Analysis of Patient Diaries in the NETTER1 Study


- Slides: 10

Analysis of Patient Diaries in the NETTER-1 Study Abstract T 2 P Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Hobday T, Hendifar A, Santoro P, Broberg P, Demange A, Öberg K, Ruszniewski P, Ravasi L, Krennin E; on behalf of the NETTER-1 Trial Investigators

Introduction and Aim • In the Neuroendocrine Tumors Therapy (NETTER-1) study, patients with progressive midgut NETs treated with 177 Lu-DOTATATE had a clinically and statistically significant PFS benefit over those treated with high-dose octreotide alone[a] • Patients in the 177 Lu-DOTATATE arm also experienced a statistically significant delay in time to deterioration in HRQo. L compared with patients in the high-dose octreotide arm[b] • Patients taking part in the NETTER-1 trial completed daily symptom diaries • Here, we report the results of these diaries ; HRQo. L = health-related quality of life; NETs = neuroendocrine tumors; PFS = progression-free survival. a. Strosberg J, et al. N Engl J Med. 2017; 376: 125 -135; b. Strosberg J, et al. J Clin Oncol. 2018; 36: 2578 -2584. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

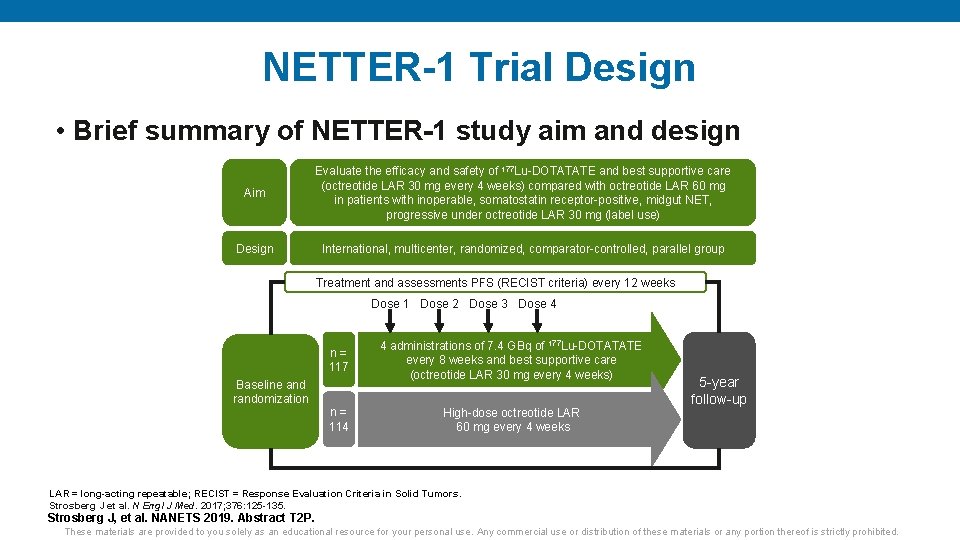
NETTER-1 Trial Design • Brief summary of NETTER-1 study aim and design Aim Evaluate the efficacy and safety of 177 Lu-DOTATATE and best supportive care (octreotide LAR 30 mg every 4 weeks) compared with octreotide LAR 60 mg in patients with inoperable, somatostatin receptor-positive, midgut NET, progressive under octreotide LAR 30 mg (label use) Design International, multicenter, randomized, comparator-controlled, parallel group Treatment and assessments PFS (RECIST criteria) every 12 weeks Dose 1 Dose 2 Dose 3 Dose 4 n= 117 Baseline and randomization n= 114 4 administrations of 7. 4 GBq of 177 Lu-DOTATATE every 8 weeks and best supportive care (octreotide LAR 30 mg every 4 weeks) High-dose octreotide LAR 60 mg every 4 weeks 5 -year follow-up LAR = long-acting repeatable; RECIST = Response Evaluation Criteria in Solid Tumors. Strosberg J et al. N Engl J Med. 2017; 376: 125 -135. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

Endpoints • Primary endpoint PFS • Key symptom-related secondary endpoints: –HRQo. L (Measured by EORTC QLQ C-30 and GI. NET-21 questionnaires) –Symptom frequency on patient diaries (number of days with symptoms averaged over preceding 4 weeks) EORTC QLQ = European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire. Strosberg J, et al. J Clin Oncol. 2018; 36: 2578 -2584. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

Diary Methods • Patients in both treatment arms were asked to record the presence or absence of a range of symptoms in a daily diary • Changes from baseline in the mean number of days with symptoms were determined using mixed model for repeated measures, adjusting for baseline symptom status, week, treatment, and the interaction between week and treatment • At baseline, the number of days with symptoms was determined using diary data from the 6 weeks before randomization Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

Diary Methods • During the randomized treatment phase, the number of days with symptoms during the previous 4 weeks was determined from diary data at each 4 -weekly clinic visit • Pearson and repeated measures correlation analyses were conducted for symptoms recorded in the diaries and corresponding symptoms reported using the EORTC QLQ-C 30 or QLQ-GI. NET-21 • Symptoms considered as particularly relevant for evaluating overall disease status were abdominal pain, diarrhea, and cutaneous flushing Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

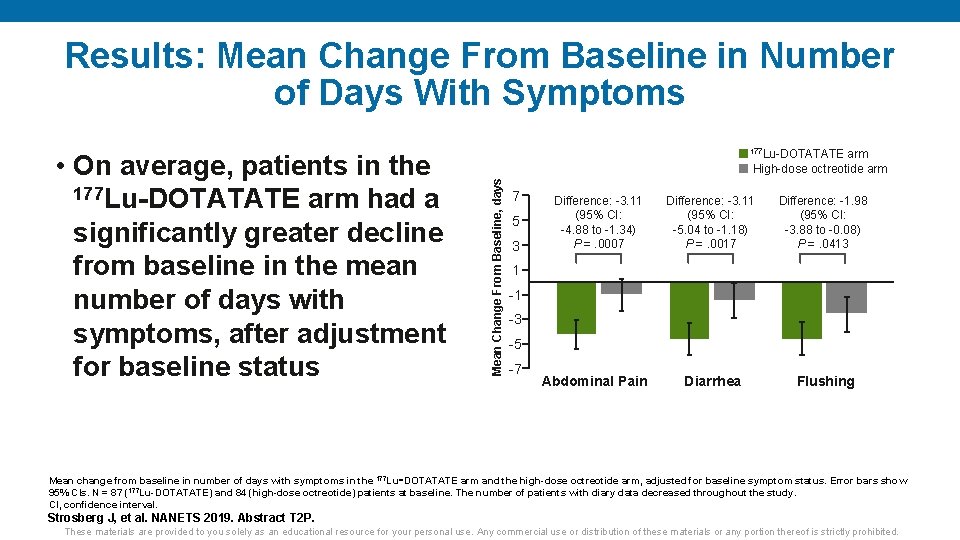
Results: Mean Change From Baseline in Number of Days With Symptoms arm High-dose octreotide arm Mean Change From Baseline, days • On average, patients in the 177 Lu-DOTATATE arm had a significantly greater decline from baseline in the mean number of days with symptoms, after adjustment for baseline status 177 Lu-DOTATATE 7 5 3 Difference: -3. 11 (95% CI: -4. 88 to -1. 34) P =. 0007 Difference: -3. 11 (95% CI: -5. 04 to -1. 18) P =. 0017 Difference: -1. 98 (95% CI: -3. 88 to -0. 08) P =. 0413 Diarrhea Flushing 1 -1 -3 -5 -7 Abdominal Pain Mean change from baseline in number of days with symptoms in the 177 Lu‑DOTATATE arm and the high-dose octreotide arm, adjusted for baseline symptom status. Error bars show 95% CIs. N = 87 (177 Lu-DOTATATE) and 84 (high-dose octreotide) patients at baseline. The number of patients with diary data decreased throughout the study. CI, confidence interval. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

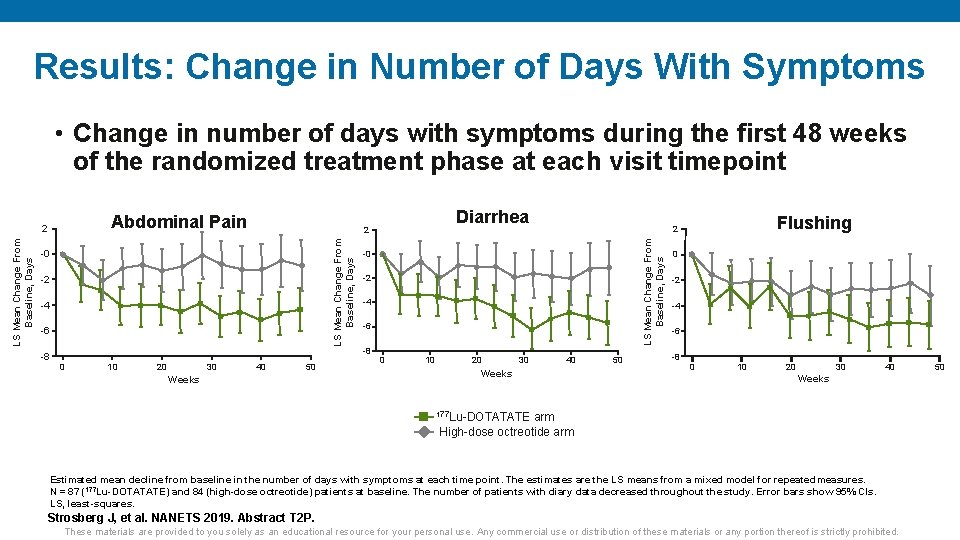
Results: Change in Number of Days With Symptoms • Change in number of days with symptoms during the first 48 weeks of the randomized treatment phase at each visit timepoint Abdominal Pain -2 -4 -6 0 10 20 30 40 50 Weeks -0 -2 -4 -6 -8 0 10 20 30 Flushing 2 LS Mean Change From Baseline, Days -0 -8 Diarrhea 2 LS Mean Change From Baseline, Days 2 40 Weeks 50 0 -2 -4 -6 -8 0 10 20 30 40 Weeks 177 Lu-DOTATATE arm High-dose octreotide arm Estimated mean decline from baseline in the number of days with symptoms at each time point. The estimates are the LS means from a mixed model for repeated measures. N = 87 (177 Lu-DOTATATE) and 84 (high-dose octreotide) patients at baseline. The number of patients with diary data decreased throughout the study. Error bars show 95% CIs. LS, least-squares. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited. 50

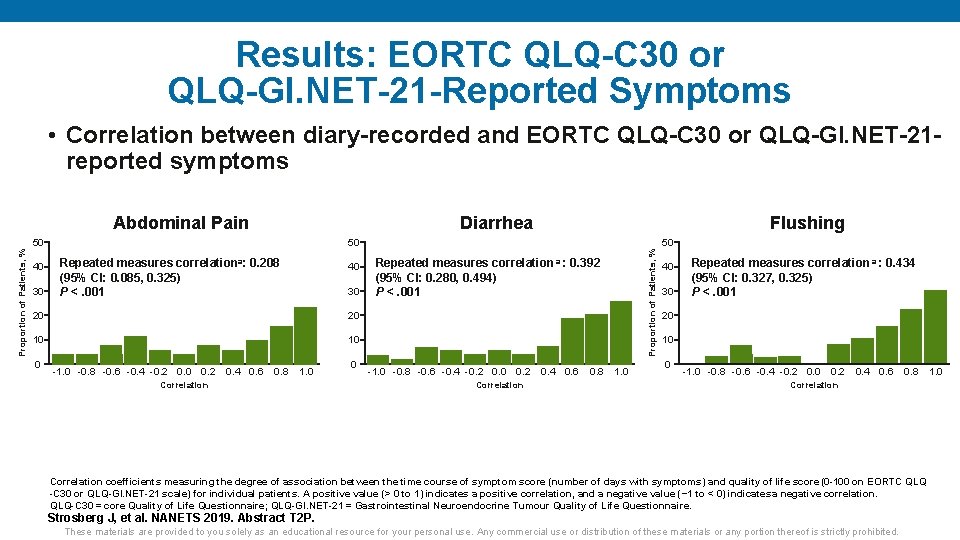
Results: EORTC QLQ-C 30 or QLQ-GI. NET-21 -Reported Symptoms • Correlation between diary-recorded and EORTC QLQ-C 30 or QLQ-GI. NET-21 reported symptoms Diarrhea Abdominal Pain 40 30 50 Repeated measures correlationa: 0. 208 (95% CI: 0. 085, 0. 325) P <. 001 40 30 20 20 10 10 0 -1. 0 -0. 8 -0. 6 -0. 4 -0. 2 0. 0 0. 2 0. 4 0. 6 0. 8 1. 0 Correlation 0 50 Repeated measures correlation a : 0. 392 (95% CI: 0. 280, 0. 494) P <. 001 -1. 0 -0. 8 -0. 6 -0. 4 -0. 2 0. 0 0. 2 0. 4 0. 6 0. 8 1. 0 Correlation Proportion of Patients, % 50 Flushing 40 30 Repeated measures correlation a : 0. 434 (95% CI: 0. 327, 0. 325) P <. 001 20 10 0 -1. 0 -0. 8 -0. 6 -0. 4 -0. 2 0. 0 0. 2 0. 4 0. 6 0. 8 1. 0 Correlation coefficients measuring the degree of association between the time course of symptom score (number of days with symptoms) and quality of life score (0 -100 on EORTC QLQ -C 30 or QLQ-GI. NET-21 scale) for individual patients. A positive value (> 0 to 1) indicates a positive correlation, and a negative value (− 1 to < 0) indicates a negative correlation. QLQ-C 30 = core Quality of Life Questionnaire; QLQ-GI. NET-21 = Gastrointestinal Neuroendocrine Tumour Quality of Life Questionnaire. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.

Conclusions • Analysis of symptom diaries from the NETTER-1 study confirms that 177 Lu-DOTATATE can palliate clinically relevant symptoms compared with high-dose octreotide • Symptom diary data support previously reported results from NETTER-1, which showed a statistically significant delay in decline of HRQo. L for patients in the 177 Lu-DOTATATE arm compared with those in the high-dose octreotide arma a. Strosberg J, et al. J Clin Oncol. 2018; 36: 2578 -2584. Strosberg J, et al. NANETS 2019. Abstract T 2 P. These materials are provided to you solely as an educational resource for your personal use. Any commercial use or distribution of these materials or any portion thereof is strictly prohibited.