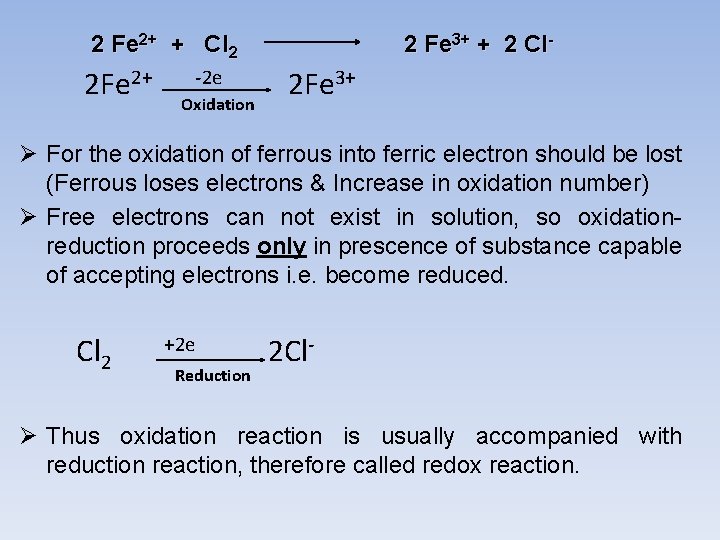
2 Fe 2+ + Cl 2 2 Fe 2+ -2 e Oxidation 2 Fe 3+ + 2 Cl- 2 Fe 3+ Ø For the oxidation of ferrous into ferric electron should be lost (Ferrous loses electrons & Increase in oxidation number) Ø Free electrons can not exist in solution, so oxidationreduction proceeds only in prescence of substance capable of accepting electrons i. e. become reduced. Cl 2 +2 e Reduction 2 Cl- Ø Thus oxidation reaction is usually accompanied with reduction reaction, therefore called redox reaction.

Reducing agent Substance that losses electrons(Electron Donor) and changes to higher valency. E. g. Na 2 S 2 O 3, Metallic iron, Oxalic acid, Sod. oxalate Oxidizing agent Substance that gains electrons (Electron Acceptor) and changes to lower valency. E. g. KMn. O 4, K 2 Cr 2 O 7, I 2, KIO 3, KBr. O 3 Example 2: Cl 2 + 2 Br- = Br 2 + 2 Cl-

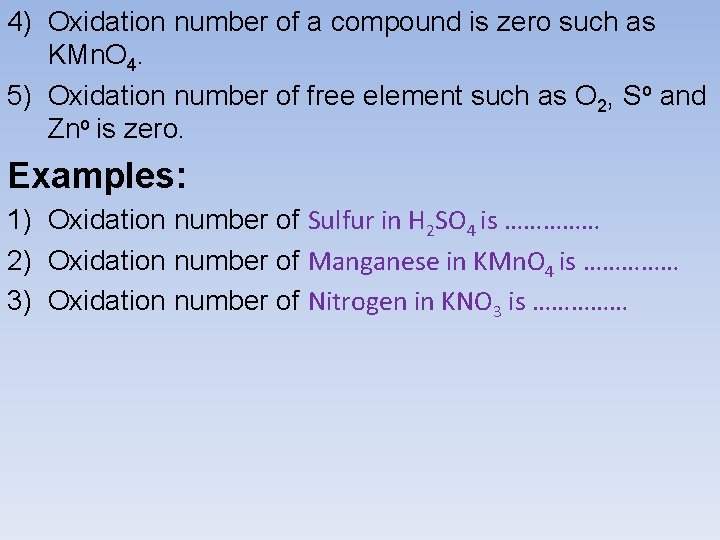
Oxidation number: It is the number of charges an atom appears to have when it is changed to an ion. Notes: 1) Oxidation number of any ion is its electrical charge such as Fe 2+ or Zn 2+. 2) Oxidation number of O is -2 ( except in peroxides). 3) Oxidation number of H is +1 ( except in hydrides).

4) Oxidation number of a compound is zero such as KMn. O 4. 5) Oxidation number of free element such as O 2, So and Zno is zero. Examples: 1) Oxidation number of Sulfur in H 2 SO 4 is …………… 2) Oxidation number of Manganese in KMn. O 4 is …………… 3) Oxidation number of Nitrogen in KNO 3 is ……………

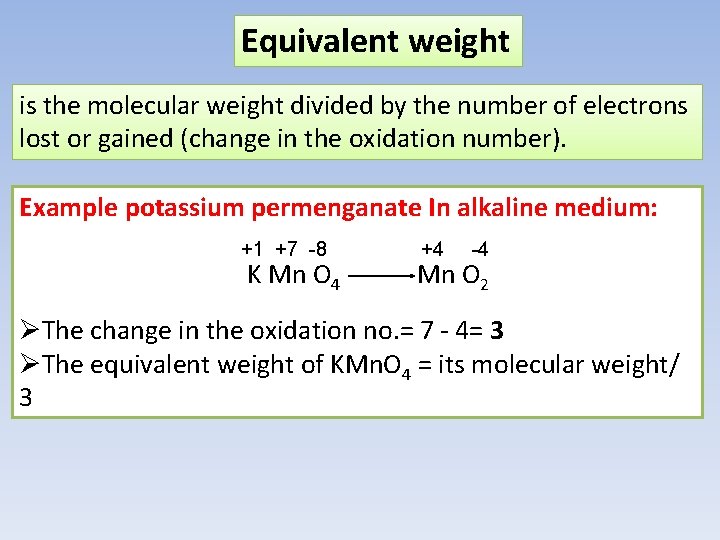
Equivalent weight is the molecular weight divided by the number of electrons lost or gained (change in the oxidation number). Example potassium permenganate In alkaline medium: +1 +7 -8 K Mn O 4 +4 -4 Mn O 2 ØThe change in the oxidation no. = 7 - 4= 3 ØThe equivalent weight of KMn. O 4 = its molecular weight/ 3

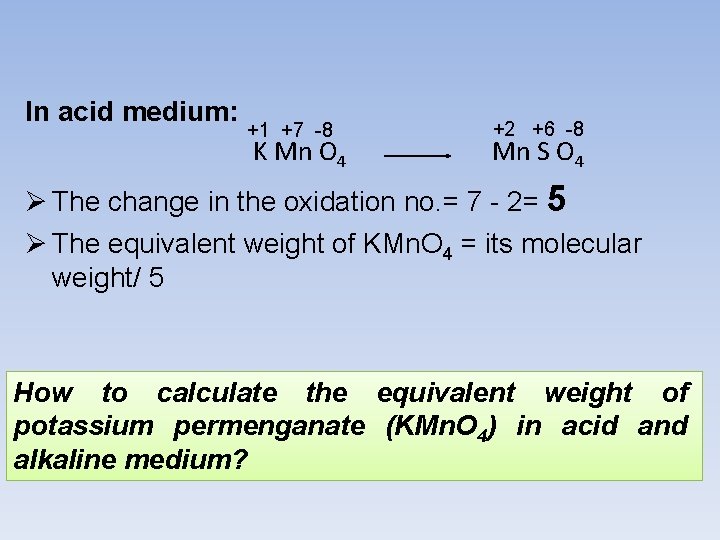
In acid medium: +1 +7 -8 K Mn O 4 +2 +6 -8 Mn S O 4 Ø The change in the oxidation no. = 7 - 2= 5 Ø The equivalent weight of KMn. O 4 = its molecular weight/ 5 How to calculate the equivalent weight of potassium permenganate (KMn. O 4) in acid and alkaline medium?

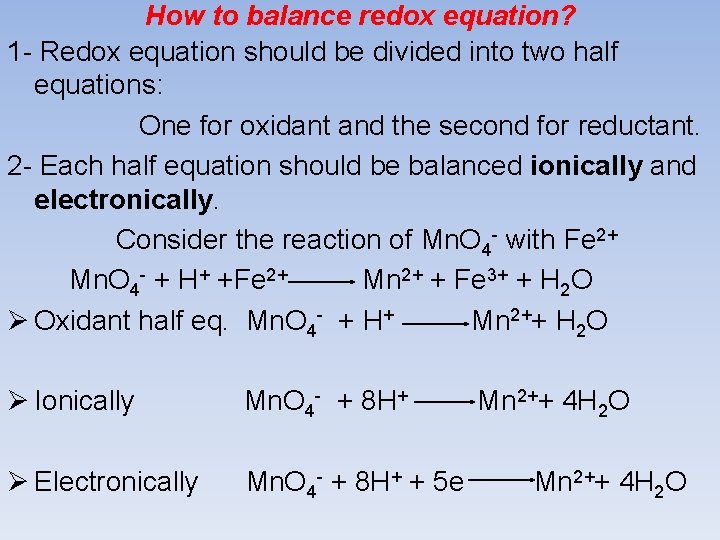
How to balance redox equation? 1 - Redox equation should be divided into two half equations: One for oxidant and the second for reductant. 2 - Each half equation should be balanced ionically and electronically. Consider the reaction of Mn. O 4 - with Fe 2+ Mn. O 4 - + H+ +Fe 2+ Mn 2+ + Fe 3+ + H 2 O Ø Oxidant half eq. Mn. O 4 - + H+ Mn 2++ H 2 O Ø Ionically Mn. O 4 - + 8 H+ Ø Electronically Mn. O 4 - + 8 H+ + 5 e Mn 2++ 4 H 2 O

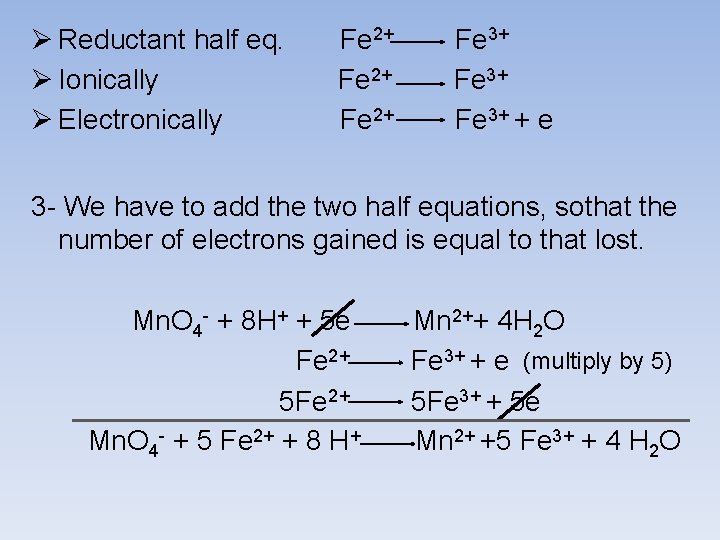
Ø Reductant half eq. Ø Ionically Ø Electronically Fe 2+ Fe 3+ + e 3 - We have to add the two half equations, sothat the number of electrons gained is equal to that lost. Mn. O 4 - + 8 H+ + 5 e Fe 2+ 5 Fe 2+ Mn. O 4 - + 5 Fe 2+ + 8 H+ Mn 2++ 4 H 2 O Fe 3+ + e (multiply by 5) 5 Fe 3+ + 5 e Mn 2+ +5 Fe 3+ + 4 H 2 O

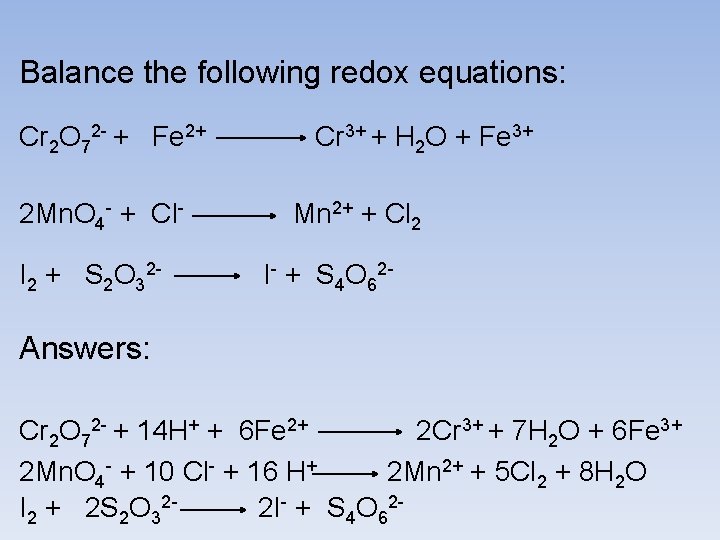
Balance the following redox equations: Cr 2 O 72 - + Fe 2+ 2 Mn. O 4 - + Cl. I 2 + S 2 O 32 - Cr 3+ + H 2 O + Fe 3+ Mn 2+ + Cl 2 I- + S 4 O 62 - Answers: Cr 2 O 72 - + 14 H+ + 6 Fe 2+ 2 Cr 3+ + 7 H 2 O + 6 Fe 3+ 2 Mn. O 4 - + 10 Cl- + 16 H+ 2 Mn 2+ + 5 Cl 2 + 8 H 2 O I 2 + 2 S 2 O 322 I- + S 4 O 62 -