Analysis of FerromagneticMultiferroic interfaces in Epitaxial Multilayers of











- Slides: 34

Analysis of Ferromagnetic-Multiferroic interfaces in Epitaxial Multilayers of LSMO and BFO Student: Peter Knapp Research Advisor: Professor Jeremiah Abiade

Overview I. Bilayers were fabricated from ferromagnetic (FM) LSMO (La 0. 7 Sr 0. 3 Mn. O 3) and antiferromagnetic (AFM) BFO (Bi. Fe. O 3) via Pulsed Laser Deposition (PLD) II. Layers were analyzed using TEM (Transmission Electron Microscopy), XRD (X-ray Diffraction), and XPS (X-ray Photoelectron Spectroscopy) in order to confirm composition and observe structural detiails

Motivation For Project • Need to control the structure of oxide thin films and multilayers • Understand effects of structure & layering on magnetic interaction • Preliminary work for future experiments on properties of ferromagnetic/ferroelectric systems

Introduction to Multiferroic Bilayers • Materials where electric polarization influences ferromagnetic polarization, allowing manipulation of electric/magnetic order 1 • Contemporary research focuses on bilayers of FM and AFM materials • These structures demonstrate exchange bias (EB), exchange enhancement (EE), and exchange coupling (EC)

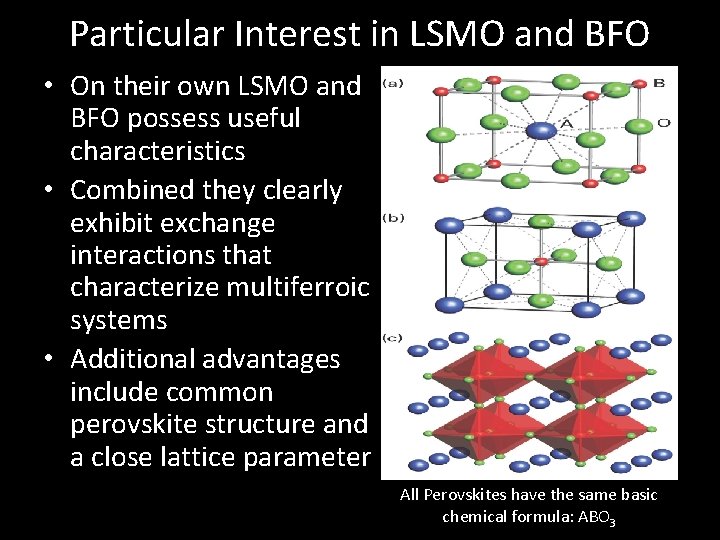
Particular Interest in LSMO and BFO • On their own LSMO and BFO possess useful characteristics • Combined they clearly exhibit exchange interactions that characterize multiferroic systems • Additional advantages include common perovskite structure and a close lattice parameter (A) (B) All Perovskites have the same basic chemical formula: ABO 3

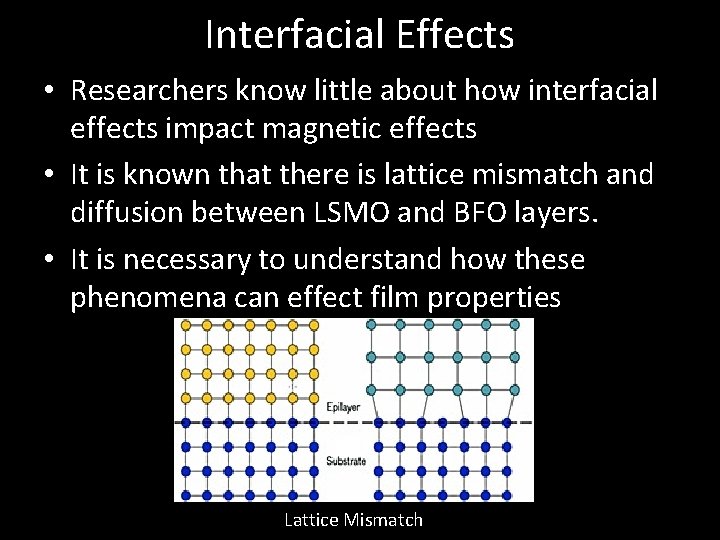
Interfacial Effects • Researchers know little about how interfacial effects impact magnetic effects • It is known that there is lattice mismatch and diffusion between LSMO and BFO layers. • It is necessary to understand how these phenomena can effect film properties Lattice Mismatch

Controlling Structure • These experiments will focus on achieving structural control during deposition • Substrate will be varied between La. Al. O 3 or Sr. Ti. O 3 • The thickness of the layers will be varied • Layer order will be varied

Potential Applications of Work • Could help demonstrate novel uses for materials like LMSO and BFO in memory devices and sensors, for instance Hard Drives and SQUIDs (superconducting quantum interference devices) • Development of novel heterostructures for unusual uses i. e. LMSO as electrode for ferroelectric films • Tailor structures to realize multicomponent multiferroic systems (e. g. electrical control of magnetism)

Experimental Procedures I. PLD for synthesis of the Bilayers. II. TEM to observe local characteristics II. XRD to observe interlayer interaction and structural characteristics III. XPS to confirm composition

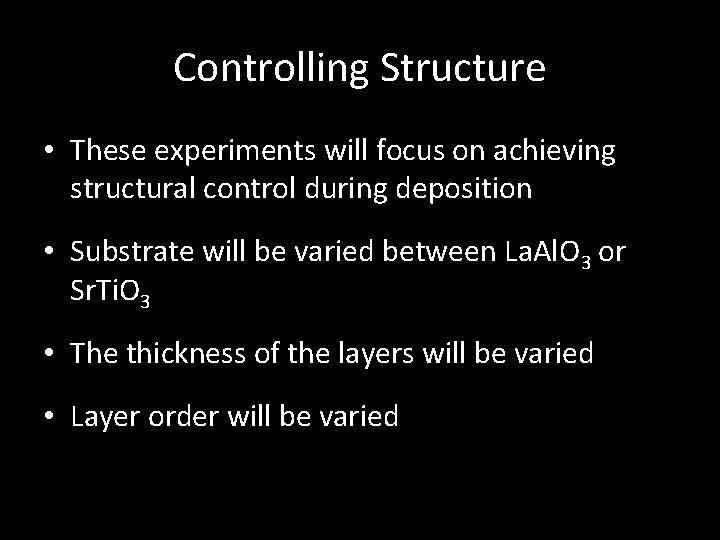
Pulsed Laser Deposition 1. Physical Vapor Deposition Technique 2. High Powered (Excimer Laser) focused on target (material to be deposited) in vacuum 3. Material is vaporized into plasma plume which extends from target 4. Proceeds to land on substrate forming a thin film 5. Highly Advantageous

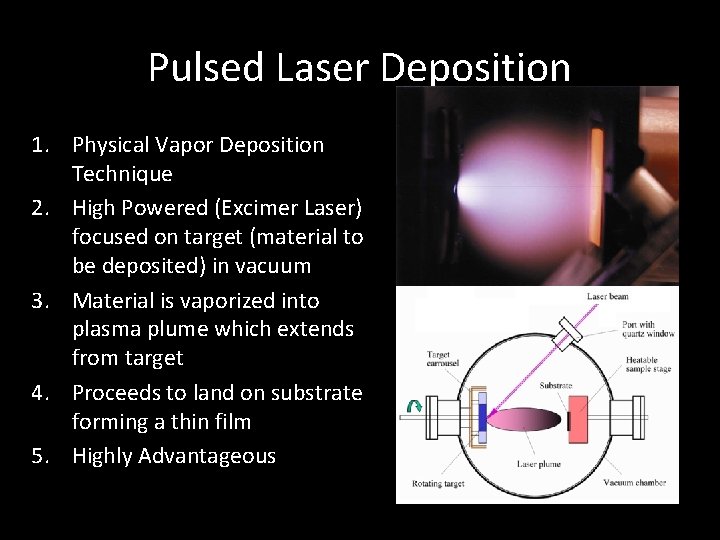
Transmission Electron Microscopy • Beam of Electrons fired through specimen • Electrons interact with material in film • Image created on photographic film or a CCD camera

II. X-Ray Reflectivity • Measurement: Specular reflection as a function of angle of incidence. Thin Film or Multilayer • Result: electron density profile along substrate normal • Thickness and average electron density of the film. • Thickness and electron density can be used to infer roughness and structural defects like diffusion and lattice mismatch • X-ray techniques can also be used to analyze strain in the films

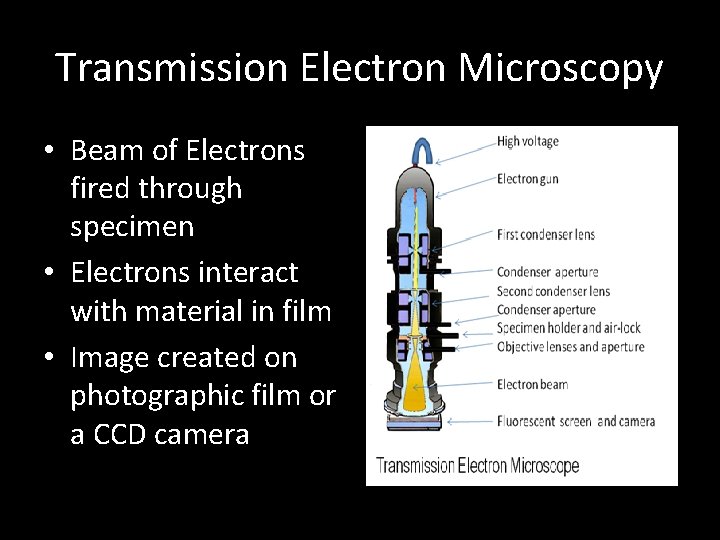
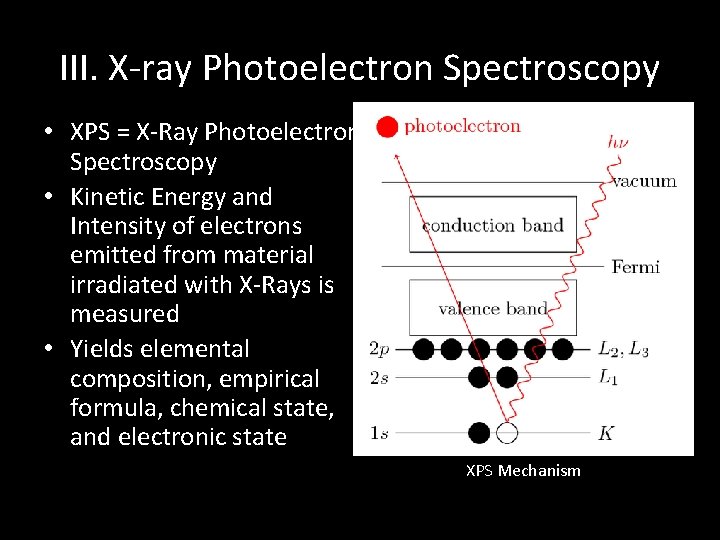
III. X-ray Photoelectron Spectroscopy • XPS = X-Ray Photoelectron Spectroscopy • Kinetic Energy and Intensity of electrons emitted from material irradiated with X-Rays is measured • Yields elemental composition, empirical formula, chemical state, and electronic state XPS Mechanism

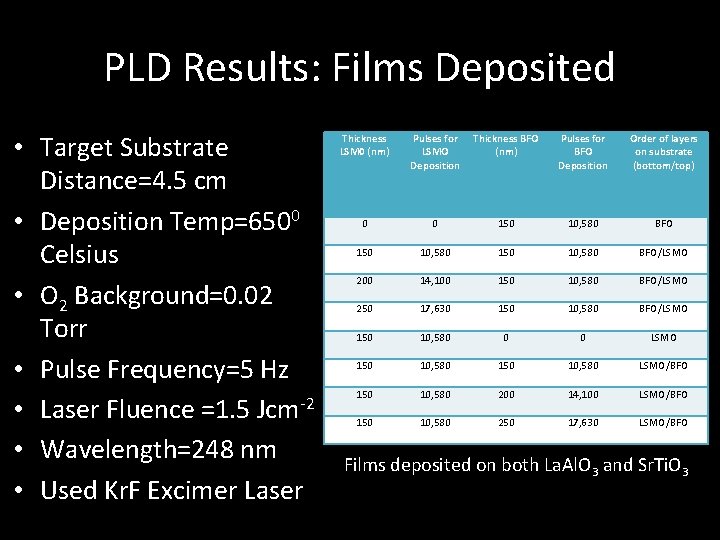
PLD Results: Films Deposited • Target Substrate Distance=4. 5 cm • Deposition Temp=6500 Celsius • O 2 Background=0. 02 Torr • Pulse Frequency=5 Hz • Laser Fluence =1. 5 Jcm-2 • Wavelength=248 nm • Used Kr. F Excimer Laser Thickness LSM 0 (nm) Pulses for LSMO Deposition Thickness BFO (nm) Pulses for BFO Deposition Order of layers on substrate (bottom/top) 0 0 150 10, 580 BFO/LSMO 200 14, 100 150 10, 580 BFO/LSMO 250 17, 630 150 10, 580 BFO/LSMO 150 10, 580 0 0 LSMO 150 10, 580 LSMO/BFO 150 10, 580 200 14, 100 LSMO/BFO 150 10, 580 250 17, 630 LSMO/BFO Films deposited on both La. Al. O 3 and Sr. Ti. O 3

TEM Results – 150 nm_BFO_La. Al. O 3 BFO

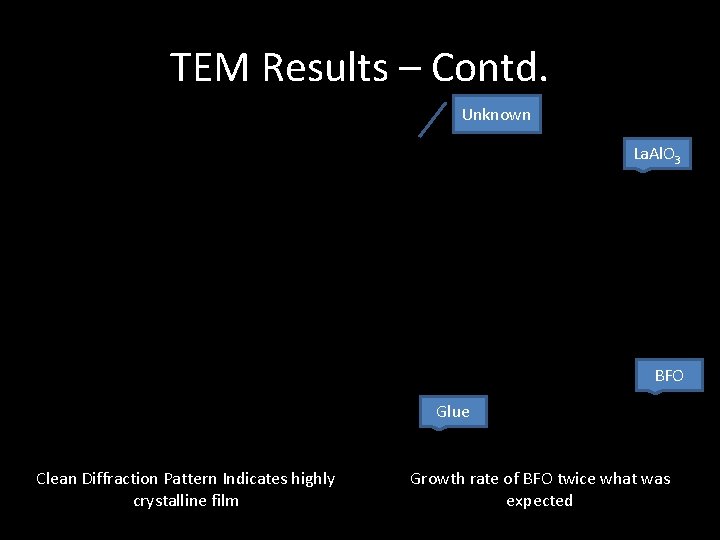
TEM Results – Contd. Unknown La. Al. O 3 BFO Glue Clean Diffraction Pattern Indicates highly crystalline film Growth rate of BFO twice what was expected

TEM - Results 1. PLD Allowed for deposition of films that are highly crystalline 2. At the interface there is a slight rotation (30 o to 40 o) between the crystalline plane of the substrate and film 3. Growth Rate of BFO is twice that of LSMO

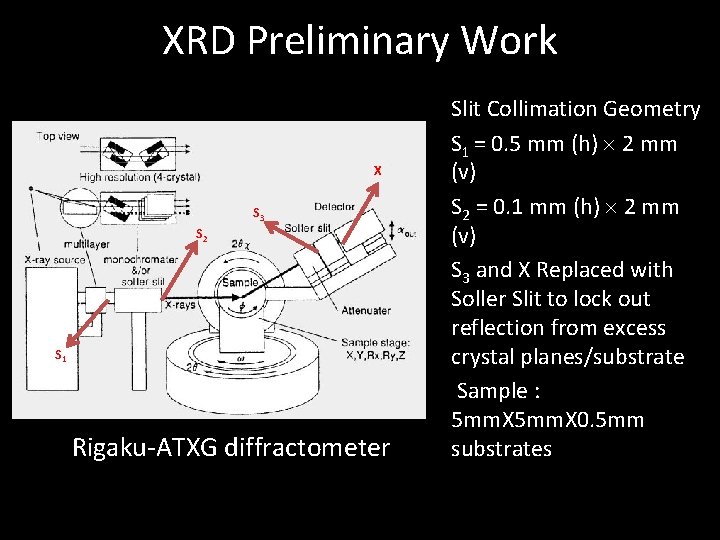
XRD Preliminary Work X S 3 S 2 S 1 Rigaku-ATXG diffractometer Slit Collimation Geometry S 1 = 0. 5 mm (h) 2 mm (v) S 2 = 0. 1 mm (h) 2 mm (v) S 3 and X Replaced with Soller Slit to lock out reflection from excess crystal planes/substrate Sample : 5 mm. X 0. 5 mm substrates

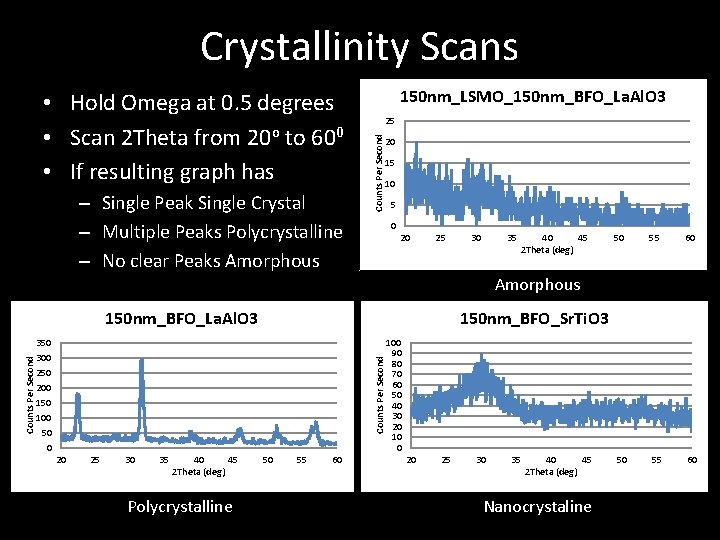
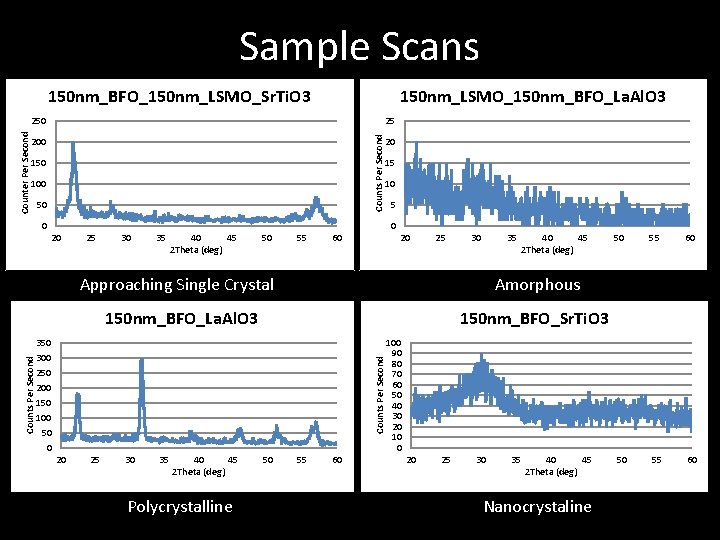
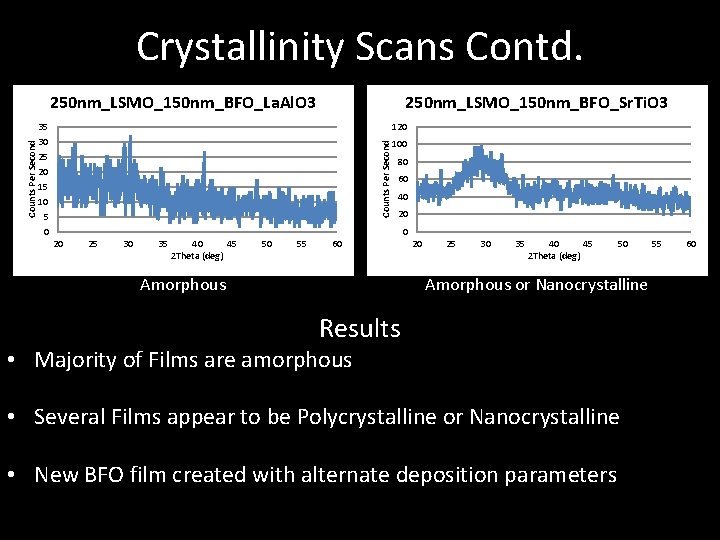
Crystallinity Scans – Single Peak Single Crystal – Multiple Peaks Polycrystalline – No clear Peaks Amorphous 150 nm_LSMO_150 nm_BFO_La. Al. O 3 25 Counts Per Second • Hold Omega at 0. 5 degrees • Scan 2 Theta from 20 o to 600 • If resulting graph has 20 15 10 5 0 20 25 30 35 40 45 2 Theta (deg) 50 55 60 Amorphous 150 nm_BFO_Sr. Ti. O 3 350 300 250 200 150 100 50 0 Counts Per Second 150 nm_BFO_La. Al. O 3 20 25 30 35 40 45 2 Theta (deg) Polycrystalline 50 55 60 100 90 80 70 60 50 40 30 20 10 0 20 25 30 35 40 45 2 Theta (deg) Nanocrystaline

Sample Scans 150 nm_BFO_150 nm_LSMO_Sr. Ti. O 3 150 nm_LSMO_150 nm_BFO_La. Al. O 3 25 Counts Per Second Counter Per Second 250 200 150 100 50 20 15 10 0 0 20 25 30 35 40 45 2 Theta (deg) 50 55 60 20 30 35 40 45 2 Theta (deg) Amorphous 150 nm_BFO_La. Al. O 3 150 nm_BFO_Sr. Ti. O 3 350 300 250 200 150 100 50 0 20 25 Approaching Single Crystal Counts Per Second 5 25 30 35 40 45 2 Theta (deg) Polycrystalline 50 55 60 100 90 80 70 60 50 40 30 20 10 0 20 25 30 35 40 45 2 Theta (deg) Nanocrystaline

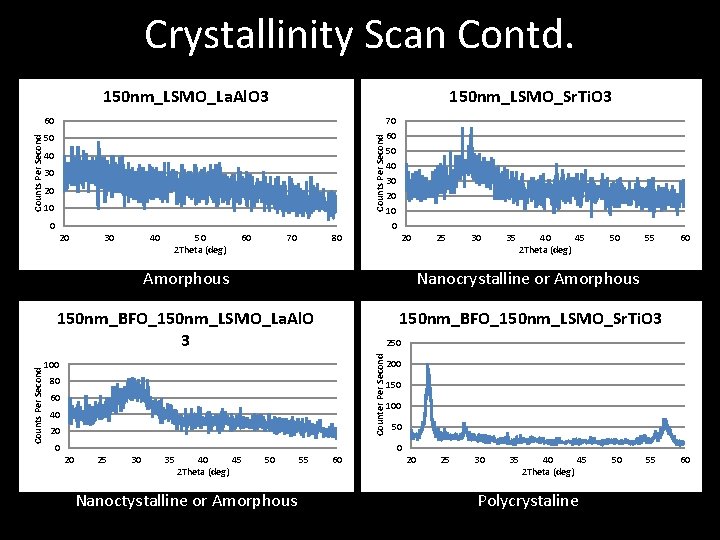
Crystallinity Scan Contd. 150 nm_LSMO_La. Al. O 3 150 nm_LSMO_Sr. Ti. O 3 50 Counts Per Second 60 40 30 20 10 0 30 40 50 2 Theta (deg) 60 70 80 20 25 30 35 40 45 2 Theta (deg) 50 55 Amorphous Nanocrystalline or Amorphous 150 nm_BFO_150 nm_LSMO_La. Al. O 3 150 nm_BFO_150 nm_LSMO_Sr. Ti. O 3 60 250 Counter Per Second Counts Per Second 20 70 60 50 40 30 20 100 80 60 40 200 150 100 50 0 20 25 30 35 40 45 2 Theta (deg) 50 Nanoctystalline or Amorphous 55 60 20 25 30 35 40 45 2 Theta (deg) Polycrystaline 50 55 60

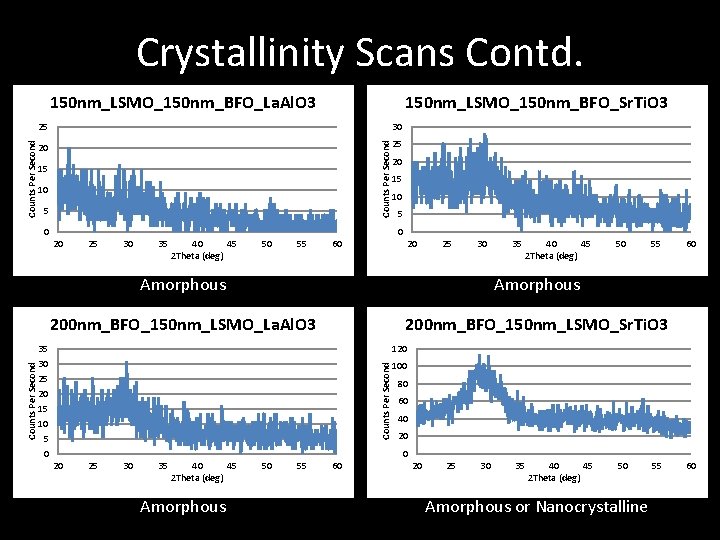
Crystallinity Scans Contd. 150 nm_LSMO_150 nm_BFO_Sr. Ti. O 3 25 30 20 25 Counts Per Second 150 nm_LSMO_150 nm_BFO_La. Al. O 3 15 10 5 0 20 25 30 35 40 45 2 Theta (deg) 50 55 60 20 25 30 35 40 45 2 Theta (deg) 50 55 Amorphous 200 nm_BFO_150 nm_LSMO_La. Al. O 3 200 nm_BFO_150 nm_LSMO_Sr. Ti. O 3 35 30 25 20 15 10 5 0 60 120 Counts Per Second 20 100 80 60 40 20 25 30 35 40 45 2 Theta (deg) Amorphous 50 55 60 20 25 30 35 40 45 2 Theta (deg) 50 Amorphous or Nanocrystalline 55 60

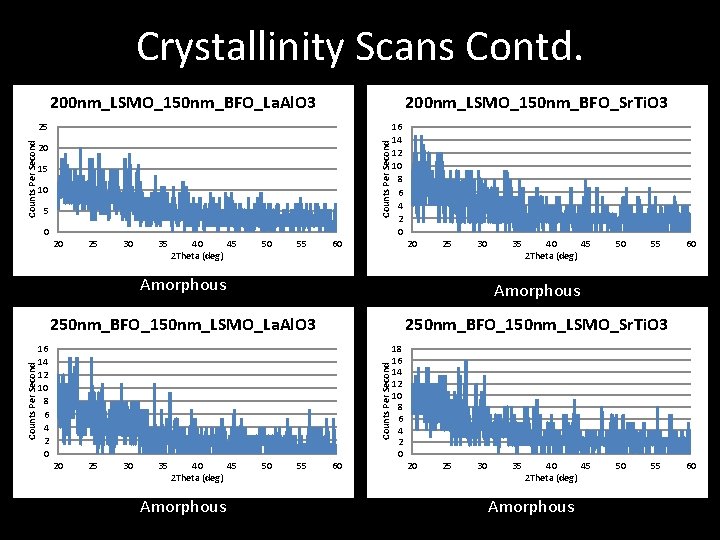
Crystallinity Scans Contd. 200 nm_LSMO_150 nm_BFO_La. Al. O 3 200 nm_LSMO_150 nm_BFO_Sr. Ti. O 3 Counts Per Second 25 20 15 10 5 0 25 30 35 40 45 2 Theta (deg) 50 55 60 20 25 30 35 40 45 2 Theta (deg) 50 55 Amorphous 250 nm_BFO_150 nm_LSMO_La. Al. O 3 250 nm_BFO_150 nm_LSMO_Sr. Ti. O 3 16 14 12 10 8 6 4 2 0 Counts Per Second 20 16 14 12 10 8 6 4 2 0 20 25 30 35 40 45 2 Theta (deg) Amorphous 50 55 60 60 18 16 14 12 10 8 6 4 2 0 20 25 30 35 40 45 2 Theta (deg) Amorphous 50 55 60

Crystallinity Scans Contd. 250 nm_LSMO_150 nm_BFO_Sr. Ti. O 3 35 30 25 20 15 10 5 0 120 Counts Per Second 250 nm_LSMO_150 nm_BFO_La. Al. O 3 100 80 60 40 20 25 30 35 40 45 2 Theta (deg) 50 55 60 Amorphous 20 25 30 35 40 45 2 Theta (deg) 50 Amorphous or Nanocrystalline Results • Majority of Films are amorphous • Several Films appear to be Polycrystalline or Nanocrystalline • New BFO film created with alternate deposition parameters 55 60

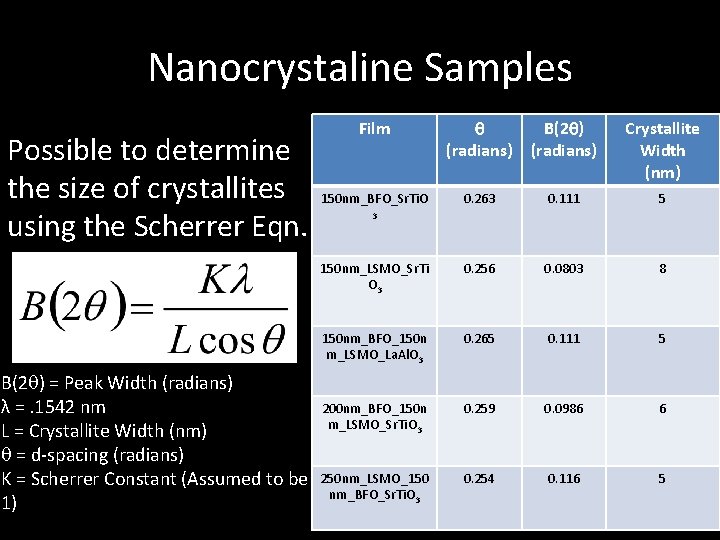
Nanocrystaline Samples Possible to determine the size of crystallites using the Scherrer Eqn. B(2 ) = Peak Width (radians) λ =. 1542 nm L = Crystallite Width (nm) = d-spacing (radians) K = Scherrer Constant (Assumed to be 1) Film (radians) B(2 ) (radians) Crystallite Width (nm) 150 nm_BFO_Sr. Ti. O 0. 263 0. 111 5 150 nm_LSMO_Sr. Ti O 3 0. 256 0. 0803 8 150 nm_BFO_150 n m_LSMO_La. Al. O 3 0. 265 0. 111 5 200 nm_BFO_150 n m_LSMO_Sr. Ti. O 3 0. 259 0. 0986 6 250 nm_LSMO_150 nm_BFO_Sr. Ti. O 3 0. 254 0. 116 5 3

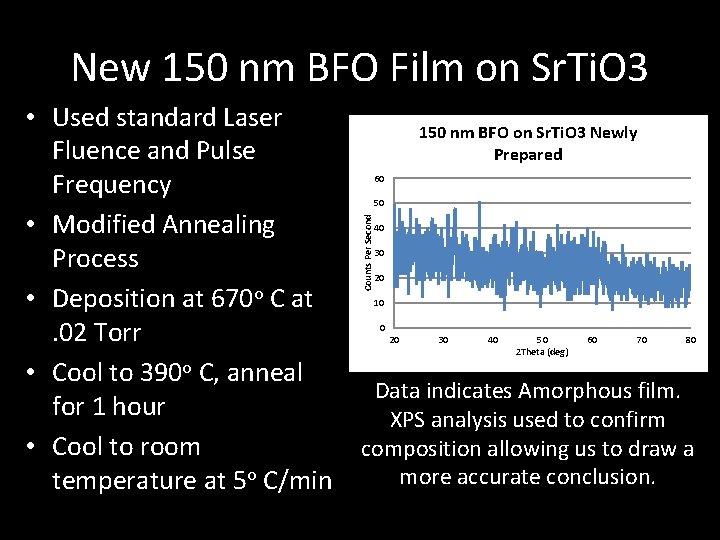
New 150 nm BFO Film on Sr. Ti. O 3 150 nm BFO on Sr. Ti. O 3 Newly Prepared 60 50 Counts Per Second • Used standard Laser Fluence and Pulse Frequency • Modified Annealing Process • Deposition at 670 o C at . 02 Torr • Cool to 390 o C, anneal for 1 hour • Cool to room temperature at 5 o C/min 40 30 20 10 0 20 30 40 50 2 Theta (deg) 60 70 80 Data indicates Amorphous film. XPS analysis used to confirm composition allowing us to draw a more accurate conclusion.

Crystallinity Scans - Results • Majority of films are amorphous with some polycrystalline and nanocrystalline samples • Likely due to diffusion of oxygen during annealing • Indicated deposition process still requires optimization

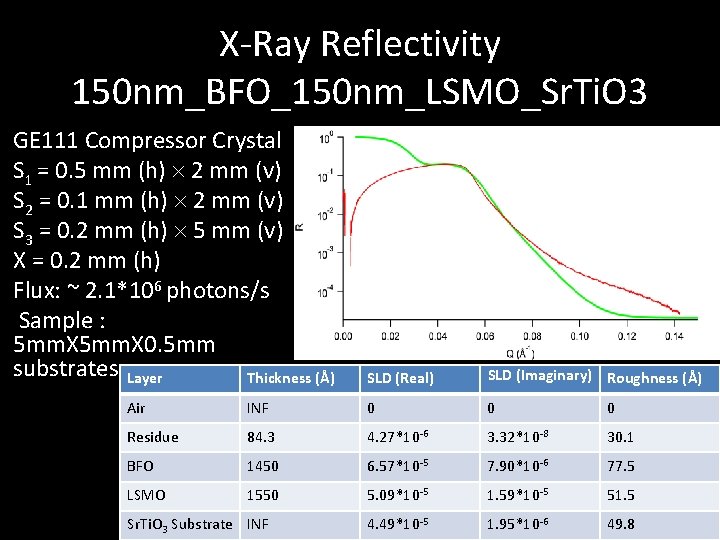
X-Ray Reflectivity 150 nm_BFO_150 nm_LSMO_Sr. Ti. O 3 GE 111 Compressor Crystal S 1 = 0. 5 mm (h) 2 mm (v) S 2 = 0. 1 mm (h) 2 mm (v) S 3 = 0. 2 mm (h) 5 mm (v) X = 0. 2 mm (h) Flux: ~ 2. 1*106 photons/s Sample : 5 mm. X 0. 5 mm substrates Layer Thickness (Å) SLD (Real) SLD (Imaginary) Roughness (Å) Air INF 0 0 0 Residue 84. 3 4. 27*10 -6 3. 32*10 -8 30. 1 BFO 1450 6. 57*10 -5 7. 90*10 -6 77. 5 LSMO 1550 5. 09*10 -5 1. 59*10 -5 51. 5 4. 49*10 -5 1. 95*10 -6 49. 8 Sr. Ti. O 3 Substrate INF

Conclusion - XRR • Thickness and SLD data seems reasonable but contrasts with data on growth rate from TEM • Unfitted drop results from having a high roughness film and low X-ray intensity during scanning • Top residue Layer is Likely a Combination of organics and silver particles from adhesive

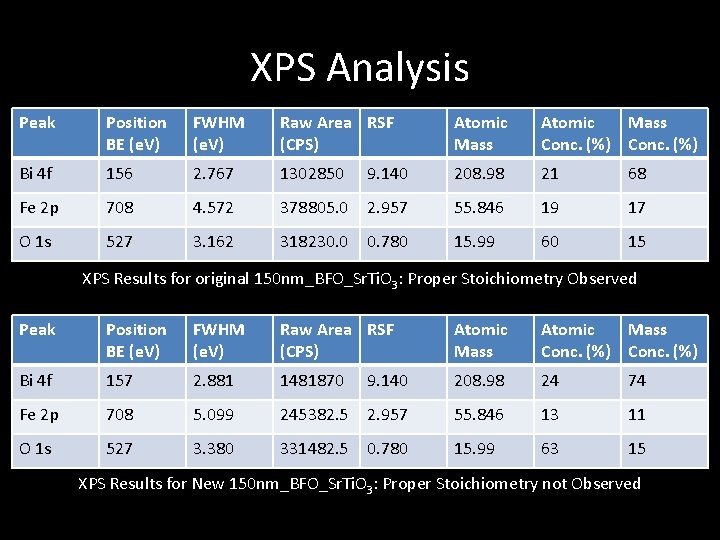
XPS Analysis Peak Position BE (e. V) FWHM (e. V) Raw Area RSF (CPS) Atomic Mass Conc. (%) Bi 4 f 156 2. 767 1302850 9. 140 208. 98 21 68 Fe 2 p 708 4. 572 378805. 0 2. 957 55. 846 19 17 O 1 s 527 3. 162 318230. 0 0. 780 15. 99 60 15 XPS Results for original 150 nm_BFO_Sr. Ti. O 3: Proper Stoichiometry Observed Peak Position BE (e. V) FWHM (e. V) Raw Area RSF (CPS) Atomic Mass Conc. (%) Bi 4 f 157 2. 881 1481870 9. 140 208. 98 24 74 Fe 2 p 708 5. 099 245382. 5 2. 957 55. 846 13 11 O 1 s 527 3. 380 331482. 5 0. 780 15. 99 63 15 XPS Results for New 150 nm_BFO_Sr. Ti. O 3: Proper Stoichiometry not Observed

XPS - Results • Stoichiometry of films very similar to target material • Currently no explanation for iron deficiency in the new BFO film

Summary/Conclusion • While the constructed films were not epitaxial many were highly crystalline • The Stoichiometry of films examined by XPS was consistent with the target material • XRR indicated the films have a large roughness • The deposition process for LSMO and BFO still requires optimization.

Acknowledgements The financial support from the National Science Foundation, EEC-NSF Grant # 1062943 is gratefully acknowledged. I would like to thank Professors Jursich and Takoudis for organizing the REU Program. I would like to thank the LORE lab in general and Professor Jeremiah Abiade specifically for providing me with the opportunity to work in their lab.

Sources 1 P. S. Sankara Rama Krishnan, M. Arredondo, M. Saunders, Q. M. Ramase, M. Valanoor: ‘Microstructural analysis of interfaces in a ferromagnetic-multiferroic epitaxial heterostructure’, J. Appl. Phys. , 2011, 109 034103 (2011), 1 -7. 2 L. W. Martin, Y-H. Chu, M. b. Holcomb, M. Huijben. P. Yu, S-J. Han, D. Lee, S. X. Wang, R. Ramesh: ‘Nanoscale Control of Exchange Bias with Bi. Fe. O 3 Thin Films’, Nano Letters, 2008, Vol. 8, No. 7, 2050 -2055. 3 X. Ke, L. J. Belenkey, C. B. Eom, M. S. Rzchowski: ‘Antiferromagnetic exchange-bias in epitaxial ferromagnetic La 0. 67 Sr 0. 33 Mn. O 3 /Sr. Ru. O 3 bilayers’, J. Appl. Phys. , 2005, 97 10 k 115 (2005), 1 -3. 4 M. Kharrasov, I. Kyzyrgulov, F. Iskahkov: ‘Exchange enhancement of the magnetoelastic interaction in a La. Mn. O< sub> 3< /sub> crystal’, Doklady Physics, 2003, Vol. 48, No. 9, 499 -500. 5 S. Habouti, R. K. Shiva, C-H. Solterbeck, M. Es-Souni, V. Zaporojtcheko: ‘La 0. 8 Sr 0. 2 Mn. O 3 buffer layer effects on microstructure, leakage current, polarization, and magnetic properties of Bi. Fe. O 3 thin films’, J. Appl. Phys. , 2007, 102 044113 (2007), 1 -6. 6 Esteve, D. , Postava, K. , Gogol, P. , Niu, G. , Vilquin, B. and Lecoeur, P. (2010), In situ monitoring of La 0. 67 Sr 0. 33 Mn. O 3 monolayers grown by pulsed laser deposition. Phys. Status Solidi B, 247: 1956– 1959. doi: 10. 1002/pssb. 200983960 7 G-Z. Liu, C. Wang, C-C. Wang, J. Qiu, M. He, J. Xing, K-J Jin, H-B Lu, G-Z. Yang: ‘Effects of interfacial polarization on the dielectric properties of Bi. Fe. O 3 thin film capacitors’, Appl. Phys Lett. , 2008. 92 122903 (2008), 1 -3 8 D. B. Chrisey, G. K. Hubbler: ‘Pulsed Laser Deposition of Thin Films’, 13 -56; 1994, New York, John Wiley & Sons.