Analysis of essential composition and quality product standard



- Slides: 44

Analysis of essential composition and quality product standard of coconut oil and downstream products Assoc. Prof. Kunchit Judprasong Quality manager of ISO 17025 laboratory Institute of Nutrition, Mahidol University (INMU), Salaya, Phuthamonthon 4, Nakhon Pathom, Thailand Kunchit. jud@mahidol. ac. th Assoc. Prof. Kunchit Judprasong Institute of Nutrition 1

Outline Ø Laboratory at Institute of Nutrition, Mahidol University (INMU) Ø Analysis of essential composition: 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) 13) Fatty acids Moisture and volatile matter and total solid Insoluble impurities Total fat Protein Codex standards: Ash - CODEX STAN 210 -1999, Minerals amendment 2015 (vegetable oils) Saponification value - CODEX STAN 240 -2003 (coconut milk & coconut cream) Iodine value - Codex CX/ASIA 99/4, 1999 Unsaponificable matter (aqueous coconut products) Peroxide value Acid value and acidity Heavy metals Ø Conclusion Assoc. Prof. Kunchit Judprasong Institute of Nutrition 2

Institute of Nutrition Mahidol University Website: http: //www. inmu. mahidol. ac. th/eng/ Bitec Assoc. Prof. Kunchit Judprasong Institute of Nutrition 3

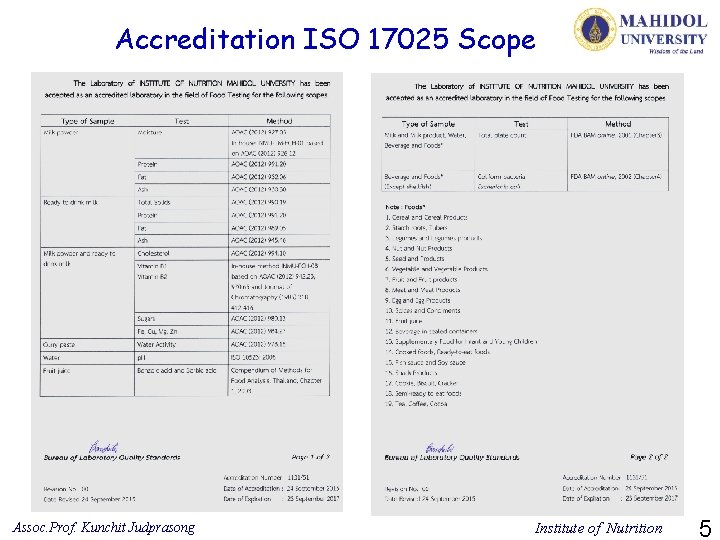
Accreditation No. 1131/51 Laboratory of Institute of Nutrition Mahidol University has been Accredited to International standard ISO/IEC 17025: 2005 from Bureau of Laboratory Quality Standards Department of Medical Sciences, Ministry of Public Health Assoc. Prof. Kunchit Judprasong Institute of Nutrition 4

Accreditation ISO 17025 Scope Assoc. Prof. Kunchit Judprasong Institute of Nutrition 5

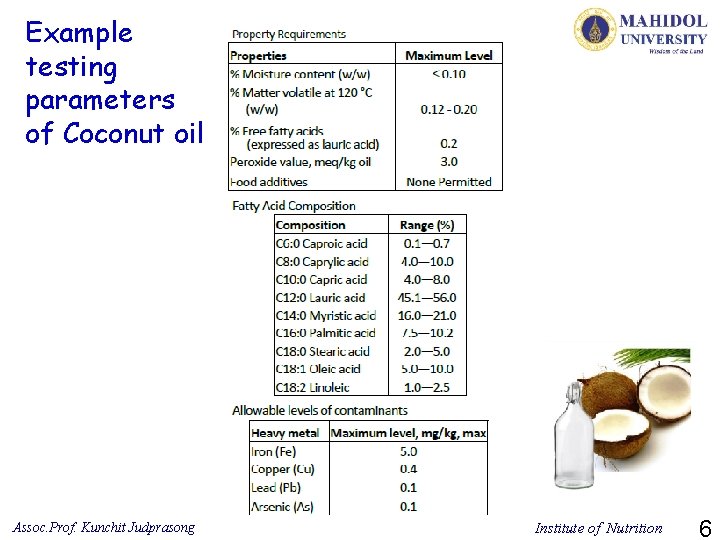
Example testing parameters of Coconut oil Assoc. Prof. Kunchit Judprasong Institute of Nutrition 6

Outline Ø Laboratory at Institute of Nutrition, Mahidol University (INMU) Ø Analysis of essential composition: 1) Fatty acids 2) Moisture and volatile matter and total solid 3) Insoluble impurities 4) Total fat 5) Protein 6) Ash 7) Minerals 8) Saponification value 9) Iodine value 10) Unsaponificable matter 11) Peroxide value 12) Acid value and acidity 13) Heavy metals Ø Conclusion Assoc. Prof. Kunchit Judprasong Institute of Nutrition 7

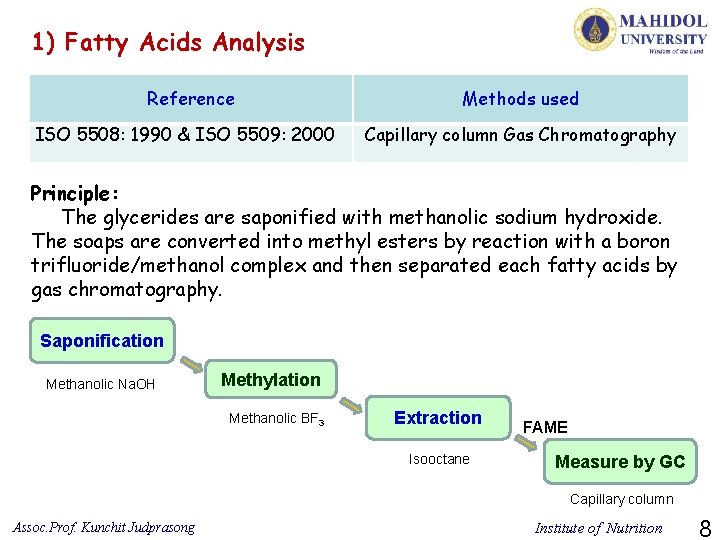
1) Fatty Acids Analysis Reference ISO 5508: 1990 & ISO 5509: 2000 Methods used Capillary column Gas Chromatography Principle: The glycerides are saponified with methanolic sodium hydroxide. The soaps are converted into methyl esters by reaction with a boron trifluoride/methanol complex and then separated each fatty acids by gas chromatography. Saponification Methanolic Na. OH Methylation Methanolic BF 3 Extraction Isooctane FAME Measure by GC Capillary column Assoc. Prof. Kunchit Judprasong Institute of Nutrition 8

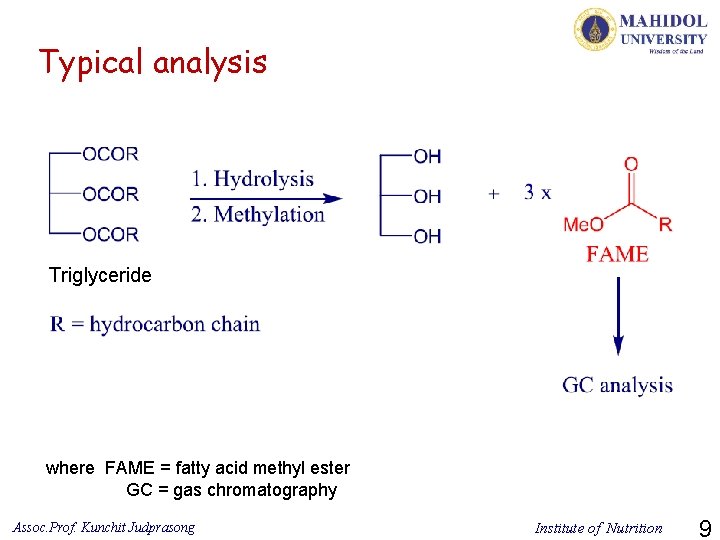
Typical analysis Triglyceride where FAME = fatty acid methyl ester GC = gas chromatography Assoc. Prof. Kunchit Judprasong Institute of Nutrition 9

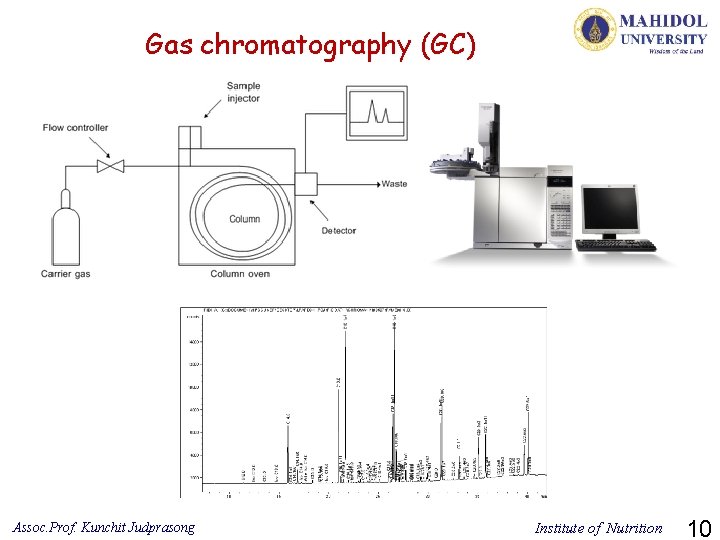
Gas chromatography (GC) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 10

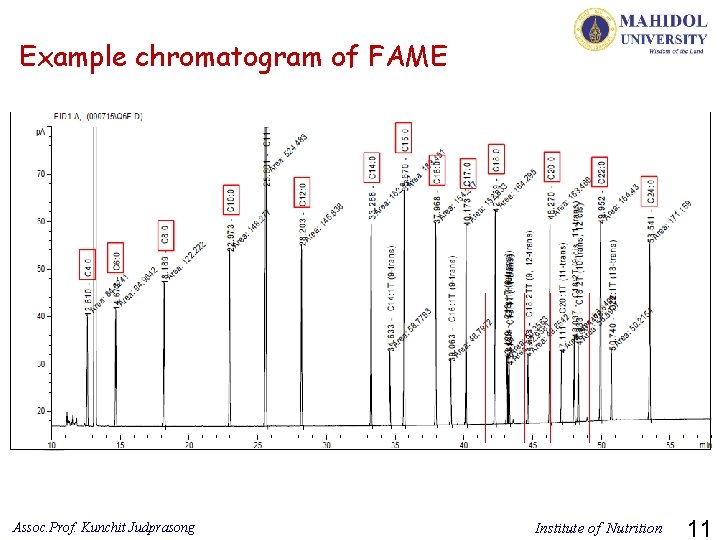
Example chromatogram of FAME Assoc. Prof. Kunchit Judprasong Institute of Nutrition 11

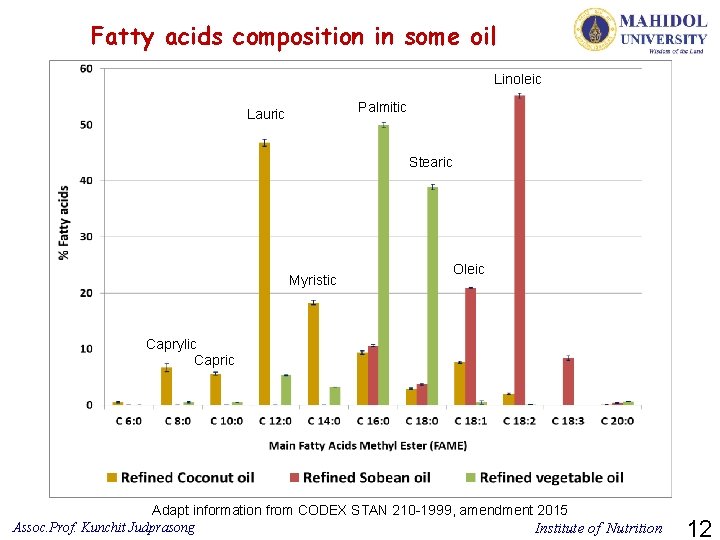
Fatty acids composition in some oil Linoleic Palmitic Lauric Stearic Myristic Oleic Caprylic Capric Adapt information from CODEX STAN 210 -1999, amendment 2015 Assoc. Prof. Kunchit Judprasong Institute of Nutrition 12

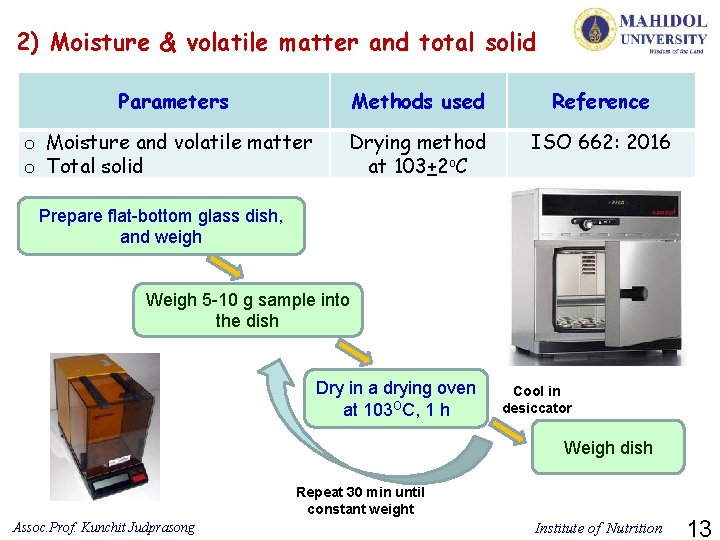
2) Moisture & volatile matter and total solid Parameters Methods used Reference o Moisture and volatile matter o Total solid Drying method at 103+2 o. C ISO 662: 2016 Prepare flat-bottom glass dish, and weigh Weigh 5 -10 g sample into the dish Dry in a drying oven at 103 OC, 1 h Cool in desiccator Weigh dish Repeat 30 min until constant weight Assoc. Prof. Kunchit Judprasong Institute of Nutrition 13

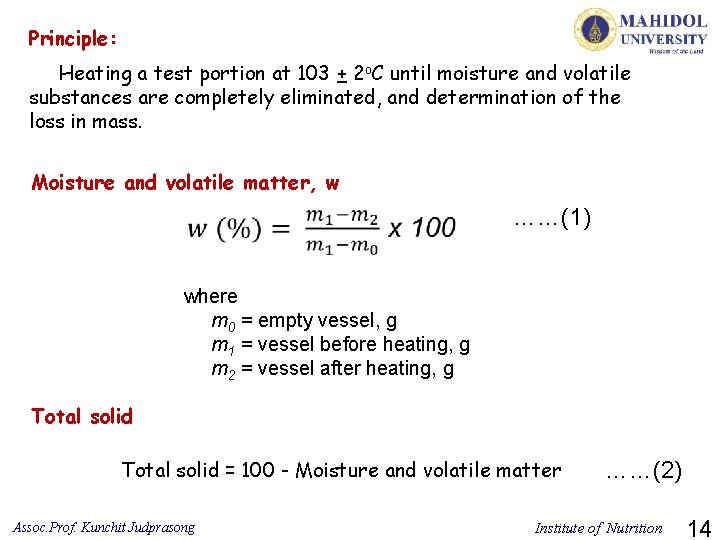
Principle: Heating a test portion at 103 + 2 o. C until moisture and volatile substances are completely eliminated, and determination of the loss in mass. Moisture and volatile matter, w ……(1) where m 0 = empty vessel, g m 1 = vessel before heating, g m 2 = vessel after heating, g Total solid = 100 - Moisture and volatile matter Assoc. Prof. Kunchit Judprasong ……(2) Institute of Nutrition 14

3) Insoluble impurities Parameters Methods used Reference Insoluble impurities Gravimetric method after solvent extraction ISO 663: 2007 Principle: A test portion is treated with an excess of n-hexane or light petroleum, then the solution obtained is filtered. The filter and residue are washed with the same solvent, then dried at 103 o. C and weighed. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 15

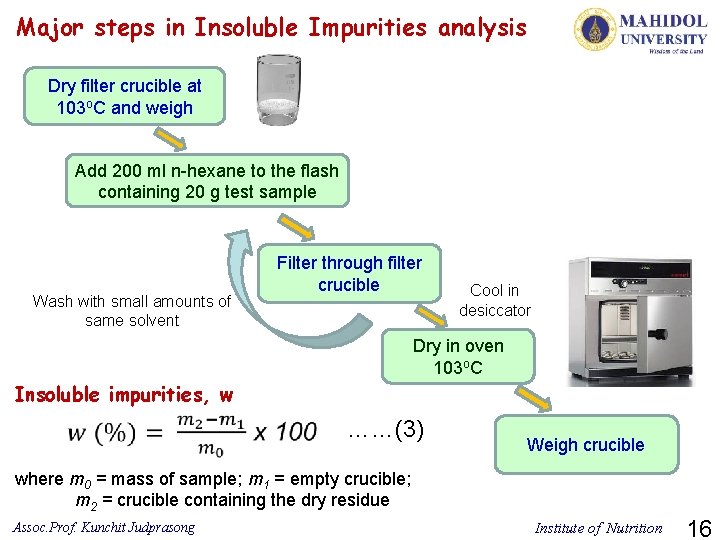
Major steps in Insoluble Impurities analysis Dry filter crucible at 103 o. C and weigh Add 200 ml n-hexane to the flash containing 20 g test sample Wash with small amounts of same solvent Filter through filter crucible Cool in desiccator Dry in oven 103 o. C Insoluble impurities, w ……(3) Weigh crucible where m 0 = mass of sample; m 1 = empty crucible; m 2 = crucible containing the dry residue Assoc. Prof. Kunchit Judprasong Institute of Nutrition 16

4) Total fat Nutrient Methods used Crude fat o Fat in Dried Milk (Alkaline hydrolysis, ether/petroleum ether) o Fat in Flour (Acid hydrolysis, ether/petroleum ether) o Nut and nut products (no hydrolysis, ether) References AOAC 2016, Method 932. 06 Method 922. 06 Method 948. 22 Principle: Total lipid extract is obtained by digesting test portion with alkaline or acid. Hydrolyzed fat components are extracted into ethyl and petroleum ethers, evaporated and then weighed. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 17

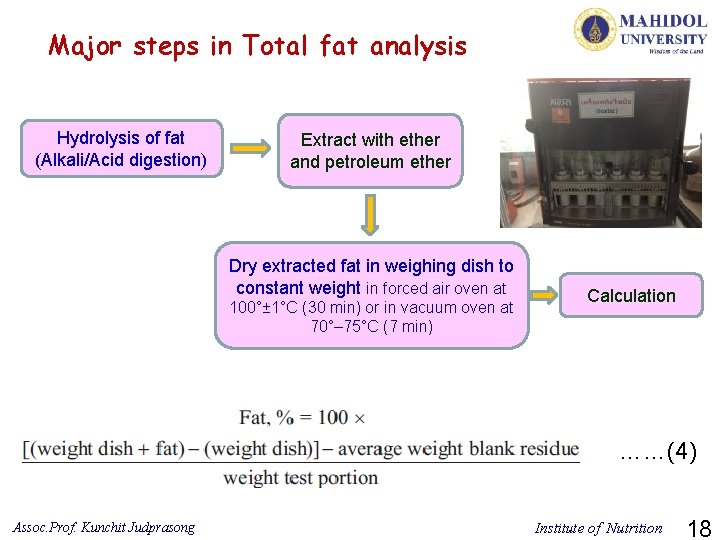
Major steps in Total fat analysis Hydrolysis of fat (Alkali/Acid digestion) Extract with ether and petroleum ether Dry extracted fat in weighing dish to constant weight in forced air oven at 100°± 1°C (30 min) or in vacuum oven at 70°– 75°C (7 min) Calculation ……(4) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 18

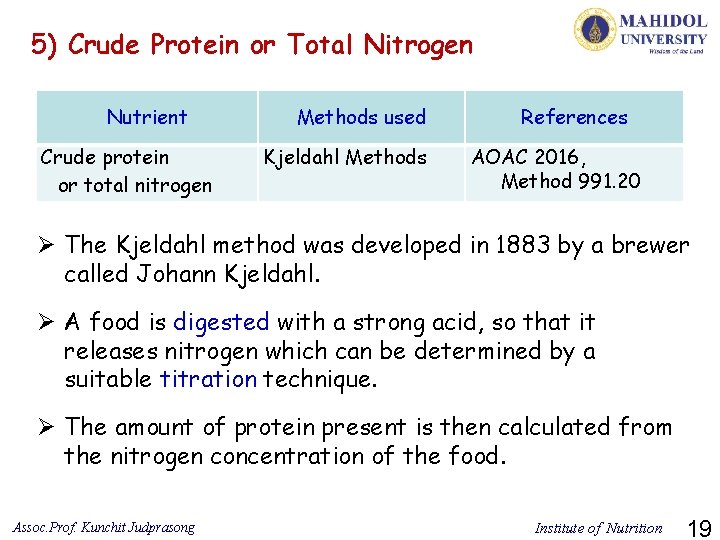
5) Crude Protein or Total Nitrogen Nutrient Crude protein or total nitrogen Methods used Kjeldahl Methods References AOAC 2016, Method 991. 20 Ø The Kjeldahl method was developed in 1883 by a brewer called Johann Kjeldahl. Ø A food is digested with a strong acid, so that it releases nitrogen which can be determined by a suitable titration technique. Ø The amount of protein present is then calculated from the nitrogen concentration of the food. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 19

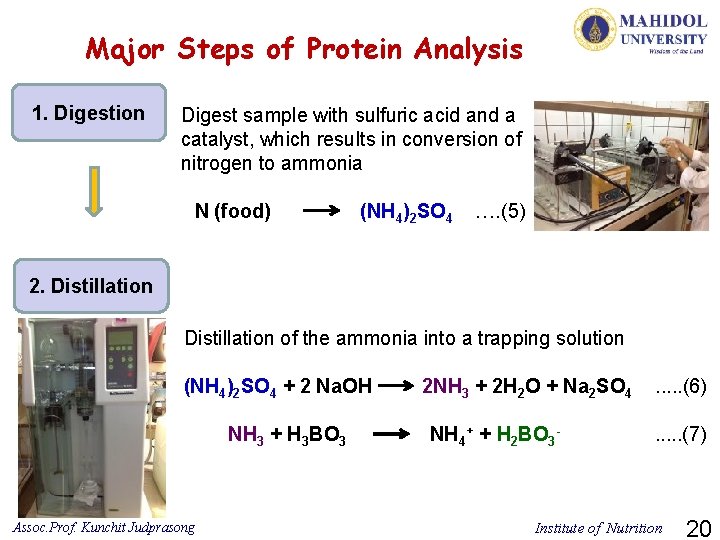
Major Steps of Protein Analysis 1. Digestion Digest sample with sulfuric acid and a catalyst, which results in conversion of nitrogen to ammonia N (food) (NH 4)2 SO 4 …. (5) 2. Distillation of the ammonia into a trapping solution (NH 4)2 SO 4 + 2 Na. OH NH 3 + H 3 BO 3 Assoc. Prof. Kunchit Judprasong 2 NH 3 + 2 H 2 O + Na 2 SO 4 NH 4+ + H 2 BO 3 - . . . (6). . . (7) Institute of Nutrition 20

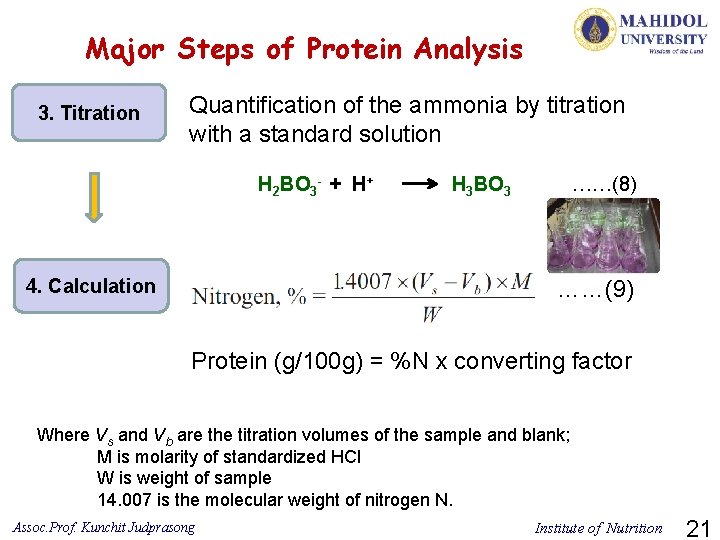
Major Steps of Protein Analysis 3. Titration Quantification of the ammonia by titration with a standard solution H 2 BO 3 - + H+ H 3 BO 3 ……(8) ……(9) 4. Calculation Protein (g/100 g) = %N x converting factor Where Vs and Vb are the titration volumes of the sample and blank; M is molarity of standardized HCl W is weight of sample 14. 007 is the molecular weight of nitrogen N. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 21

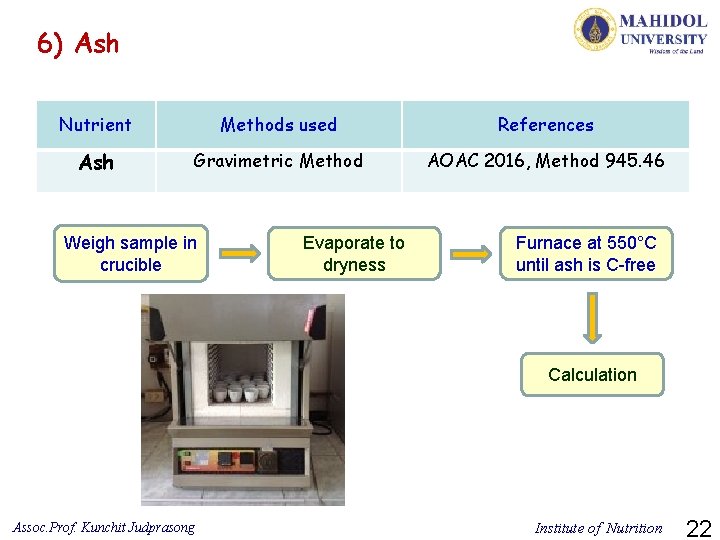
6) Ash Nutrient Methods used References Ash Gravimetric Method AOAC 2016, Method 945. 46 Weigh sample in crucible Evaporate to dryness Furnace at 550°C until ash is C-free Calculation Assoc. Prof. Kunchit Judprasong Institute of Nutrition 22

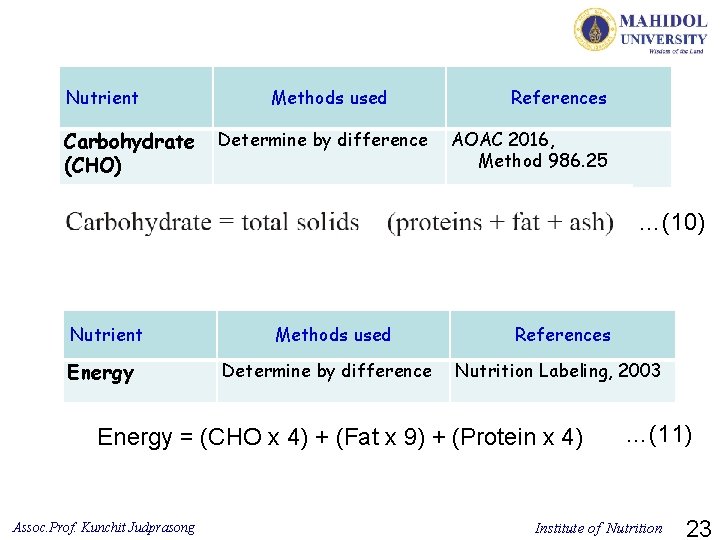
Nutrient Carbohydrate (CHO) Methods used Determine by difference References AOAC 2016, Method 986. 25 …(10) Nutrient Energy Methods used Determine by difference References Nutrition Labeling, 2003 Energy = (CHO x 4) + (Fat x 9) + (Protein x 4) Assoc. Prof. Kunchit Judprasong …(11) Institute of Nutrition 23

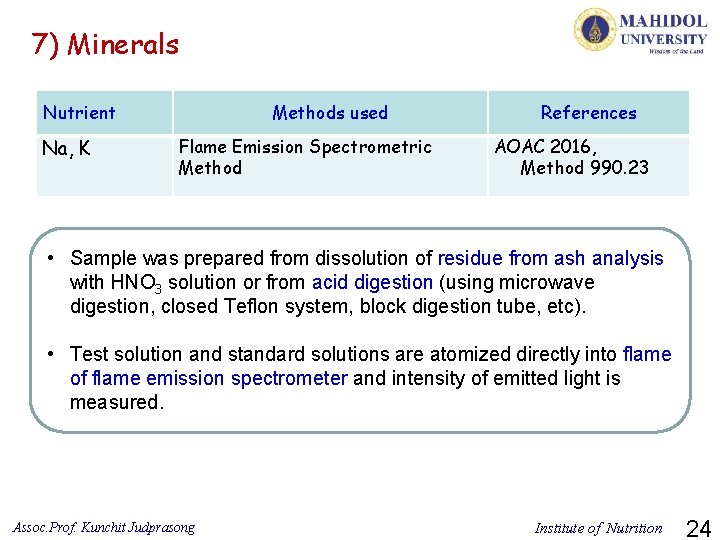
7) Minerals Nutrient Na, K Methods used Flame Emission Spectrometric Method References AOAC 2016, Method 990. 23 • Sample was prepared from dissolution of residue from ash analysis with HNO 3 solution or from acid digestion (using microwave digestion, closed Teflon system, block digestion tube, etc). • Test solution and standard solutions are atomized directly into flame of flame emission spectrometer and intensity of emitted light is measured. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 24

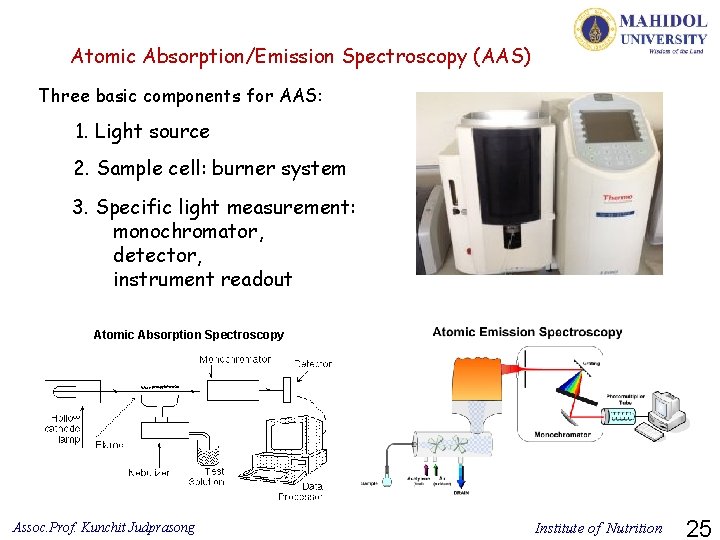
Atomic Absorption/Emission Spectroscopy (AAS) Three basic components for AAS: 1. Light source 2. Sample cell: burner system 3. Specific light measurement: monochromator, detector, instrument readout Atomic Absorption Spectroscopy Assoc. Prof. Kunchit Judprasong Institute of Nutrition 25

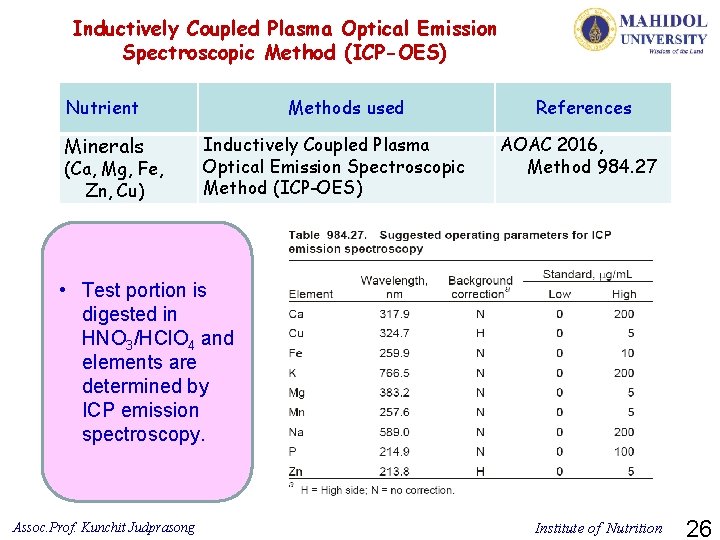
Inductively Coupled Plasma Optical Emission Spectroscopic Method (ICP-OES) Nutrient Minerals (Ca, Mg, Fe, Zn, Cu) Methods used Inductively Coupled Plasma Optical Emission Spectroscopic Method (ICP-OES) References AOAC 2016, Method 984. 27 • Test portion is digested in HNO 3/HCl. O 4 and elements are determined by ICP emission spectroscopy. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 26

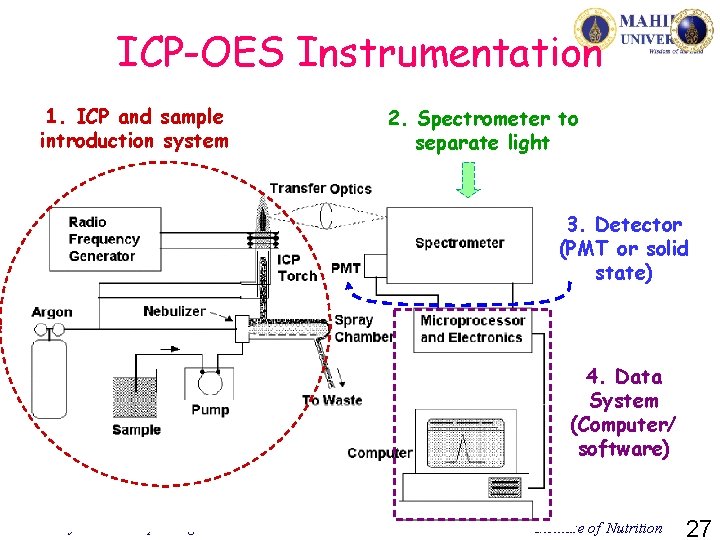
ICP-OES Instrumentation 1. ICP and sample introduction system 2. Spectrometer to separate light 3. Detector (PMT or solid state) 4. Data System (Computer/ software) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 27

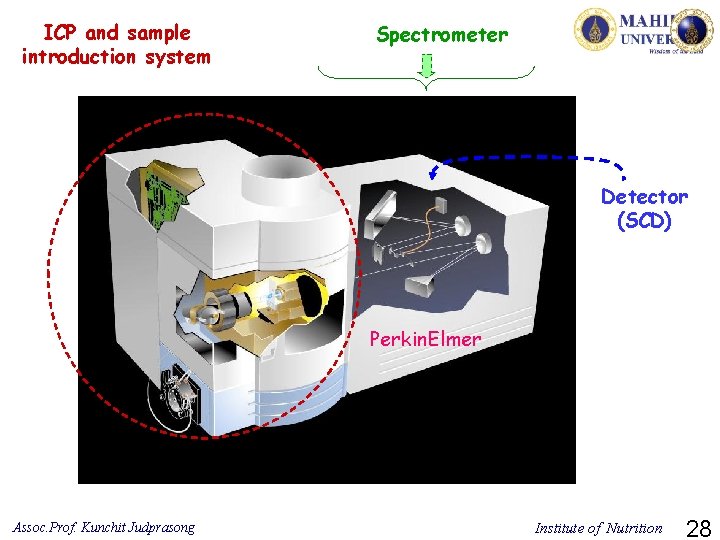
ICP and sample introduction system Spectrometer Detector (SCD) Perkin. Elmer Assoc. Prof. Kunchit Judprasong Institute of Nutrition 28

8) Saponification value Parameter Method used Saponification value Titration References ISO 3657: 2013(E) Saponification value = number pf milligrams of potassium hydroxide (KOH) required for the saponification of 1 g of the product tested. Principle of analysis: The test sample is saponified by boiling under reflux with an excess of ethanolic KOH, followed by titration of the excess KOH with standard volumetric hydrochloric acid solution. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 29

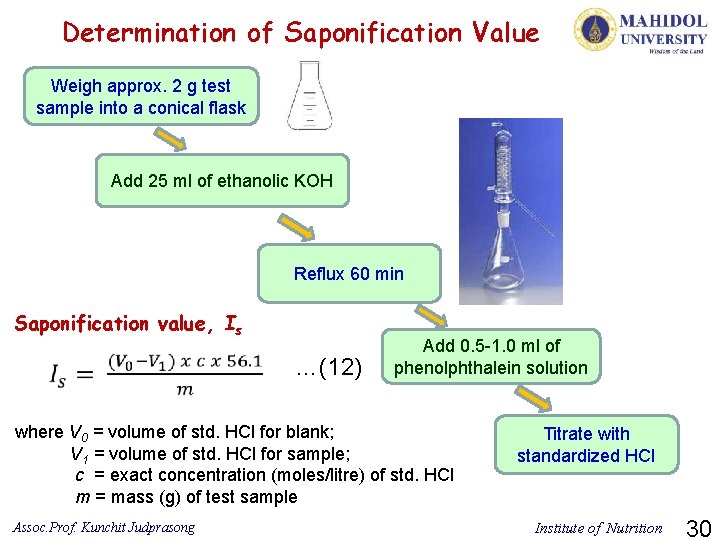
Determination of Saponification Value Weigh approx. 2 g test sample into a conical flask Add 25 ml of ethanolic KOH Reflux 60 min Saponification value, Is …(12) Add 0. 5 -1. 0 ml of phenolphthalein solution where V 0 = volume of std. HCl for blank; V 1 = volume of std. HCl for sample; c = exact concentration (moles/litre) of std. HCl m = mass (g) of test sample Assoc. Prof. Kunchit Judprasong Titrate with standardized HCl Institute of Nutrition 30

9) Iodine value Parameter Method used Iodine value Titration References ISO 3961: 2009(E) Ø Iodine numbers are often used to determine the amount of unsaturation in fatty acids. Ø This unsaturation is in the form of double bonds, which react with iodine compounds. Ø The higher the iodine number, the more C=C bonds are presented in the fat. Principle of analysis: Dissolution of a test portion in solvent and addition of Wijs reagent (iodine monochloride in acetic acid). After a specified time, addition of potassium iodide and water, and titration of the liberated iodine with sodium thiosulfate solution. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 31

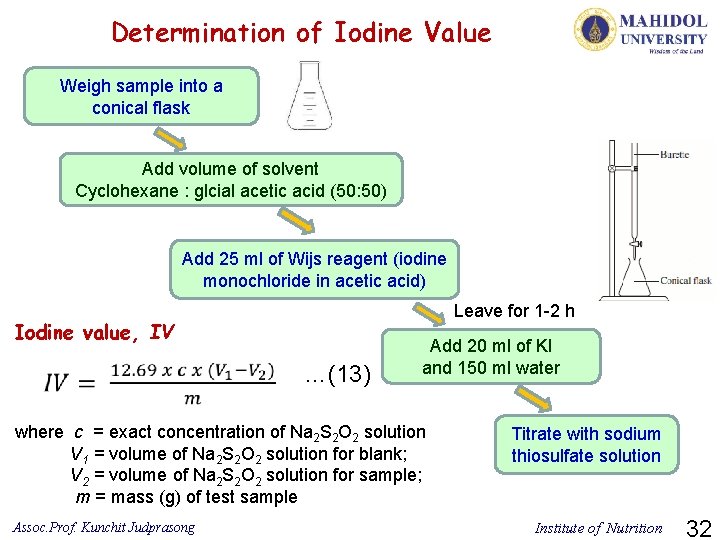
Determination of Iodine Value Weigh sample into a conical flask Add volume of solvent Cyclohexane : glcial acetic acid (50: 50) Add 25 ml of Wijs reagent (iodine monochloride in acetic acid) Leave for 1 -2 h Iodine value, IV …(13) Add 20 ml of KI and 150 ml water where c = exact concentration of Na 2 S 2 O 2 solution V 1 = volume of Na 2 S 2 O 2 solution for blank; V 2 = volume of Na 2 S 2 O 2 solution for sample; m = mass (g) of test sample Assoc. Prof. Kunchit Judprasong Titrate with sodium thiosulfate solution Institute of Nutrition 32

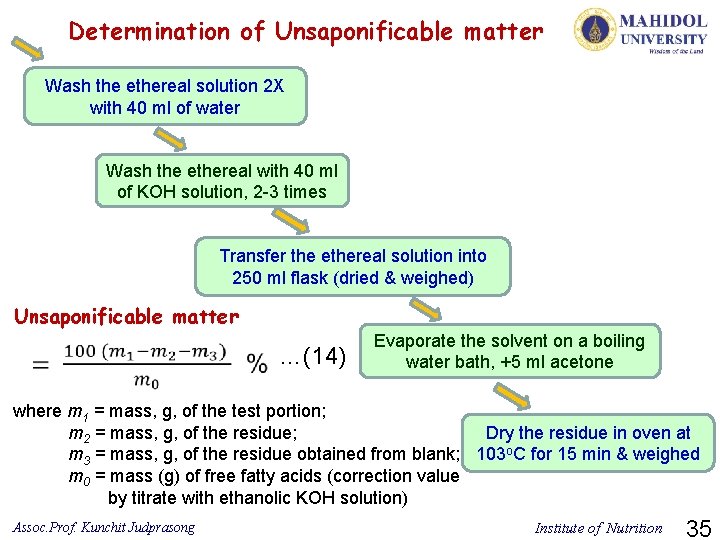
10) Unsaponificable matter Parameter Method used Unsaponificable matter Diethyl ether extraction References ISO 3596: 2000(E) Ø All the substances present in the product which, after saponification of the latter by potassium hydroxide and extraction by a specified solvent, are not volatile under the specified operating conditions. Ø Unsaponifiables are components of an oily (oil, fat, wax) mixture that fail to form soaps when blended with sodium hydroxide or potassium hydroxide. Principle of analysis: The fat or oil is saponified by boiling under reflux with an ethanolic potassium hydroxide solution. The unsaponificable matter is extracted from the soap solution by diethyl ether. The solvent is evaporated and the residue is weighed after drying. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 33

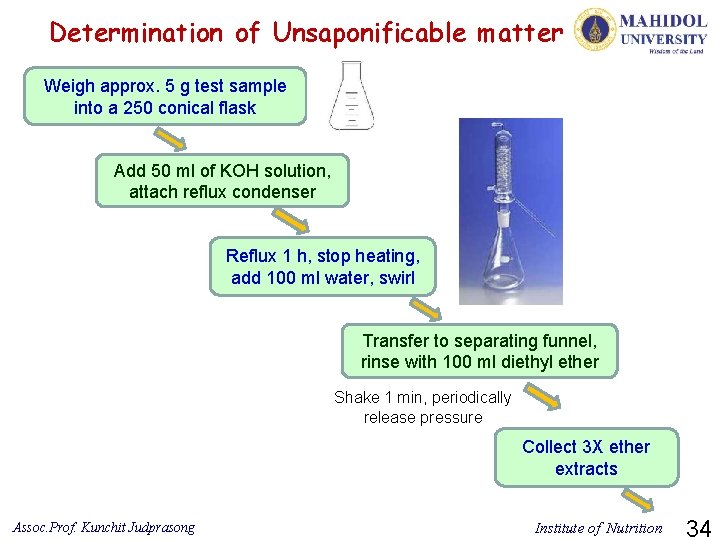
Determination of Unsaponificable matter Weigh approx. 5 g test sample into a 250 conical flask Add 50 ml of KOH solution, attach reflux condenser Reflux 1 h, stop heating, add 100 ml water, swirl Transfer to separating funnel, rinse with 100 ml diethyl ether Shake 1 min, periodically release pressure Collect 3 X ether extracts Assoc. Prof. Kunchit Judprasong Institute of Nutrition 34

Determination of Unsaponificable matter Wash the ethereal solution 2 X with 40 ml of water Wash the ethereal with 40 ml of KOH solution, 2 -3 times Transfer the ethereal solution into 250 ml flask (dried & weighed) Unsaponificable matter …(14) Evaporate the solvent on a boiling water bath, +5 ml acetone where m 1 = mass, g, of the test portion; Dry the residue in oven at m 2 = mass, g, of the residue; m 3 = mass, g, of the residue obtained from blank; 103 o. C for 15 min & weighed m 0 = mass (g) of free fatty acids (correction value by titrate with ethanolic KOH solution) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 35

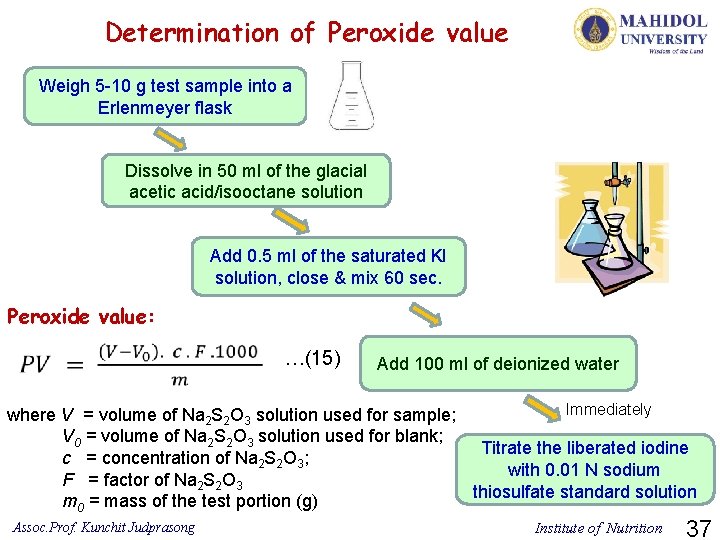
11) Peroxide value Parameter Peroxide value Method used References Iodometric (visual) endpoint ISO 3960: 2007(E) Ø PV is a quantity of those substances in the sample, expressed in terms of active oxygen, that oxidize potassium iodide under the conditions specified. Ø Detection of peroxide gives the initial evidence of rancidity in unsaturated fats and oils. Principle of analysis: The test sample is dissolved in isooctane and glacial acetic acid, and potassium iodide is added. The iodine liberated by the peroxides is determined iodometrically (visually) with a starch indicator and a sodium thiosulfate standard solution. The endpoint of the titration is determined iodometrically. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 36

Determination of Peroxide value Weigh 5 -10 g test sample into a Erlenmeyer flask Dissolve in 50 ml of the glacial acetic acid/isooctane solution Add 0. 5 ml of the saturated KI solution, close & mix 60 sec. Peroxide value: …(15) Add 100 ml of deionized water Immediately where V = volume of Na 2 S 2 O 3 solution used for sample; V 0 = volume of Na 2 S 2 O 3 solution used for blank; Titrate the liberated iodine c = concentration of Na 2 S 2 O 3; with 0. 01 N sodium F = factor of Na 2 S 2 O 3 thiosulfate standard solution m 0 = mass of the test portion (g) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 37

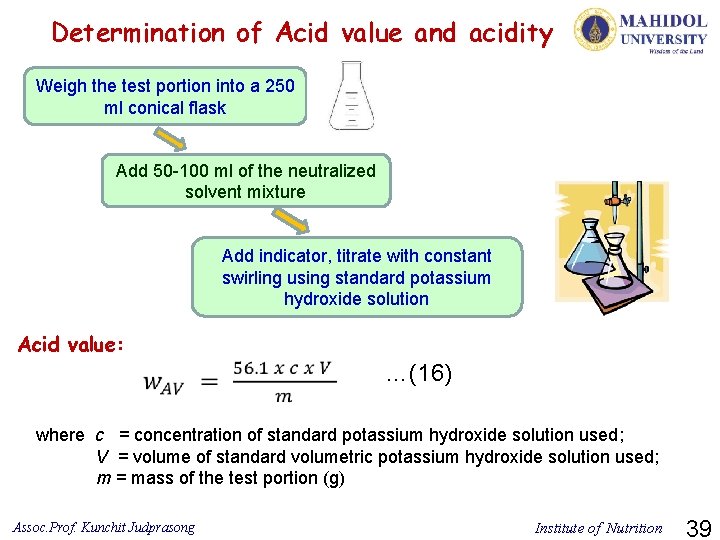
12) Acid value and acidity Parameter Method used Acid value and acidity Titration References ISO 660: 2009(E) Ø Acid value is a number of milligrams of KOH required to neutralize the free fatty acids present in 1 g of fat, when determined in accordance with the procedure specified. Ø Acidity is content of free fatty acids determined in accordance with the procedure specified. Principle of analysis: The sample is dissolved in a suitable solvent mixture, and the acids present are titrated with an ethanolic or methanolic solution of potassium or sodium hydroxide. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 38

Determination of Acid value and acidity Weigh the test portion into a 250 ml conical flask Add 50 -100 ml of the neutralized solvent mixture Add indicator, titrate with constant swirling using standard potassium hydroxide solution Acid value: …(16) where c = concentration of standard potassium hydroxide solution used; V = volume of standard volumetric potassium hydroxide solution used; m = mass of the test portion (g) Assoc. Prof. Kunchit Judprasong Institute of Nutrition 39

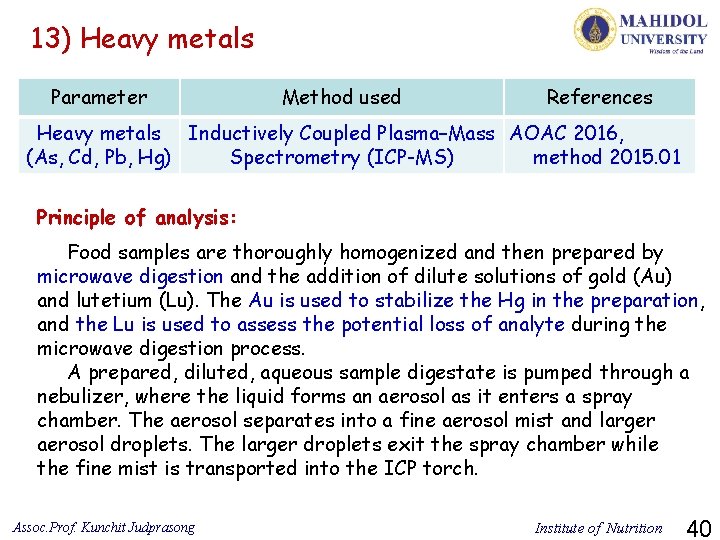
13) Heavy metals Parameter Method used References Heavy metals Inductively Coupled Plasma–Mass AOAC 2016, (As, Cd, Pb, Hg) Spectrometry (ICP-MS) method 2015. 01 Principle of analysis: Food samples are thoroughly homogenized and then prepared by microwave digestion and the addition of dilute solutions of gold (Au) and lutetium (Lu). The Au is used to stabilize the Hg in the preparation, and the Lu is used to assess the potential loss of analyte during the microwave digestion process. A prepared, diluted, aqueous sample digestate is pumped through a nebulizer, where the liquid forms an aerosol as it enters a spray chamber. The aerosol separates into a fine aerosol mist and larger aerosol droplets. The larger droplets exit the spray chamber while the fine mist is transported into the ICP torch. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 40

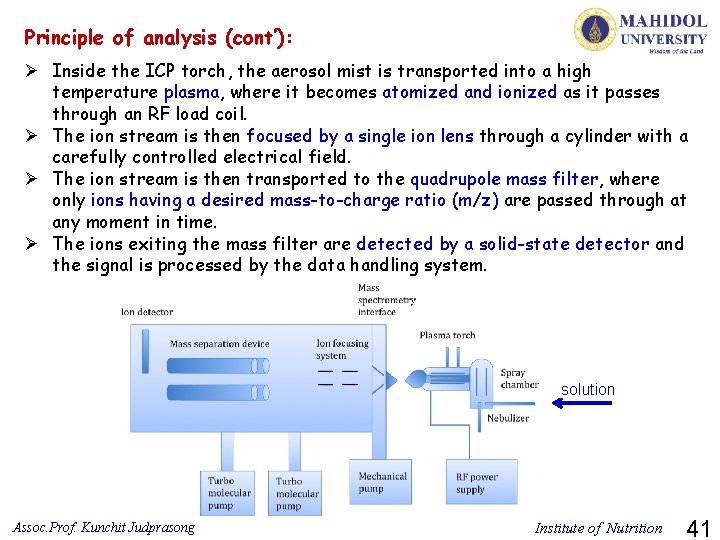
Principle of analysis (cont’): Ø Inside the ICP torch, the aerosol mist is transported into a high temperature plasma, where it becomes atomized and ionized as it passes through an RF load coil. Ø The ion stream is then focused by a single ion lens through a cylinder with a carefully controlled electrical field. Ø The ion stream is then transported to the quadrupole mass filter, where only ions having a desired mass-to-charge ratio (m/z) are passed through at any moment in time. Ø The ions exiting the mass filter are detected by a solid-state detector and the signal is processed by the data handling system. sa solution Assoc. Prof. Kunchit Judprasong Institute of Nutrition 41

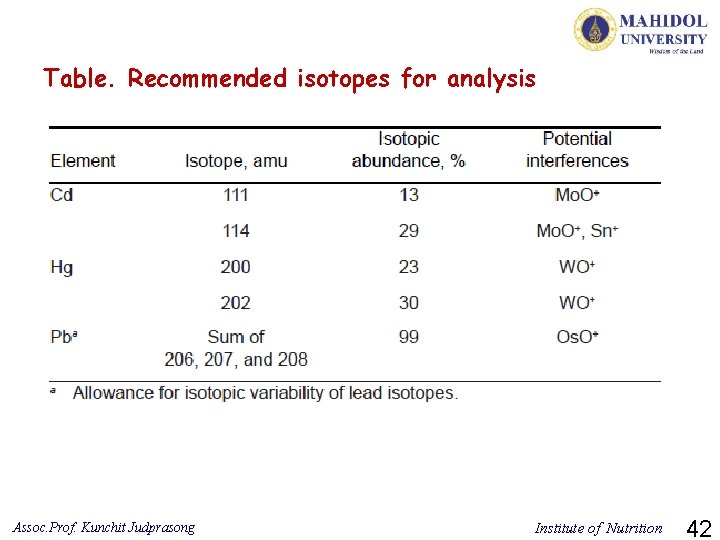
Table. Recommended isotopes for analysis Assoc. Prof. Kunchit Judprasong Institute of Nutrition 42

LABORATORY WITH CONSISTENTLY RELIABLE DATA Ø Analytical method: § Standard method e. g. ISO, AOAC, …etc. § Fully validated and documented Ø Laboratory staff: competent, well-trained Ø Environmental conditions: monitor, control, record Ø Equipment: calibrate, comply with specifications Ø Quality control system: § Internal quality assessment system : - Replicate tests - in-house QC sample /control chart - using CRM/RM for checking accuracy § External quality assessment system: -Proficiency testing / interlaboratory comparison Ø Report: accurately, clearly, and objectively Assoc. Prof. Kunchit Judprasong Institute of Nutrition 43

Conclusion ü Suitable testing method, international standard, is a key factors in the reliable final results. ü Analytical testing laboratory should be accredited with the international standard ISO/IEC 17025. ü Coconut oil and downstream products could be analyzed for essential composition and quality factors using standards and well-validated method to ensure the products meet stringent quality standards. Assoc. Prof. Kunchit Judprasong Institute of Nutrition 44