An Update on Sexually Transmitted Infections STIs C







































- Slides: 75

An Update on Sexually Transmitted Infections (STIs) C. Brock Woodis, Pharm. D, BCACP, BCPS, CDE, BC-ADM, CPP Associate Professor Campbell University College of Pharmacy & Health Sciences Duke Family Medicine September 27, 2016 brock. woodis@duke. edu

Disclosure Statement �I do not have any financial relevance related to this continuing education activity.

Objectives �Identify most likely causative pathogens for a variety of presented sexually transmitted infections (STIs) �Recommend appropriate drug therapy for a variety of STIs based on specific laboratory and patient-specific data �Formulate complete patient-specific treatment plans including monitoring for safety and efficacy for a variety of presented STIs

Clinical practice guidelines �Workowski KA and Bolan KA. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2015; 64(No. RR-3): [1137]. http: //www. cdc. gov/std/tg 2015/ Pocket guide Wall poster �Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections. World heath organization website. Updated Aug 30, 2016. Accessed Sept 22, 2016. http: //www. who. int/reproductivehealth/topics/rtis/st

Introduction �Each day > 1 million sexually transmitted infections (STIs) are acquired worldwide �Estimated 357 million new infections with 1 of 4 STIs occur each year: chlamydia, gonorrhea, syphilis and trichomoniasis �> 500 million people are estimated to have genital infection with herpes simplex virus (HSV) �> 290 million women have a human papillomavirus (HPV) infection �Majority of STIs have no symptoms or only mild symptoms that may not be recognized as an STI Sexually transmitted infections fact sheet. World Health Organization website. Updated August 2016. Accessed

Introduction �STIs such as HSV type 2 and syphilis can increase the risk of HIV acquisition �> 900 000 pregnant women were infected with syphilis resulting in approximately 350 000 adverse birth outcomes including stillbirth in 2012 �STIs can have serious reproductive health consequences beyond the immediate impact of the infection itself (e. g. , infertility or mother -to-child transmission) �Drug resistance (especially for gonorrhea) is a major threat to reducing the impact of STIs worldwide Sexually transmitted infections fact sheet. World Health Organization website. Updated August 2016. Accessed

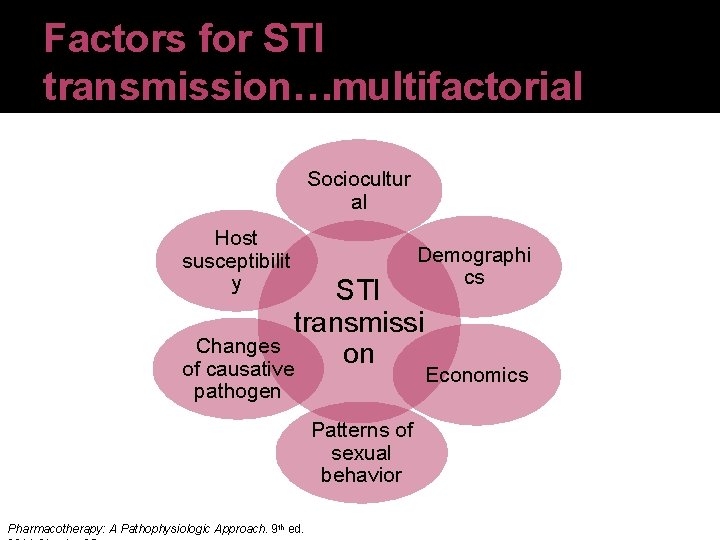
Background �STI transmission Variety of clinical syndromes and infections caused by pathogens which are acquired and transmitted through sexual activity Few STIs have been eradicated �STIs have reemerged secondary to social trends of sexual activity ↑ numbers of adolescents engaging in unsafe sexual practices ↑ incidence of men who have sex with men (MSM) and women who have sex with women (WSW) Pharmacotherapy: A Pathophysiologic Approach. 9 th ed.

Background �Optimal detection and treatment of STIs depends on knowledgeable and competent clinicians �Higher reported incidence of most major STIs in men, but complications of STIs are generally more frequent and severe in women Serious effects on maternal and child health during pregnancy Possible damage to reproductive organs, Pharmacotherapy: A Pathophysiologic Approach. 9 ed. th

The Five Ps �Partners �Practices �Prevention of pregnancy �Protection from STIs �Past history of STIs IMPORTANCE OF STI PREVENTION COUNSELING MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Factors for STI transmission…multifactorial Sociocultur al Host susceptibilit y Demographi cs STI transmissi Changes on of causative pathogen Patterns of sexual behavior Pharmacotherapy: A Pathophysiologic Approach. 9 th ed. Economics

Four desired outcomes of STI therapy �Treatment of infection based on microbiologic eradication �Alleviation of signs and symptoms �Prevention of sequelae �Prevention of transmission, including advantages such as cost-effectiveness and other advantages (e. g. single-dose formulations) MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

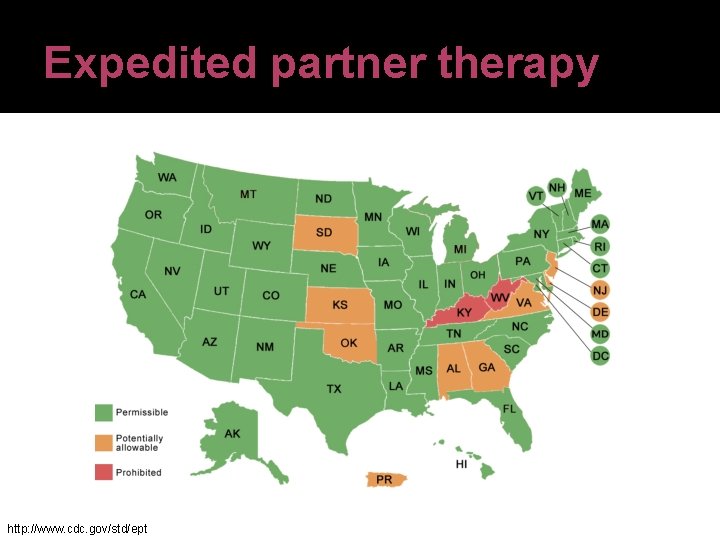
Expedited partner therapy http: //www. cdc. gov/std/ept

Behavioral considerations �Do not assume that patients (especially adolescents) consistently know how to use barrier methods of contraception �↑ of adolescents engaging in unsafe sexual practices, as well as men who have sex with men (MSM) and women who have sex with women (WSW) �Optimal to treat both partners for an STI simultaneously Pharmacotherapy: A Pathophysiologic Approach. 9 th ed.

USPSTF STI Screening recommendations �All sexually active females aged 25 and younger, and older women at risk for STIs should be screened for chlamydia and gonorrhea �At-risk women include those with new sexual partners, multiple partners, and inconsistent condom use �USPSTF says not enough evidence to weigh the benefits and harms of such screening in men �Recommends intensive behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs

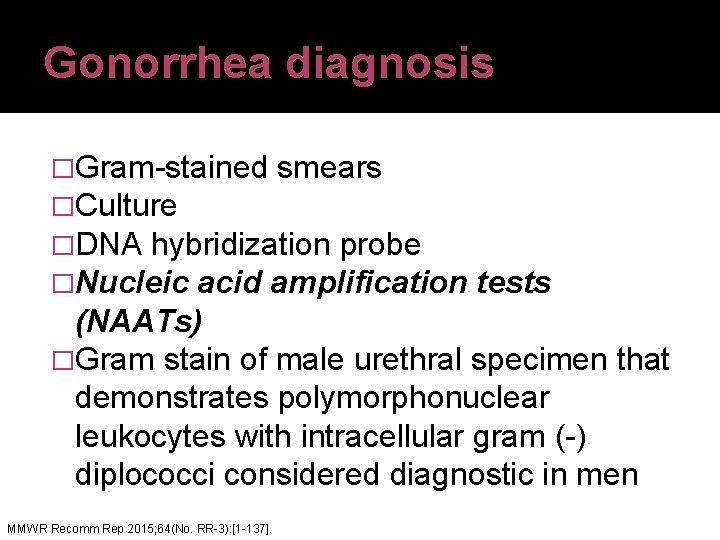
Gonorrhea diagnosis �Gram-stained smears �Culture �DNA hybridization probe �Nucleic acid amplification tests (NAATs) �Gram stain of male urethral specimen that demonstrates polymorphonuclear leukocytes with intracellular gram (-) diplococci considered diagnostic in men. MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

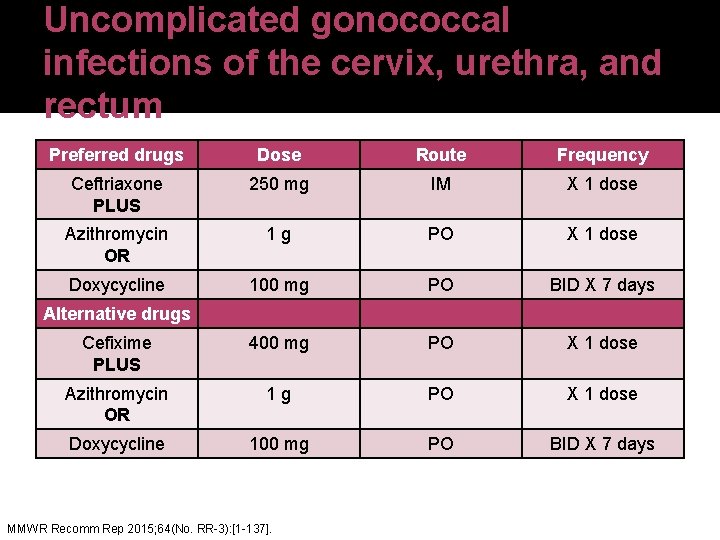
Uncomplicated gonococcal infections of the cervix, urethra, and rectum Preferred drugs Dose Route Frequency Ceftriaxone PLUS 250 mg IM X 1 dose Azithromycin OR 1 g PO X 1 dose Doxycycline 100 mg PO BID X 7 days Cefixime PLUS 400 mg PO X 1 dose Azithromycin OR 1 g PO X 1 dose Doxycycline 100 mg PO BID X 7 days Alternative drugs MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

MMWR update (August 10, 2012) � CDC recommends combination therapy with ceftriaxone 250 mg IM X 1 dose + azithromycin 1 g PO X 1 dose OR doxycycline 100 mg PO BID (in place of azithromycin) X 7 days as the most reliably effective treatment for uncomplicated gonorrhea � CDC no longer recommends cefixime at any dose as a 1 st-line regimen for treatment of gonococcal infections � Cefixime Alternative agent Centers for disease control and prevention. 25 Sept 2012

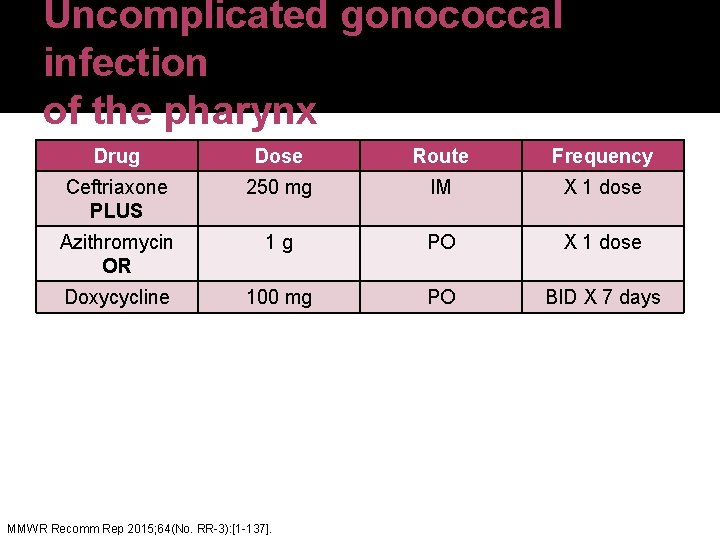
Uncomplicated gonococcal infection of the pharynx Drug Dose Route Frequency Ceftriaxone PLUS 250 mg IM X 1 dose Azithromycin OR 1 g PO X 1 dose Doxycycline 100 mg PO BID X 7 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Gonococcal treatment recommendations �Follow-up No “test-of-cure” unless cefixime used Retest 3 months after treatment to assess REINFECTION �Cephalosporins are safe in pregnancy �Management of sex partners MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Additional gonococcal infections � Gonococcal conjunctivitis-ceftriaxone 1 g IM X 1 dose PLUS azithromycin 1 g PO X 1 dose � Disemminated gonococcal infection (DGI) Results in petechial or pustular acral lesions, symmetrical arthralgia, tenosynsovitis, or septic arthritis Hospitalization initially Rule out endocarditis and meningitis Recommended-ceftriaxone 1 g IM or IV Q 24 H PLUS azithromycin 1 g PO X 1 dose then switch to cefixime 400 mg PO BID X 7 days Alternatives (in combination with azithromycin 1 g PO X 1 dose) MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

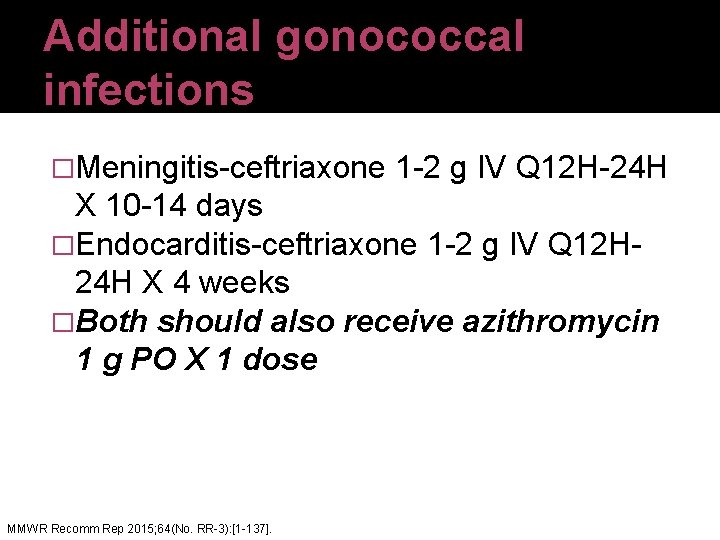
Additional gonococcal infections �Meningitis-ceftriaxone 1 -2 g IV Q 12 H-24 H X 10 -14 days �Endocarditis-ceftriaxone 1 -2 g IV Q 12 H 24 H X 4 weeks �Both should also receive azithromycin 1 g PO X 1 dose MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

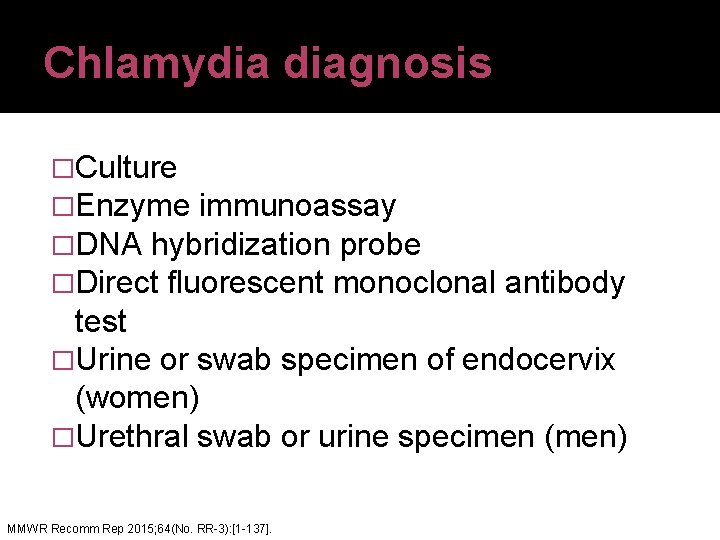
Chlamydia diagnosis �Culture �Enzyme immunoassay �DNA hybridization probe �Direct fluorescent monoclonal antibody test �Urine or swab specimen of endocervix (women) �Urethral swab or urine specimen (men) MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Gonorrhea resistance � Resistance to azithromycin is emerging according to CDC findings � CDC currently recommends a combination gonorrhea treatment with two antibiotics – an oral dose of azithromycin and single shot of ceftriaxone � Combination therapy currently recommended by CDC is still effective No treatment failures have been reported in the United States Signs of emerging resistance to azithromycin suggests that this drug will be next in the long line of antibiotics to which gonorrhea bacteria have become resistant – Antibiotic resistance threatens gonorrhea treatment. Centers for disease control and prevention website. Updated July 14, 2016.

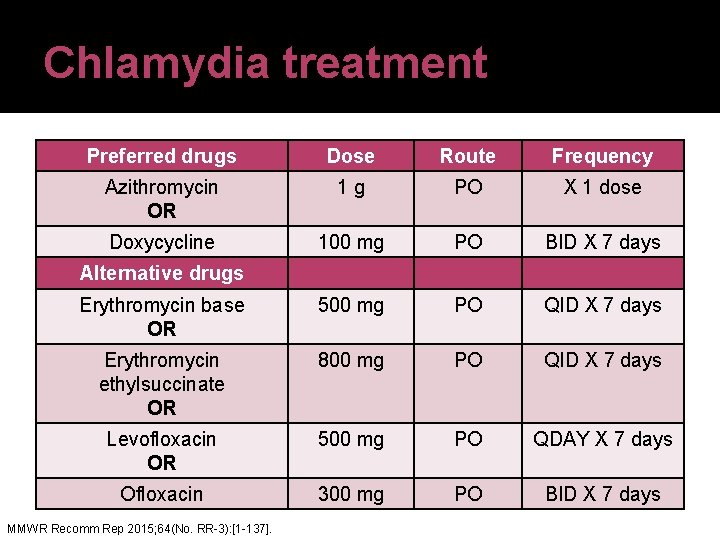
Chlamydia treatment Preferred drugs Dose Route Frequency Azithromycin OR 1 g PO X 1 dose Doxycycline 100 mg PO BID X 7 days Erythromycin base OR 500 mg PO QID X 7 days Erythromycin ethylsuccinate OR 800 mg PO QID X 7 days Levofloxacin OR 500 mg PO QDAY X 7 days Ofloxacin 300 mg PO BID X 7 days Alternative drugs MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Chlamydia treatment recommendations �Abstinence from intercourse for 7 days from when therapy was initiated �Test-of-cure is not recommended �Unlike test-of-cure, repeat C. trachomatis testing of recently infected men and women should be conducted 3 months after treatment �Azithromycin recommended for pregnancy and chlamydial infection MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

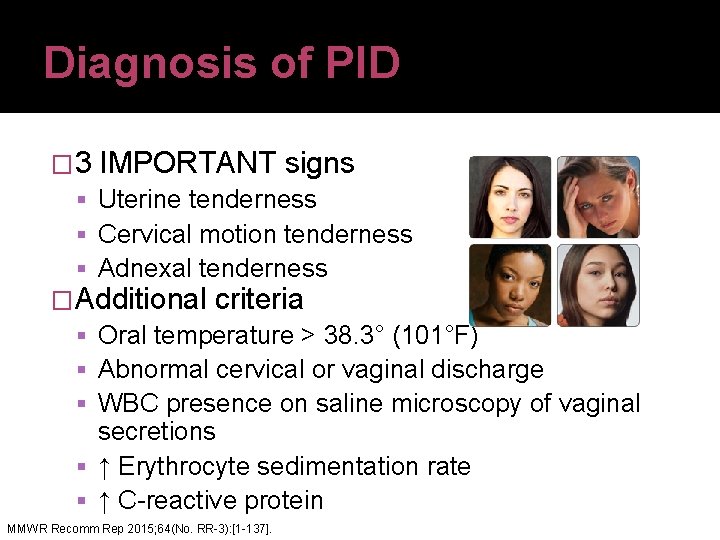
Diagnosis of PID � 3 IMPORTANT signs Uterine tenderness Cervical motion tenderness Adnexal tenderness �Additional criteria Oral temperature > 38. 3° (101°F) Abnormal cervical or vaginal discharge WBC presence on saline microscopy of vaginal secretions ↑ Erythrocyte sedimentation rate ↑ C-reactive protein MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Clinical presentation of PID �General-signs/symptoms may vary from mild to severe �Signs-vague �Symptoms Lower abdominal or pelvic pain Malodorous vaginal discharge Abnormal uterine bleeding Dyspareunia Nausea and/or vomiting Fever MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

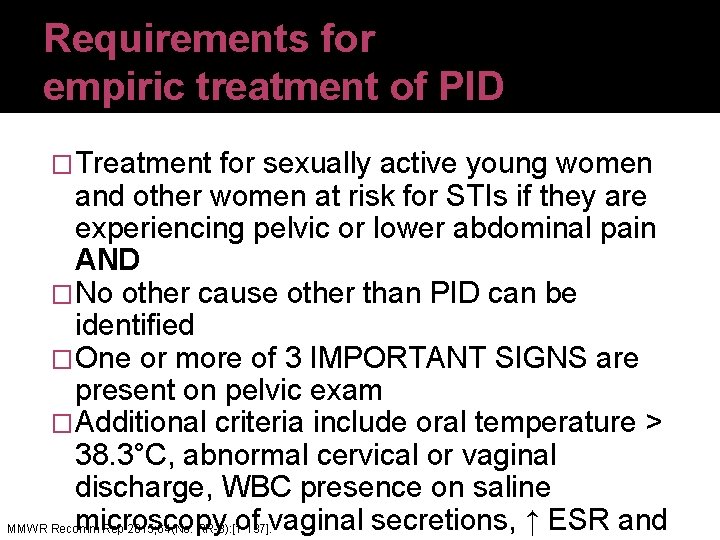
Requirements for empiric treatment of PID �Treatment for sexually active young women and other women at risk for STIs if they are experiencing pelvic or lower abdominal pain AND �No other cause other than PID can be identified �One or more of 3 IMPORTANT SIGNS are present on pelvic exam �Additional criteria include oral temperature > 38. 3°C, abnormal cervical or vaginal discharge, WBC presence on saline microscopy of vaginal secretions, ↑ ESR and MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

PID treatment �Therapy should provide empiric, broad spectrum coverage of likely pathogens N. gonorrhoeae C. trachomatis �Microorganisms which comprise vaginal flora Gardnerella vaginalis Haemophilus influenzae Enteric gram (-) rods Streptococcus agalactiae Anaerobes MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Anaerobic coverage for PID �Anaerobic bacteria have been isolated from the upper-reproductive tract of women who have PID �Data from in vitro studies have revealed some anaerobes (e. g. Bacteroides fragilis) can cause tubal and epithelial destruction �Bacterial vaginosis (BV) also present in many women who have PID �Use of regimens with anaerobic activity should be considered for PID treatment MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Inpatient versus outpatient therapy for PID �If mild to moderate clinical severity, outpatient therapy has similar outcomes to inpatient management �Hospitalize if ANY of the following factors present Surgical emergencies Pregnancy Does not respond to oral antimicrobial therapy Unable to follow or tolerate oral regimen Severe illness, N/V, high fever MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

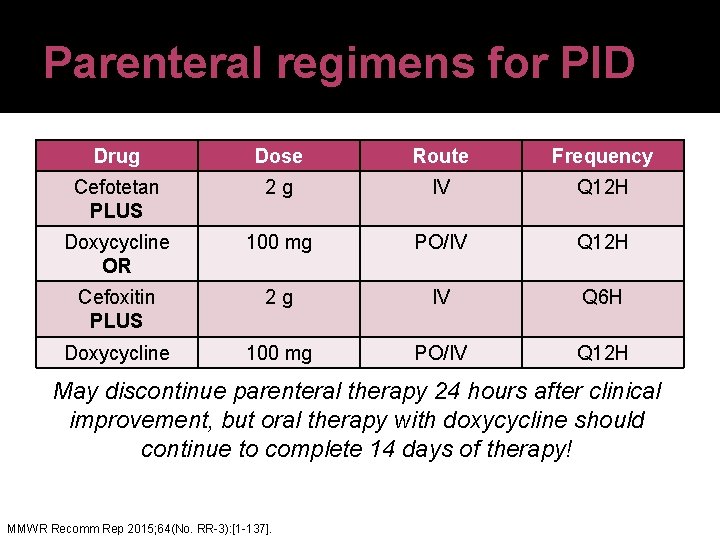
Parenteral regimens for PID Drug Dose Route Frequency Cefotetan PLUS 2 g IV Q 12 H Doxycycline OR 100 mg PO/IV Q 12 H Cefoxitin PLUS 2 g IV Q 6 H Doxycycline 100 mg PO/IV Q 12 H May discontinue parenteral therapy 24 hours after clinical improvement, but oral therapy with doxycycline should continue to complete 14 days of therapy! MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

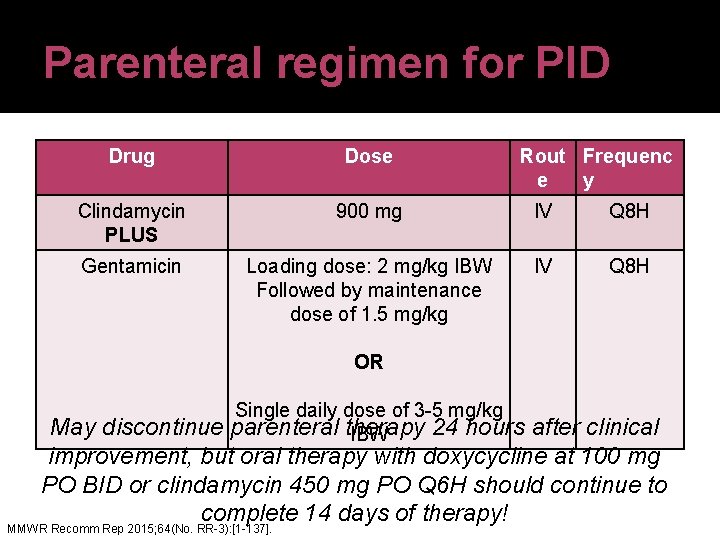
Parenteral regimen for PID Drug Dose Rout Frequenc e y Clindamycin PLUS 900 mg IV Q 8 H Gentamicin Loading dose: 2 mg/kg IBW Followed by maintenance dose of 1. 5 mg/kg IV Q 8 H OR Single daily dose of 3 -5 mg/kg parenteral therapy 24 hours IBW May discontinue after clinical improvement, but oral therapy with doxycycline at 100 mg PO BID or clindamycin 450 mg PO Q 6 H should continue to complete 14 days of therapy! MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

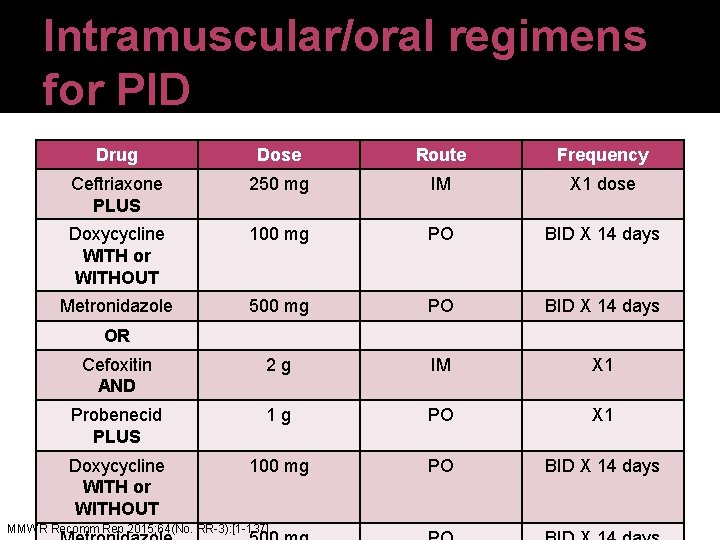
Intramuscular/oral regimens for PID Drug Dose Route Frequency Ceftriaxone PLUS 250 mg IM X 1 dose Doxycycline WITH or WITHOUT 100 mg PO BID X 14 days Metronidazole 500 mg PO BID X 14 days Cefoxitin AND 2 g IM X 1 Probenecid PLUS 1 g PO X 1 Doxycycline WITH or WITHOUT 100 mg PO BID X 14 days OR MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

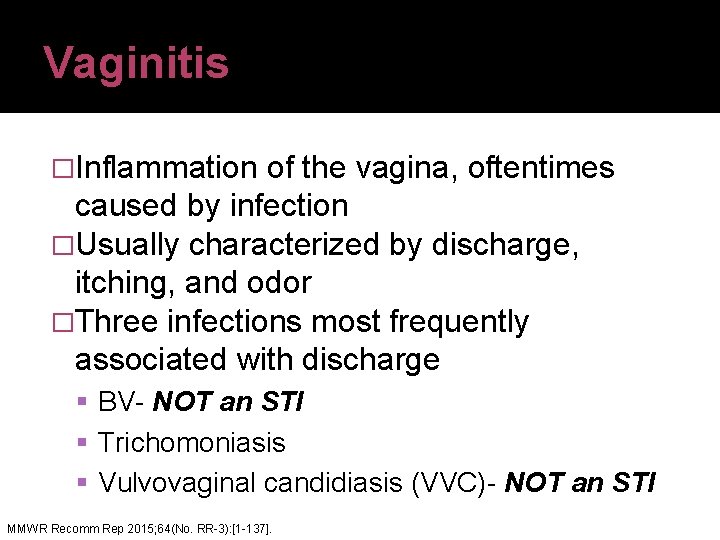
Vaginitis �Inflammation of the vagina, oftentimes caused by infection �Usually characterized by discharge, itching, and odor �Three infections most frequently associated with discharge BV- NOT an STI Trichomoniasis Vulvovaginal candidiasis (VVC)- NOT an STI MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

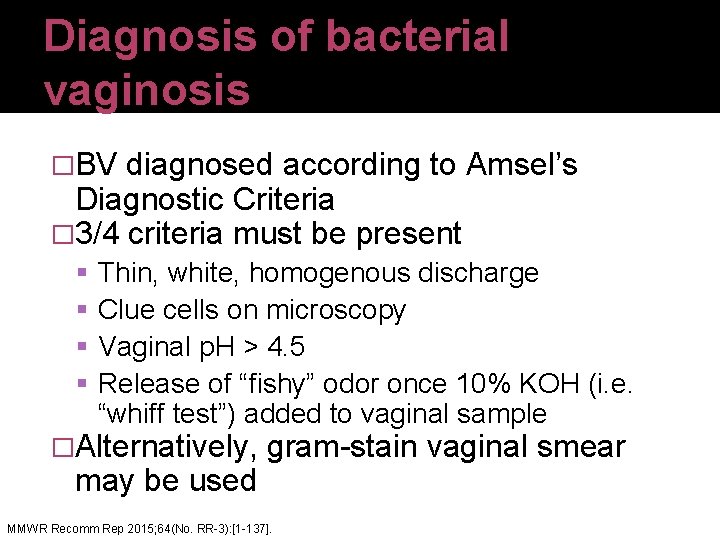
Diagnosis of bacterial vaginosis �BV diagnosed according to Amsel’s Diagnostic Criteria � 3/4 criteria must be present Thin, white, homogenous discharge Clue cells on microscopy Vaginal p. H > 4. 5 Release of “fishy” odor once 10% KOH (i. e. “whiff test”) added to vaginal sample �Alternatively, gram-stain vaginal smear may be used MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

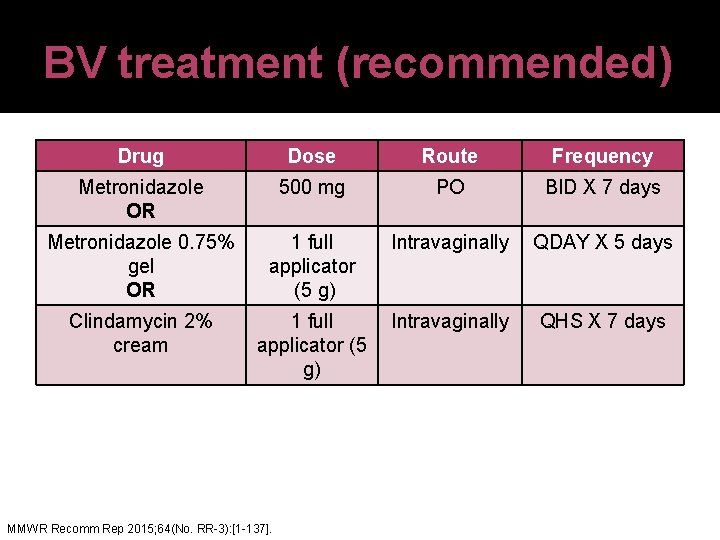
BV treatment (recommended) Drug Dose Route Frequency Metronidazole OR 500 mg PO BID X 7 days Metronidazole 0. 75% gel OR 1 full applicator (5 g) Intravaginally QDAY X 5 days Clindamycin 2% cream 1 full Intravaginally applicator (5 g) MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137]. QHS X 7 days

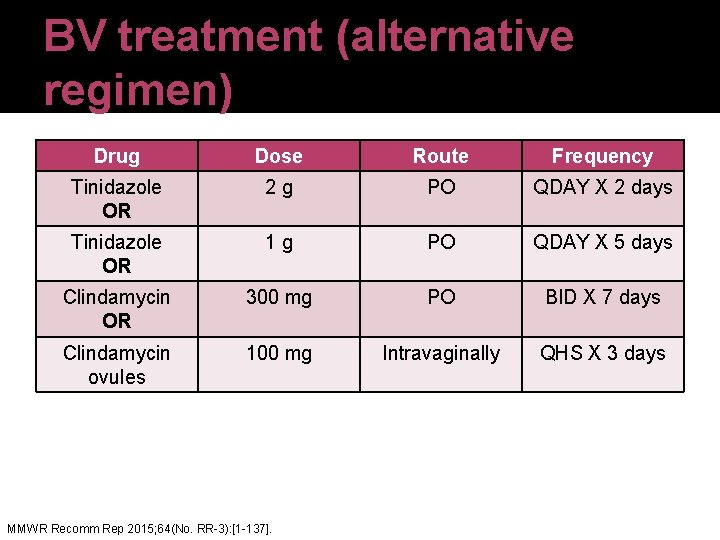
BV treatment (alternative regimen) Drug Dose Route Frequency Tinidazole OR 2 g PO QDAY X 2 days Tinidazole OR 1 g PO QDAY X 5 days Clindamycin OR 300 mg PO BID X 7 days Clindamycin ovules 100 mg Intravaginally QHS X 3 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

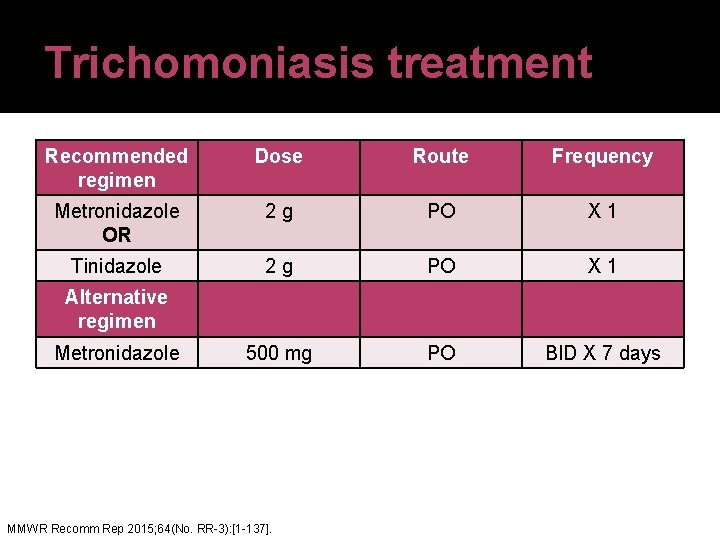
Trichomoniasis treatment Recommended regimen Dose Route Frequency Metronidazole OR 2 g PO X 1 Tinidazole 2 g PO X 1 500 mg PO BID X 7 days Alternative regimen Metronidazole MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Metronidazole counseling �A disulfiram-like reaction may occur if taken with alcohol Flushing, palpitations, tachycardia, nausea, vomiting, may occur with concurrent use Although the risk for most patients may be slight, caution is advised �Alcoholic beverages should be avoided while taking metronidazole and for at least 1 day (metronidazole tablets) or 3 days (metronidazole capsules and extended release tablets) after discontinuing MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

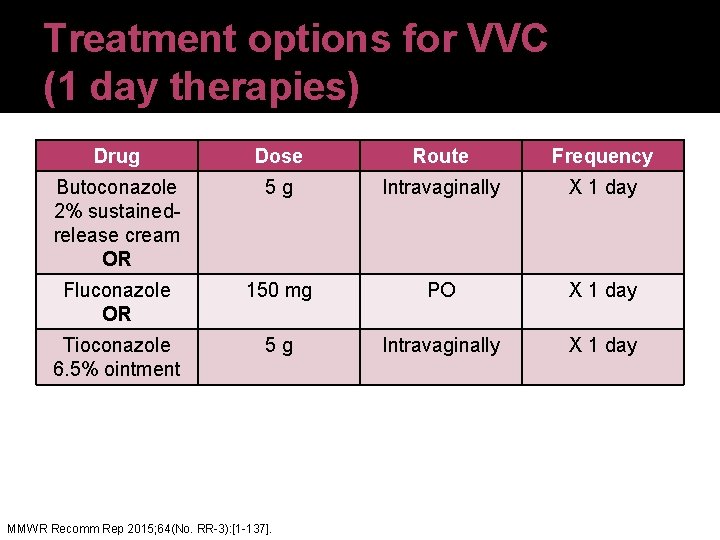
Treatment options for VVC (1 day therapies) Drug Dose Route Frequency Butoconazole 2% sustainedrelease cream OR 5 g Intravaginally X 1 day Fluconazole OR 150 mg PO X 1 day Tioconazole 6. 5% ointment 5 g Intravaginally X 1 day MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

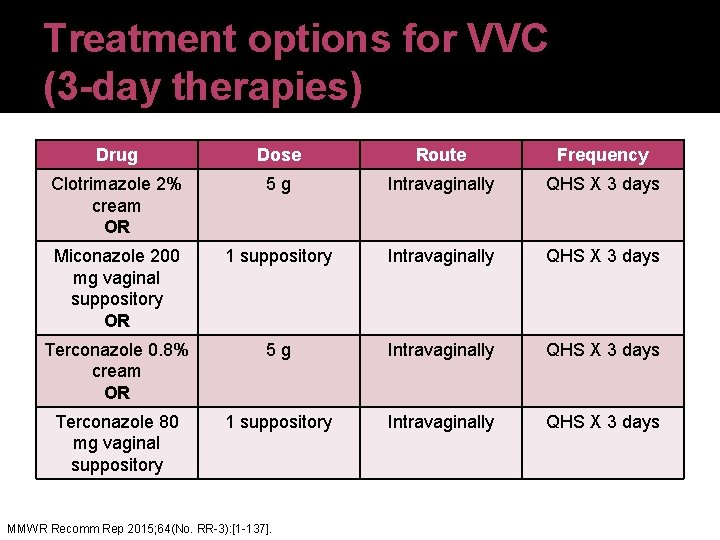
Treatment options for VVC (3 -day therapies) Drug Dose Route Frequency Clotrimazole 2% cream OR 5 g Intravaginally QHS X 3 days Miconazole 200 mg vaginal suppository OR 1 suppository Intravaginally QHS X 3 days Terconazole 0. 8% cream OR 5 g Intravaginally QHS X 3 days Terconazole 80 mg vaginal suppository 1 suppository Intravaginally QHS X 3 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Treatment options for VVC (7 -14 day therapies) Drug Dose Route Frequency Clotrimazole 1% cream OR 5 g Intravaginally QHS X 7 -14 nights Clotrimazole 100 mg vaginal tab OR 1 tablet Intravaginally QHS X 7 nights Miconazole 2% cream OR 5 g Intravaginally QHS X 7 nights Miconazole 100 mg vaginal tab OR 1 suppository Intravaginally QHS X 7 nights Terconazole 0. 4% cream 5 g Intravaginally QDAY X 7 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

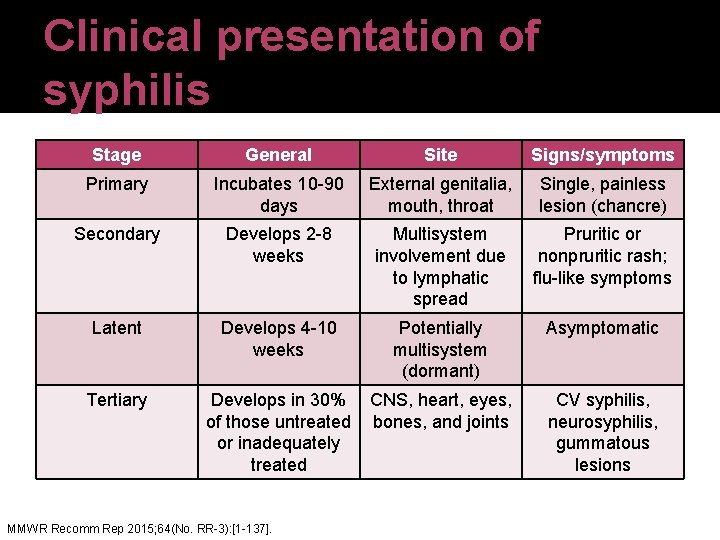
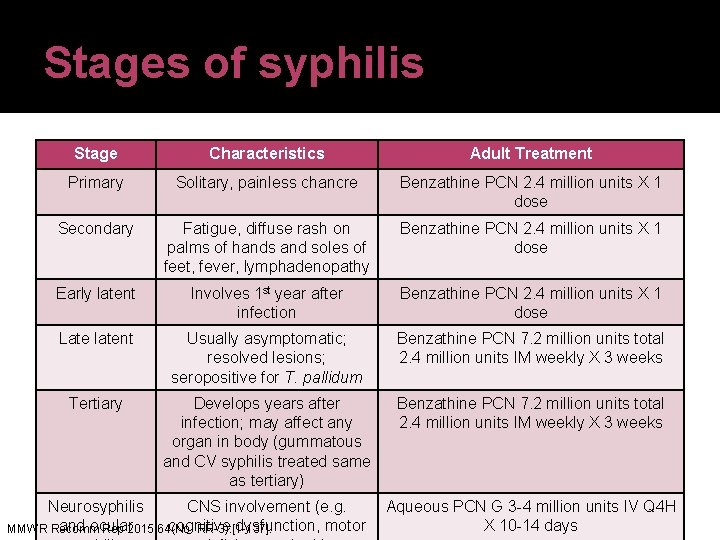
Clinical presentation of syphilis Stage General Site Signs/symptoms Primary Incubates 10 -90 days External genitalia, mouth, throat Single, painless lesion (chancre) Secondary Develops 2 -8 weeks Multisystem involvement due to lymphatic spread Pruritic or nonpruritic rash; flu-like symptoms Latent Develops 4 -10 weeks Potentially multisystem (dormant) Asymptomatic Tertiary Develops in 30% CNS, heart, eyes, of those untreated bones, and joints or inadequately treated MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137]. CV syphilis, neurosyphilis, gummatous lesions

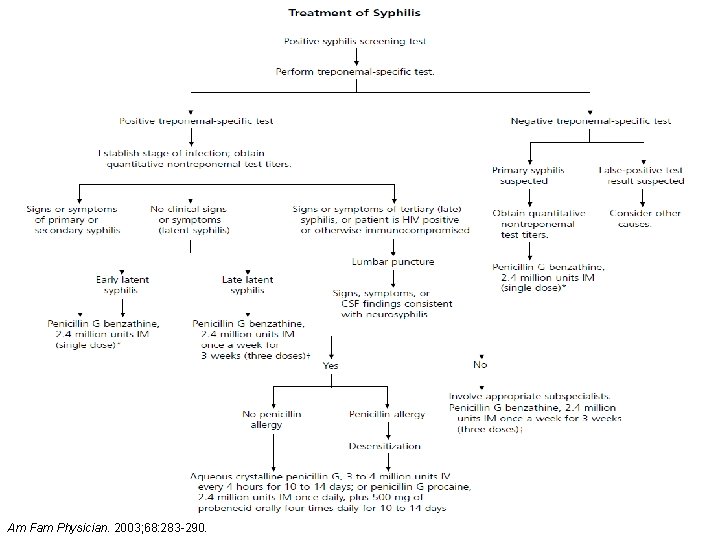
Stages of syphilis Stage Characteristics Adult Treatment Primary Solitary, painless chancre Benzathine PCN 2. 4 million units X 1 dose Secondary Fatigue, diffuse rash on palms of hands and soles of feet, fever, lymphadenopathy Benzathine PCN 2. 4 million units X 1 dose Early latent Involves 1 st year after infection Benzathine PCN 2. 4 million units X 1 dose Late latent Usually asymptomatic; resolved lesions; seropositive for T. pallidum Benzathine PCN 7. 2 million units total 2. 4 million units IM weekly X 3 weeks Tertiary Develops years after infection; may affect any organ in body (gummatous and CV syphilis treated same as tertiary) Benzathine PCN 7. 2 million units total 2. 4 million units IM weekly X 3 weeks Neurosyphilis CNS involvement (e. g. Aqueous PCN G 3 -4 million units IV Q 4 H and ocular cognitive dysfunction, motor X 10 -14 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Am Fam Physician. 2003; 68: 283 -290.

Jarisch-Herxheimer �Reaction which occurs secondary to spirochete lysis and pro-inflammatory cytokine cascades �Can transpire as early as 2 hours after PCN administration and usually resolves within 24 hours �Clinical presentation Fever Chills Tachycardia Tachypnea �Treatment is supportive MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

Increase in incidence of congenital syphilis � � � Syphilis infection during pregnancy can result in significant health problems for an infant Historical data indicate that up to 40% of pregnancies in women with untreated syphilis will result in miscarriage, stillbirth, or infant death Infants who live may develop severe illness, including skeletal abnormalities; hepatosplenomegaly; jaundice; anemia; optic atrophy; interstitial keratitis; sensorineural deafness; or meningitis, which can cause developmental delays and seizures All pregnant women should be screened for syphilis at their first prenatal visit Women at high risk should be rescreened early in their third trimester and again at delivery Pregnant women diagnosed with syphilis should be treated with penicillin immediately and should last 30 days prior to delivery Kidd S. Congenital Syphilis Is on the Rise? Reviewing Prevention Steps. Centers for disease control and prevention. Updated July 18, 2016.

Increase in incidence of congenital syphilis � Patients should be advised tell sex partners about the infection and encourage them to get tested and treated to avoid reinfection � Before discharging any newborn infant from the hospital, mothers should be tested for syphilis at least once during her pregnancy or at delivery � If a woman delivers a stillborn infant, she should be tested for syphilis � Safe sex practices should be discussed � Partner with health departments, prenatal care providers, and other local organizations to address barriers to obtaining early and adequate prenatal care for the most vulnerable pregnant women in the community Kidd S. Congenital Syphilis Is on the Rise? Reviewing Prevention Steps. Centers for disease control and prevention. Updated July 18, 2016.

Bicillin-LA (benzathine penicillin G) shortage Recommended treatment for syphilis and the only recommended treatment for pregnant women infected or exposed to syphilis is benzathine penicillin G) � Pfizer, the sole manufacturer of Bicillin L-A® (penicillin G benzathine) in the United States is experiencing a manufacturing delay of this product � CDC recommendations until shortage resolves � � Refrain from the use of Bicillin L-A® (penicillin G benzathine) for treatment of other infectious diseases (e. g. , streptococcal pharyngitis) where other effective antimicrobials are available Adhere to the recommended dosing regimen of 2. 4 million units of penicillin G benzathine IM for the treatment of primary, secondary and early latent syphilis (i. e. , early syphilis) as outlined in the 2015 STD Treatment Guidelines(http: //www. cdc. gov/std/tg 2015/syphilis. htm) Additional doses to treat early syphilis do not enhance efficacy, including in patients living with HIV infection Contact your pharmacists/distributors to procure Bicillin L-A® (penicillin G benzathine), if you do not have product readily available If product reaches a critical supply level of three weeks or less, contact Pfizer Direct questions about syphilis clinical management to an infectious disease specialist or the on-line National Network of STD Clinical Prevention Training Centers (NNPTC) STD Clinical Consultation Network (https: //www. stdccn. org ). Bicillin-LA (benzathine penicillin G) shortage. Centers for disease control and prevention. Updated June 28, 2016.

Penicillin shortage � Procaine Penicillin G IM is one of the recommended treatments for congenital syphilis and an alternative treatment for both neurosyphilis and ocular syphilis � Other recommended treatment for congenital syphilis is aqueous crystalline penicillin G IV � Pfizer, the sole manufacturer of this product in the United States, is experiencing manufacturing delays � CDC is continuing to work with FDA’s Drug Shortage Staff and Pfizer to address this situation � If Procaine Penicillin G is unavailable and until normal quantities of Procaine Penicillin G is available, CDC suggests using other available and recommended regimens of penicillin to treat congenital syphilis as outlined in the 2015 STD Treatment Guidelines(https: //www. cdc. gov/std/tg 2015/syphilis. ht Procaine penicillin shortage. Centers for disease control and prevention. Updated Sept 19, 2016.

Genital herpes treatment �Desired outcome is to curtail number of episodic prodromes and to minimize any side effects experienced due to antivirals �Treatment is based on several factors Likelihood of patient compliance Whether it is the first or recurrent episode Host immunity Pregnancy MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

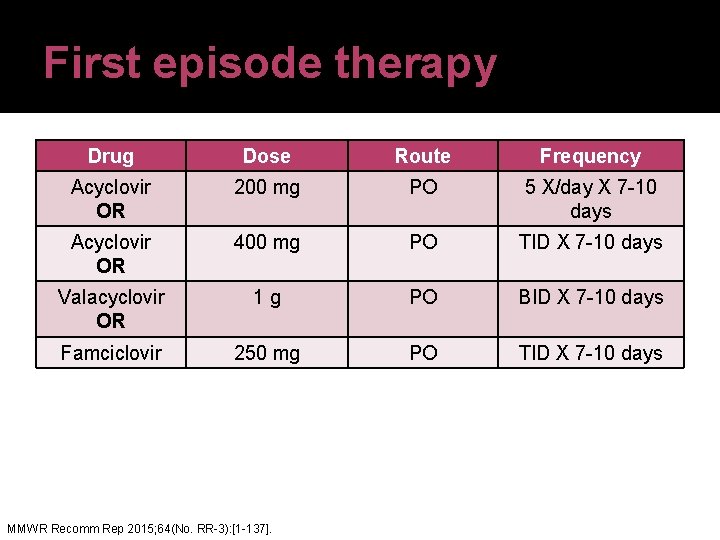
First episode therapy Drug Dose Route Frequency Acyclovir OR 200 mg PO 5 X/day X 7 -10 days Acyclovir OR 400 mg PO TID X 7 -10 days Valacyclovir OR 1 g PO BID X 7 -10 days Famciclovir 250 mg PO TID X 7 -10 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

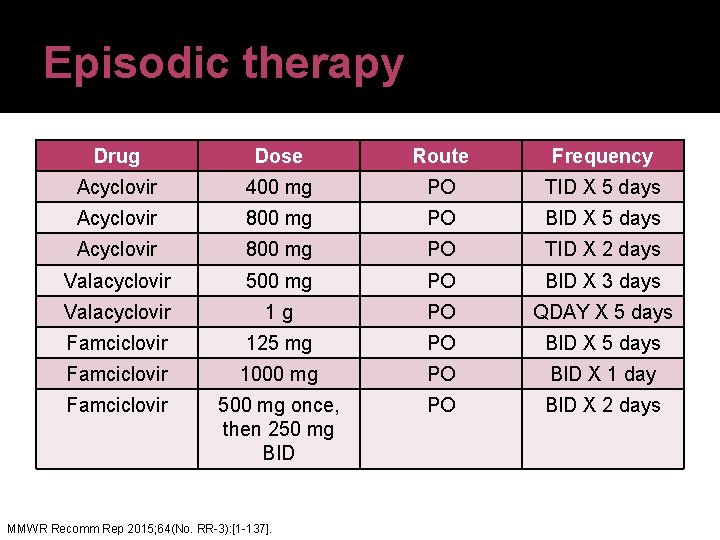
Episodic therapy Drug Dose Route Frequency Acyclovir 400 mg PO TID X 5 days Acyclovir 800 mg PO BID X 5 days Acyclovir 800 mg PO TID X 2 days Valacyclovir 500 mg PO BID X 3 days Valacyclovir 1 g PO QDAY X 5 days Famciclovir 125 mg PO BID X 5 days Famciclovir 1000 mg PO BID X 1 day Famciclovir 500 mg once, then 250 mg BID PO BID X 2 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

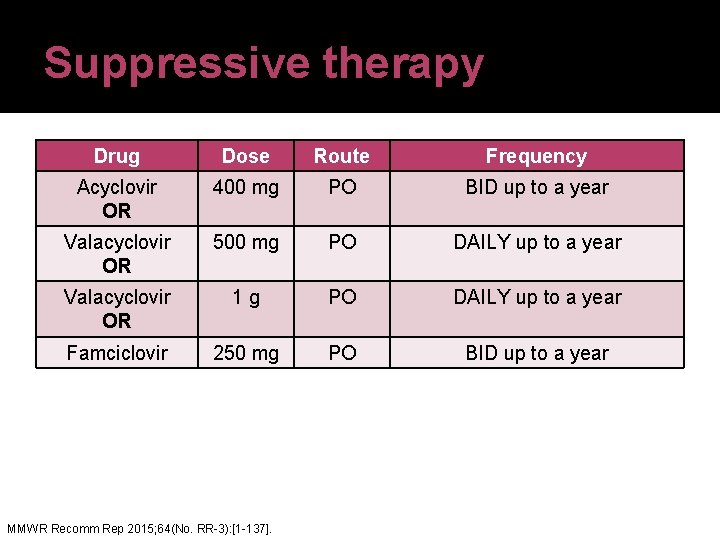
Suppressive therapy Drug Dose Route Frequency Acyclovir OR 400 mg PO BID up to a year Valacyclovir OR 500 mg PO DAILY up to a year Valacyclovir OR 1 g PO DAILY up to a year Famciclovir 250 mg PO BID up to a year MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

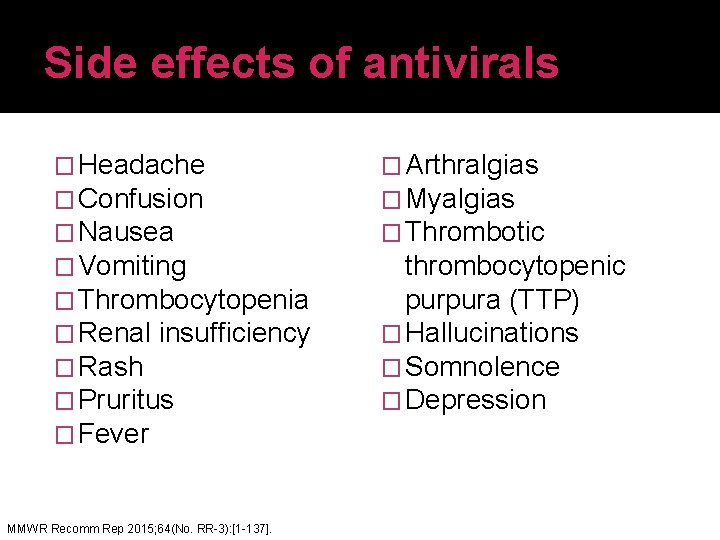
Side effects of antivirals � Headache � Confusion � Nausea � Vomiting � Thrombocytopenia � Renal insufficiency � Rash � Pruritus � Fever MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137]. � Arthralgias � Myalgias � Thrombotic thrombocytopenic purpura (TTP) � Hallucinations � Somnolence � Depression

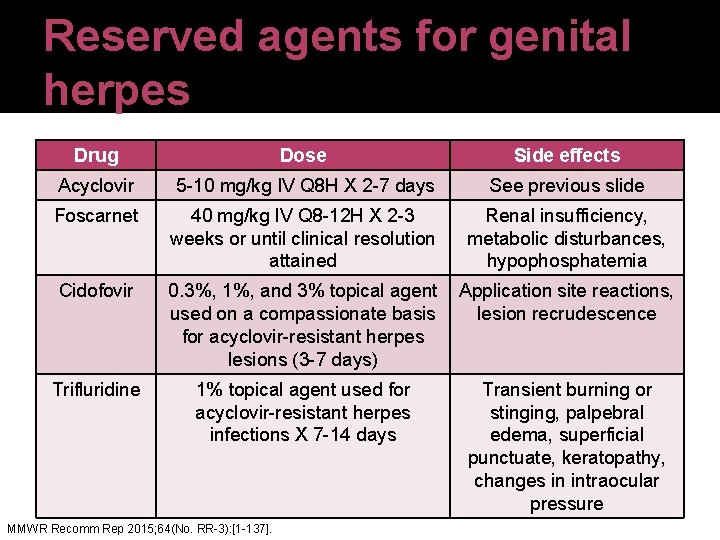
Reserved agents for genital herpes Drug Dose Side effects Acyclovir 5 -10 mg/kg IV Q 8 H X 2 -7 days See previous slide Foscarnet 40 mg/kg IV Q 8 -12 H X 2 -3 weeks or until clinical resolution attained Renal insufficiency, metabolic disturbances, hypophosphatemia Cidofovir 0. 3%, 1%, and 3% topical agent Application site reactions, used on a compassionate basis lesion recrudescence for acyclovir-resistant herpes lesions (3 -7 days) Trifluridine 1% topical agent used for acyclovir-resistant herpes infections X 7 -14 days MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137]. Transient burning or stinging, palpebral edema, superficial punctuate, keratopathy, changes in intraocular pressure

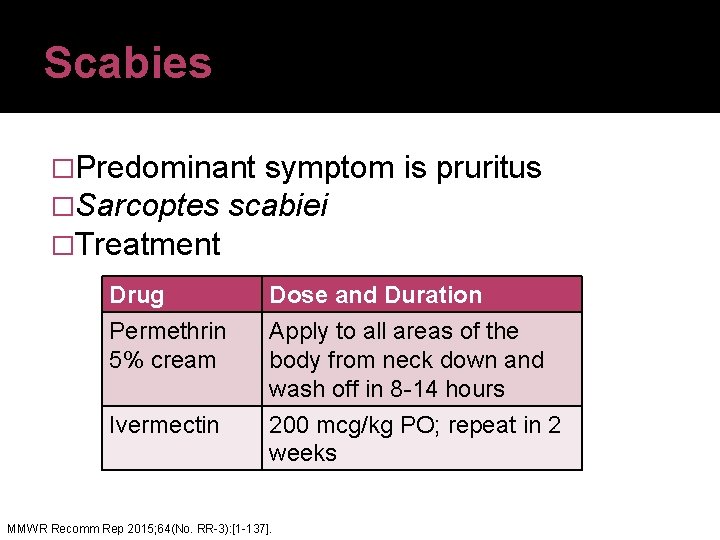
Scabies �Predominant symptom is pruritus �Sarcoptes scabiei �Treatment Drug Permethrin 5% cream Dose and Duration Apply to all areas of the body from neck down and wash off in 8 -14 hours Ivermectin 200 mcg/kg PO; repeat in 2 weeks MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

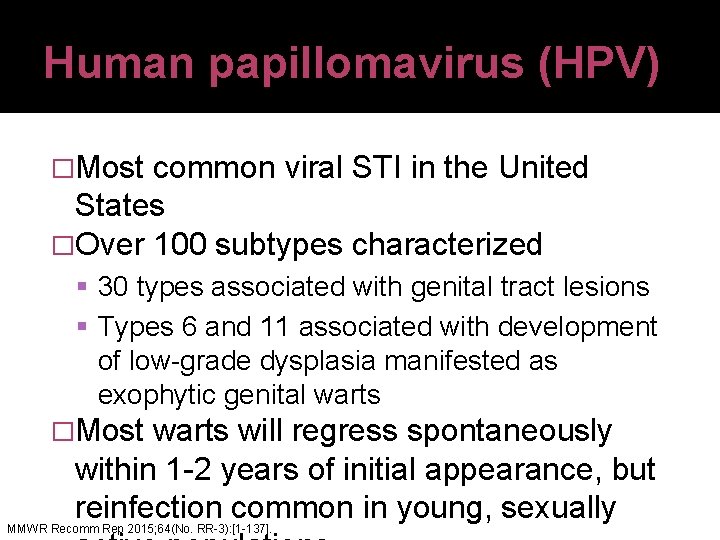
Human papillomavirus (HPV) �Most common viral STI in the United States �Over 100 subtypes characterized 30 types associated with genital tract lesions Types 6 and 11 associated with development of low-grade dysplasia manifested as exophytic genital warts �Most warts will regress spontaneously within 1 -2 years of initial appearance, but reinfection common in young, sexually MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

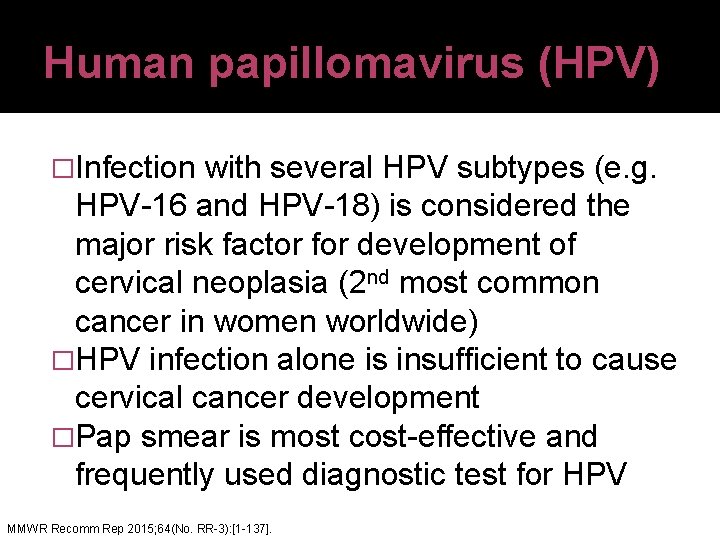
Human papillomavirus (HPV) �Infection with several HPV subtypes (e. g. HPV-16 and HPV-18) is considered the major risk factor for development of cervical neoplasia (2 nd most common cancer in women worldwide) �HPV infection alone is insufficient to cause cervical cancer development �Pap smear is most cost-effective and frequently used diagnostic test for HPV MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

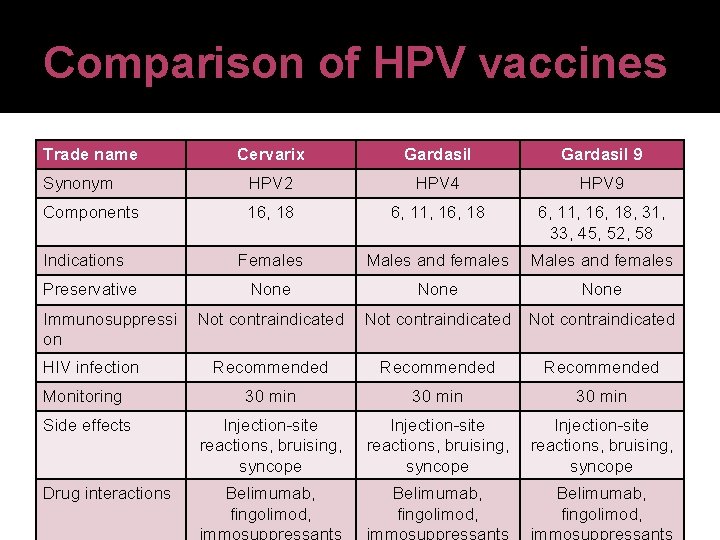
Human papillomavirus (HPV) vaccines � Three HPV vaccines marketed in the United States Cervarix® (HPV 2)-bivalent vaccine for HPV-16 and HPV-18 Gardasil® (HPV 4)-quadrivalent vaccine for HPV-6, 11, -16, and -18 Gardasil-9 (HPV 9)-nine-valent vaccine for HPV 6, 11, 16, 18, 31, 33, 45, 52, 58 � Vaccines are indicated for preventing cervical precancers and cervical cancer in females 11 or 12 through 26 years of age (but can be started as early as 9) � HPV 4 and HPV 9 also indicated in males to Centers for disease control and prevention. 06 Oct 2015 <http: //www. cdc. gov/std>

Comparison of HPV vaccines Trade name Cervarix Gardasil 9 Synonym HPV 2 HPV 4 HPV 9 Components 16, 18 6, 11, 16, 18, 31, 33, 45, 52, 58 Females Males and females None Not contraindicated Recommended Monitoring 30 min Side effects Injection-site reactions, bruising, syncope Indications Preservative Immunosuppressi on HIV infection Drug interactions Belimumab, fingolimod, Injection-site reactions, bruising, syncope Belimumab, fingolimod,

HPV vaccine recommendations � Females All vaccines are recommended in a 3 -dose series for routine vaccination at age 11 or 12 years For those aged 13 through 26 years, if not previously vaccinated � Males HPV 4 and HPV 9 are recommended in a 3 -dose series for routine vaccination at age 11 or 12 years, and for those aged 13 through 21 years, if not previously vaccinated Males aged 22 through 26 years may be vaccinated � HPV 4 is recommended for MSM through age 26 years or those who did not get any or all doses Centers for disease control and prevention. 9 Oct 2015

HPV vaccine recommendations � Vaccination is recommended for immunocompromised persons (including those with HIV infection) through age 26 years for those who did not get any or all doses when they were younger � A complete series for all vaccines consists of 3 doses 2 nd dose should be administered 1– 2 months after the first dose 3 rd dose should be administered 6 months after the first dose (at least 24 weeks after the first dose) � HPV vaccines are not recommended for use in pregnant women Pregnancy testing is not needed before vaccination If a woman is found to be pregnant after initiating the vaccination series, no intervention is needed Remainder of the 3 -dose series should be delayed until completion of pregnancy Centers for disease control and prevention. 9 Oct 2015

HPV and cancer � Cervical cancer Most common HPV-associated cancer Almost all cervical cancer is caused by HPV � Vulvar cancer-~50% are linked to HPV � Vaginal cancer-~65% are linked to HPV � Penile cancer-~35% are linked to HPV � Anal cancer-~95% are linked to HPV � Oropharyngeal cancers Cancers of the back of the throat, including the base of the tongue and tonsils ~60% are linked to HPV Many of these cancers may be related to tobacco and alcohol use Centers for disease control and prevention. 9 Oct 2015

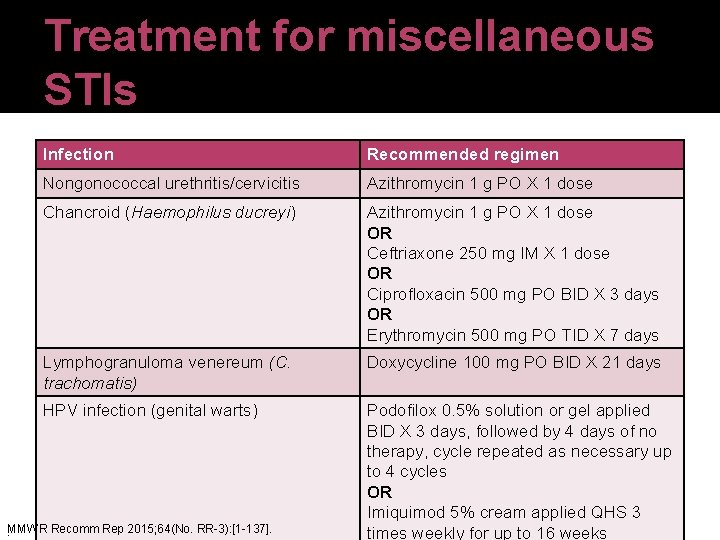
Treatment for miscellaneous STIs Infection Recommended regimen Nongonococcal urethritis/cervicitis Azithromycin 1 g PO X 1 dose Chancroid (Haemophilus ducreyi) Azithromycin 1 g PO X 1 dose OR Ceftriaxone 250 mg IM X 1 dose OR Ciprofloxacin 500 mg PO BID X 3 days OR Erythromycin 500 mg PO TID X 7 days Lymphogranuloma venereum (C. trachomatis) Doxycycline 100 mg PO BID X 21 days HPV infection (genital warts) Podofilox 0. 5% solution or gel applied BID X 3 days, followed by 4 days of no therapy, cycle repeated as necessary up to 4 cycles OR Imiquimod 5% cream applied QHS 3 times weekly for up to 16 weeks . MMWR Recomm Rep 2015; 64(No. RR-3): [1 -137].

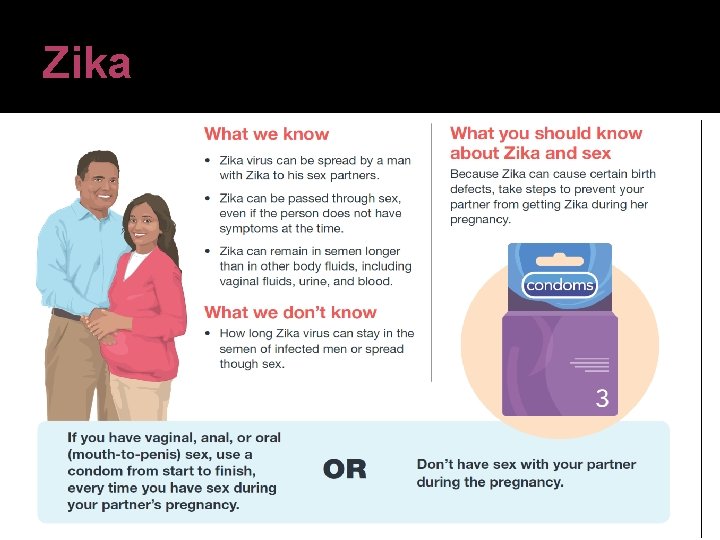
Basics of Zika transmission � Zika can be passed through sex from a person who has Zika to his or her sex partners � Sex includes vaginal, anal, oral sex, and the sharing of sex toys Zika can be passed through sex, even if the person does not have symptoms at the time It can be passed from a person with Zika before their symptoms start, while they have symptoms, and after their symptoms end Though not well documented, the virus may also be passed by a person who carries the virus but never develops symptoms � Studies are underway to find out how long Zika stays in the semen and vaginal fluids of people who have Zika, and how long it can be passed to sex partners � It is known that Zika can remain in semen longer than in other body fluids, including vaginal fluids, urine, and Zika and sexual transmission. Centers for disease control and prevention. Updated Sept 1, 2016.

Zika prevention �Condoms can reduce the chance of getting Zika from sex Condoms include male and female condoms Dental dams (latex or polyurethane sheets) may also be used for certain types of oral sex (mouth to vagina or mouth to anus) �Condoms should be used from start to finish, every time during vaginal, and oral sex �Not sharing sex toys can also reduce the risk of spreading Zika to sex partners �Not having sex eliminates the risk of getting Zika from sex Zika and sexual transmission. Centers for disease control and prevention. Updated Sept 1, 2016.

Zika



WHO STI recently updated recommendations WHO has released new guidelines for treating gonorrhea, syphilis, and chlamydia given their increasing resistance to treatment � Gonorrhea treatment changes � Quinolones are no longer recommended given the high prevalence of resistance Dual therapy is preferred over single therapy Health authorities should advise providers to prescribe the antibiotic that would be most effective, taking into account current local patterns of resistance Oropharyngeal treatment recommendations and guidance on retreatment after treatment failure are included � Syphilis-new guidelines strongly recommend a dose of benzathine penicillin, which has been in short supply, over procaine penicillin � Chlamydia� WHO developed recommendations for treating pregnant women Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections. World heath organization website. Updated Aug 30, 2016. Recommendations for preventing and treating chlamydia

Young adults resource https: //npin. cdc. gov/KABIChroni cles

Conclusions �Several STIs may be encountered in clinical practice �Open communication with patients is essential to successful care �Variety of medications are available for treatment �Therapy must be chosen through a patient -centered approach

An Update on Sexually Transmitted Infections (STIs) C. Brock Woodis, Pharm. D, BCACP, BCPS, CDE, BC-ADM, CPP Associate Professor Campbell University College of Pharmacy & Health Sciences Duke Family Medicine September 27, 2016 brock. woodis@duke. edu
 Chapter 25 sexually transmitted infections and hiv/aids
Chapter 25 sexually transmitted infections and hiv/aids Sexually transmitted diseases
Sexually transmitted diseases Chapter 24 lesson 1 sexually transmitted diseases
Chapter 24 lesson 1 sexually transmitted diseases Std
Std Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Sexually transmitted diseases
Sexually transmitted diseases What is an alternative of log based recovery
What is an alternative of log based recovery Genital infections
Genital infections Methotrexate yeast infection
Methotrexate yeast infection Bacterial vaginosis
Bacterial vaginosis Storch infections
Storch infections Amber blumling
Amber blumling Opportunistic infections
Opportunistic infections Postpartum infections
Postpartum infections Bone and joint infections
Bone and joint infections Opsonization
Opsonization Understanding the mirai botnet
Understanding the mirai botnet Retroviruses and opportunistic infections
Retroviruses and opportunistic infections Neurosiphyllis
Neurosiphyllis Opportunistic infections
Opportunistic infections Acute gingival infections
Acute gingival infections Storch infections
Storch infections Do flatback sea turtles reproduce sexually or asexually
Do flatback sea turtles reproduce sexually or asexually Run away selection
Run away selection How do birds mate
How do birds mate What is an annelid
What is an annelid Sea urchin reproduction
Sea urchin reproduction Whale phylum
Whale phylum Radial symmetry
Radial symmetry What is sexual coersion
What is sexual coersion Sexually dimorphic meaning
Sexually dimorphic meaning Have i been sexually harassed quiz
Have i been sexually harassed quiz Platyhelminthes classification
Platyhelminthes classification What is frustrated cargo
What is frustrated cargo Protists habitat
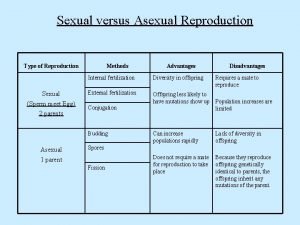
Protists habitat How protists reproduce
How protists reproduce Sexually dangerous persons act illinois
Sexually dangerous persons act illinois Sexual propagation
Sexual propagation Parasitism phylum
Parasitism phylum Spongia officinalis
Spongia officinalis Orgasm
Orgasm Was it sexual abuse quiz
Was it sexual abuse quiz Electronically transmitted postal ballot system
Electronically transmitted postal ballot system Oblique light examination in questioned document
Oblique light examination in questioned document The totality of learned socially transmitted behavior
The totality of learned socially transmitted behavior A song transmitted orally which tells a story:
A song transmitted orally which tells a story: In a differential band brake mcq
In a differential band brake mcq Flowchart proses update secara berurutan adalah
Flowchart proses update secara berurutan adalah Fsu freight status update
Fsu freight status update Ffm update
Ffm update Sql queries for insert update and delete
Sql queries for insert update and delete Data redundancy and update anomalies
Data redundancy and update anomalies Leslie intervention update
Leslie intervention update Ddns-update-style none;
Ddns-update-style none; Adk bcsr sakti
Adk bcsr sakti @microsoft/mgt
@microsoft/mgt Igel management console
Igel management console Microsoft windows 10 security update
Microsoft windows 10 security update Position update formula
Position update formula [email protected]
[email protected] Fidelity quarterly market update q1 2018
Fidelity quarterly market update q1 2018 University community plan update
University community plan update Windows update herstellen
Windows update herstellen Project status update examples
Project status update examples Chocolate countable or uncountable
Chocolate countable or uncountable Deferred update
Deferred update Gx55 gps
Gx55 gps Unity connection to the update server failed
Unity connection to the update server failed Mentor update yorkshire
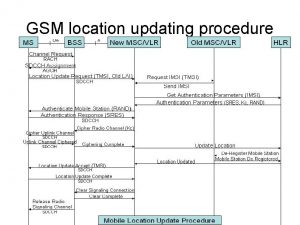
Mentor update yorkshire Location update procedure
Location update procedure Oracle release management
Oracle release management A tomato flames
A tomato flames Dokumen deskripsi sdmk
Dokumen deskripsi sdmk Microsoft security essential tidak bisa update
Microsoft security essential tidak bisa update Flash player update
Flash player update Noc update
Noc update