AN OVERVIEW OF PROTOCOL WRITING AND FDA PROTOCOL












































- Slides: 49

AN OVERVIEW OF PROTOCOL WRITING AND FDA PROTOCOL FEEDBACK Teresa Armstrong, ELS Indiana Chapter of the American Medical Writers Association February 20, 2013

AGENDA Protocols • Who • What • How FDA Feedback • When/What/How

Protocol - Who • Ethical or Institutional Review Boards • Study Investigator/Study Coordinator • Regulatory Authorities • Clinical Trial Registries (Public Domain)

Protocol - What • TITLE 21 --FOOD AND DRUGS • CHAPTER I--FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES • SUBCHAPTER D--DRUGS FOR HUMAN USEPART 312 -- INVESTIGATIONAL NEW DRUG APPLICATION Subpart B--Investigational New Drug Application (IND) • Sec. 312. 23 IND content and format. • (ii) In Phases 2 and 3, detailed protocols describing all aspects of the study should be submitted. A protocol for a Phase 2 or 3 investigation should be designed in such a way that, if the sponsor anticipates that some deviation from the study design may become necessary as the investigation progresses, alternatives or contingencies to provide for such deviation are built into the protocols at the outset. For example, a protocol for a controlled short-term study might include a plan for an early crossover of nonresponders to an alternative therapy.

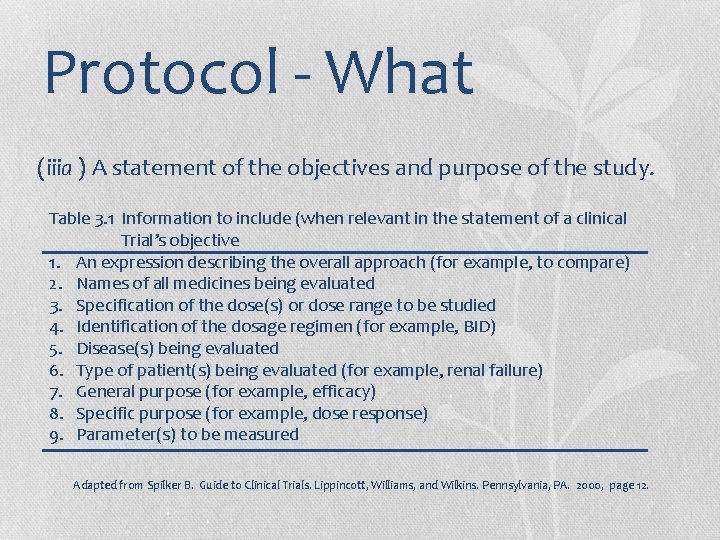
Protocol - What (iiia ) A statement of the objectives and purpose of the study. Table 3. 1 Information to include (when relevant in the statement of a clinical Trial’s objective 1. An expression describing the overall approach (for example, to compare) 2. Names of all medicines being evaluated 3. Specification of the dose(s) or dose range to be studied 4. Identification of the dosage regimen (for example, BID) 5. Disease(s) being evaluated 6. Type of patient(s) being evaluated (for example, renal failure) 7. General purpose (for example, efficacy) 8. Specific purpose (for example, dose response) 9. Parameter(s) to be measured Adapted from Spilker B. Guide to Clinical Trials. Lippincott, Williams, and Wilkins. Pennsylvania, PA. 2000, page 12.

Protocol - What (b ) The name and address and a statement of the qualifications (curriculum vitae or other statement of qualifications) of each investigator, and the name of each subinvestigator (e. g. , research fellow, resident) working under the supervision of the investigator; the name and address of the research facilities to be used; and the name and address of each reviewing Institutional Review Board.

Protocol - What (c ) The criteria for patient selection and for exclusion of patients and an estimate of the number of patients to be studied.

Protocol - What (d ) A description of the design of the study, including the kind of control group to be used, if any, and a description of methods to be used to minimize bias on the part of subjects, investigators, and analysts.

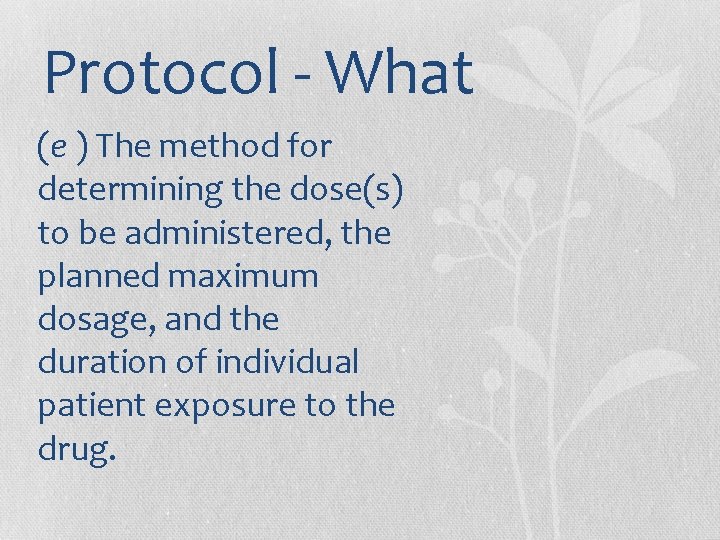
Protocol - What (e ) The method for determining the dose(s) to be administered, the planned maximum dosage, and the duration of individual patient exposure to the drug.

Protocol - What (f ) A description of the observations and measurements to be made to fulfill the objectives of the study.

Protocol - What (g ) A description of clinical procedures, laboratory tests, or other measures to be taken to monitor the effects of the drug in human subjects and to minimize risk.

Protocol - What do you think is missing from the CFR?

Protocol - What ICH E 6 6. 1 General Information 6. 1. 1 Protocol title, protocol identifying number, and date. Any amendment(s) should also bear the amendment number(s) and date(s).

Protocol - What 6. 1. 2 Name and address of the sponsor and monitor (if other than the sponsor). 6. 1. 3 Name and title of the person(s) authorized to sign the protocol and the protocol amendment(s) for the sponsor.

Protocol - What 6. 2 Background Information 6. 2. 1 Name and description of the investigational product(s). 6. 2. 2 A summary of findings from nonclinical studies that potentially have clinical significance and from clinical trials that are relevant to the trial. 6. 2. 3 Summary of the known and potential risks and benefits, if any, to human subjects.

Protocol - What 6. 2. 5 A statement that the trial will be conducted in compliance with the protocol, GCP and the applicable regulatory requirement(s). 6. 2. 7 References to literature and data that are relevant to the trial, and that provide background for the trial.

Protocol - What 6. 4 Trial Design 6. 4. 5 The expected duration of subject participation, and a description of the sequence and duration of all trial periods, including follow-up, if any. 6. 4. 6 A description of the "stopping rules" or "discontinuation criteria" for individual subjects, parts of trial and entire trial.

Protocol - What 6. 4. 7 Accountability procedures for the investigational product(s), including the placebo(s) and comparator(s), if any. 6. 4. 8 Maintenance of trial treatment randomization codes and procedures for breaking codes.

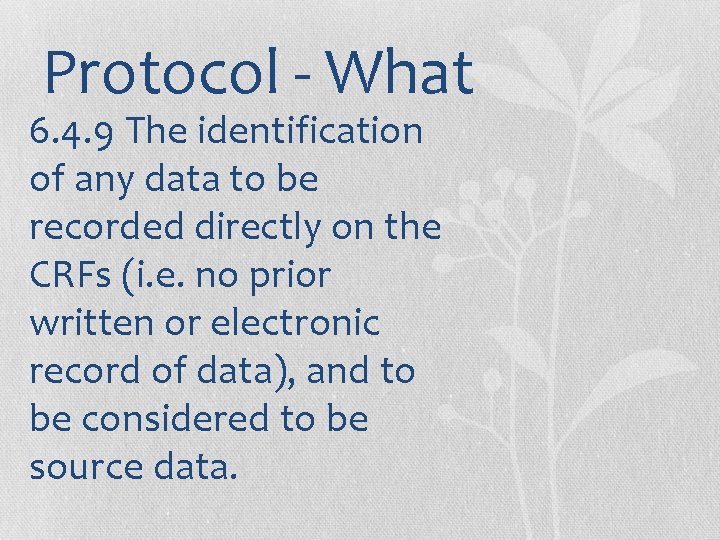
Protocol - What 6. 4. 9 The identification of any data to be recorded directly on the CRFs (i. e. no prior written or electronic record of data), and to be considered to be source data.

Protocol - What 6. 5 Selection and Withdrawal of Subjects 6. 5. 3 Subject withdrawal criteria (i. e. terminating investigational product treatment/trial treatment) and procedures specifying:

Protocol - What (a) When and how to withdraw subjects from the trial/ investigational product treatment. (b) The type and timing of the data to be collected for withdrawn subjects. (c) Whether and how subjects are to be replaced. (d) The follow-up for subjects withdrawn from investigational product treatment/trial treatment.

Protocol - What 6. 6 Treatment of Subjects 6. 6. 1 The treatment(s) to be administered, including the name(s) of all the product(s), the dose(s), the dosing schedule(s), the route/mode(s) of administration, and the treatment period(s), including the follow-up period(s) for subjects for each investigational product treatment/trial treatment group/arm of the trial.

Protocol - What 6. 6. 2 Medication(s)/treatment(s) permitted (including rescue medication) and not permitted before and/or during the trial.

Protocol - What 6. 6. 3 Procedures for monitoring subject compliance

Protocol - What 6. 7 Assessment of Efficacy 6. 7. 1 Specification of the efficacy parameters. 6. 7. 2 Methods and timing for assessing, recording, and analysing of efficacy parameters.

Protocol - What 6. 8 Assessment of Safety 6. 8. 1 Specification of safety parameters. 6. 8. 2 The methods and timing for assessing, recording, and analysing safety parameters.

Protocol - What 6. 8. 3 Procedures for eliciting reports of and for recording and reporting adverse event and intercurrent illnesses. 6. 8. 4 The type and duration of the follow-up of subjects after adverse events.

Protocol - What 6. 9 Statistics 6. 9. 1 A description of the statistical methods to be employed, including timing of any planned interim analysis(ses).

Protocol - What 6. 9. 3 The level of significance to be used. 6. 9. 4 Criteria for the termination of the trial. 6. 9. 5 Procedure for accounting for missing, unused, and spurious data.

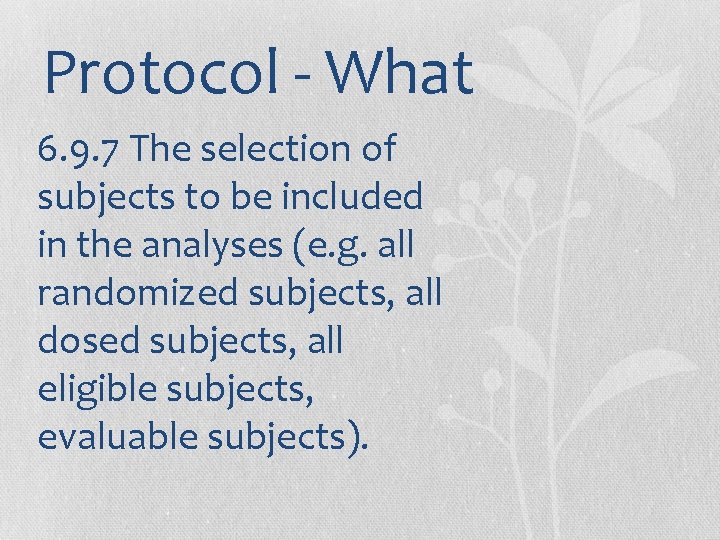
Protocol - What 6. 9. 7 The selection of subjects to be included in the analyses (e. g. all randomized subjects, all dosed subjects, all eligible subjects, evaluable subjects).

Protocol - What 6. 10 Direct Access to Source Data/Documents The sponsor should ensure that it is specified in the protocol or other written agreement that the investigator(s)/institution(s) will permit trial-related monitoring, audits, IRB/IEC review, and regulatory inspection(s), providing direct access to source data/documents.

Protocol - What • 6. 11 Quality Control and Quality Assurance • 6. 12 Ethics • Description of ethical considerations relating to the trial. • 6. 13 Data Handling and Record Keeping • 6. 14 Financing and Insurance • Financing and insurance if not addressed in a separate agreement.

Protocol - What 6. 15 Publication Policy Publication policy, if not addressed in a separate agreement. 6. 16 Supplements (NOTE: Since the protocol and the clinical trial/study report are closely related, further relevant information can be found in the ICH Guideline for Structure and Content of Clinical Study Reports. )

Protocol - How “Never express yourself more clearly than you are able to think. ” Niels Bohr As quoted in Values of the Wise: Humanity’s Highest Aspirations (Jason Merchey, 2004)

Protocol – How - Exercise Take a few minutes to read your hand out. Now enjoy this performance of the exercise as described on the hand out performed by Skyler and me. Video Redacted

Protocol – How - Exercise Video Redacted

Protocol – How - Exercise What do you find wrong with the AKC instructions you read in the hand out, assuming that Skyler and I did the exercise correctly? Video Redacted

Protocol - How Table 34. 1 General Considerations in writing and polishing a protocol 1. 2. 3. 4. 5. Avoid excessively long sentences Use correct and consistent tenses Limit the use of adjectives, adverbs, and other modifiers Use concrete, graphic, and precise words Eliminate unnecessary and hedge words (e. g. , very, seemed, etc. , presumably) 6. Eliminate clichés and trite phrases 7. Eliminate unnecessary sentences or paragraphs 8. Do not begin numerous sentences or paragraphs with the same word 9. Develop thoughts logically and in an orderly way 10. Describe each significant step in a complete thought or procedure 11. Define ambiguous or potentially ambiguous words or phrases Adapted from Spilker B. Guide to Clinical Trials. Lippincott, Williams, and Wilkins. Pennsylvania, PA. 2000, page 259.

FDA Feedback Type C Meeting Type B Meeting Special Protocol Assessment (Type A Meeting)

FDA Feedback • 75 days • 60 days and the number is limited

FDA Feedback CDER and CBER generally recommend that a sponsor submit a protocol intended for special protocol assessment to the Agency at least 90 days prior to the anticipated start of the study.

FDA Feedback FDA should be aware of the development context of the protocol.

FDA Feedback The sponsor should include information to assess the role of the study in the overall development of the drug.

FDA Feedback The sponsor should submit information supporting the proposed trial, including power calculations, the choice of study endpoints, and other critical design features (e. g. , choice of control, duration, methods of assessment).

FDA Feedback The sponsor should clearly describe any anticipated regulatory outcomes (e. g. , approval of a specific claim, breaking orphan exclusivity, a comparative claim) and proposed labeling that the sponsor believes would be supported by the results of the study.

FDA Feedback SPA is designed to answer specific questions regarding the protocol from the sponsor.

FDA Feedback FDA will provide comments on issues related to protocol design, study conduct and execution, data analysis, and implications for labeling. The Agency's assessment will be based primarily on the questions posed by the sponsor, the underlying data, assumptions, information described by the sponsor, and relevant Agency policies and guidance documents.

FDA Feedback If the Sponsor requests a meeting in response to the SPA letter, a Type A meeting can be scheduled within 30 days.

Resources FDA Guidance: • Guidance for Industry: Special Protocol Assessment • Guidance for Industry: Formal Meetings Between the FDA and Sponsors or Applicants Code of Federal Regulations ICH E 3, E 6, and E 9 Spilker B. Guide to Clinical Trials. Lippincott, Williams, and Wilkins. Pennsylvania, PA. 2000.