AN OVERVIEW OF PHOTOSYNTHESIS Autotrophs are the producers



- Slides: 26

AN OVERVIEW OF PHOTOSYNTHESIS Autotrophs are the producers of the biosphere • Plants are autotrophs, producing their own food and sustaining themselves without eating other organisms

• Plants, algae, and some bacteria are photoautotrophs – Producers of food consumed by virtually all organisms

Plants produce O 2 gas by splitting water • The O 2 liberated by photosynthesis – Is made from the oxygen in water

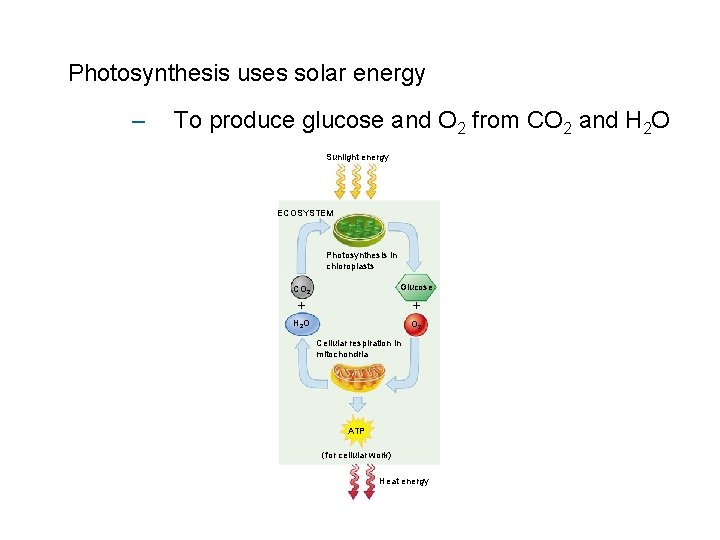
Photosynthesis uses solar energy – To produce glucose and O 2 from CO 2 and H 2 O Sunlight energy ECOSYSTEM Photosynthesis in chloroplasts CO 2 Glucose H 2 O O 2 Cellular respiration in mitochondria ATP (for cellular work) Heat energy

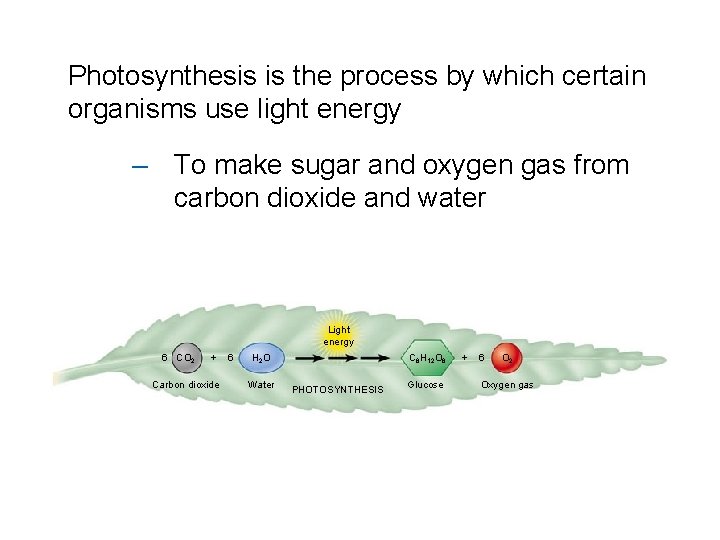
Photosynthesis is the process by which certain organisms use light energy – To make sugar and oxygen gas from carbon dioxide and water Light energy 6 CO 2 + Carbon dioxide 6 H 2 O Water C 6 H 12 O 6 PHOTOSYNTHESIS Glucose + 6 O 2 Oxygen gas

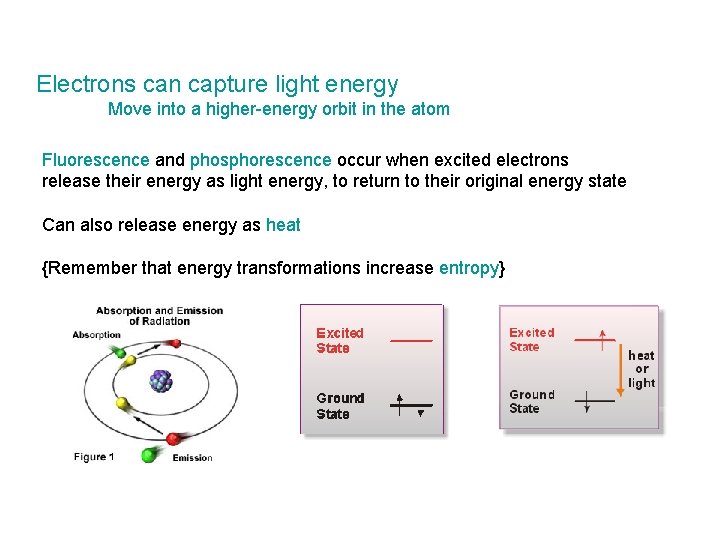
Electrons can capture light energy Move into a higher-energy orbit in the atom Fluorescence and phosphorescence occur when excited electrons release their energy as light energy, to return to their original energy state Can also release energy as heat {Remember that energy transformations increase entropy}

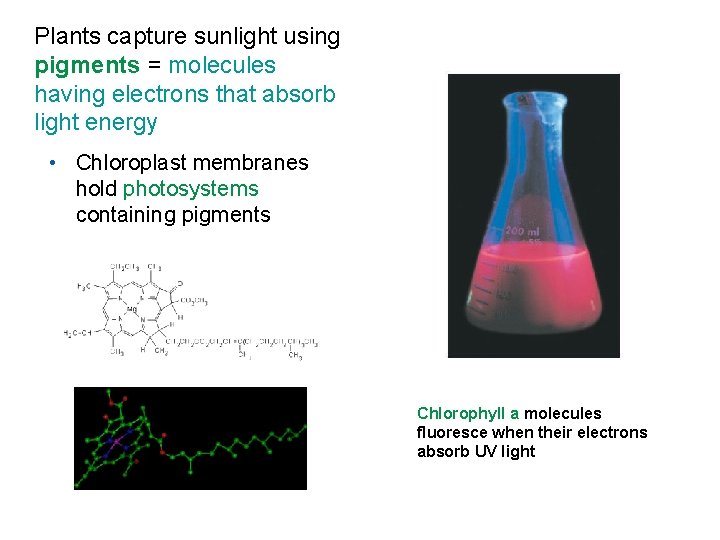
Plants capture sunlight using pigments = molecules having electrons that absorb light energy • Chloroplast membranes hold photosystems containing pigments Chlorophyll a molecules fluoresce when their electrons absorb UV light

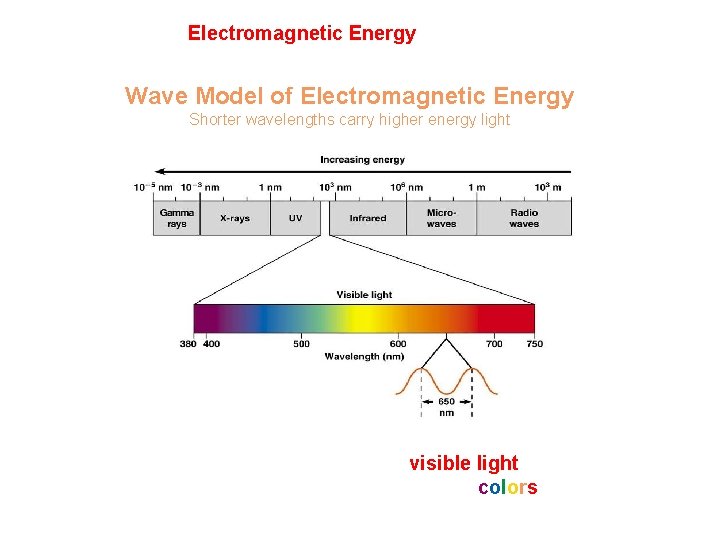
Light energy is Electromagnetic Energy Two models to describe behavior: Photon and Wave Model of Electromagnetic Energy Shorter wavelengths carry higher energy light Our eyes sense part of this energy as visible light Our brains interpret the different energies as colors

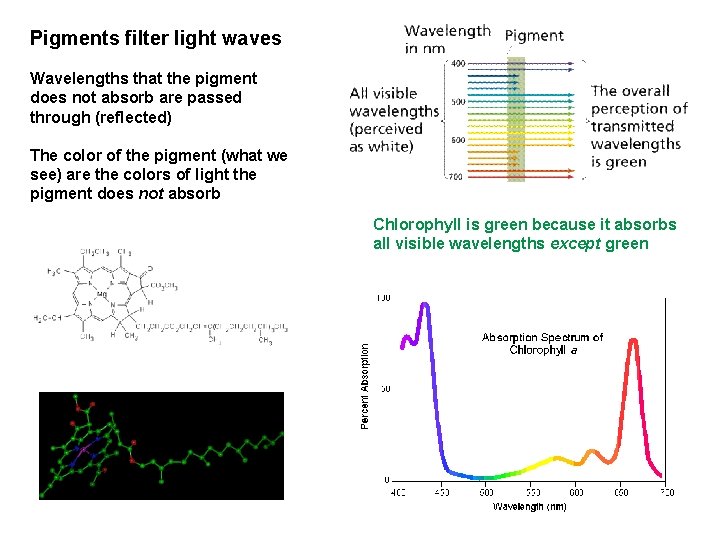
Pigments filter light waves Wavelengths that the pigment does not absorb are passed through (reflected) The color of the pigment (what we see) are the colors of light the pigment does not absorb Chlorophyll is green because it absorbs all visible wavelengths except green

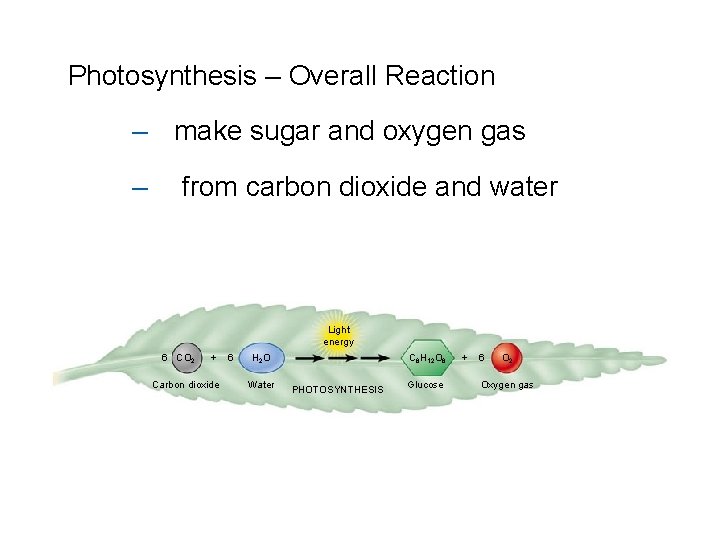
Photosynthesis – Overall Reaction – make sugar and oxygen gas – from carbon dioxide and water Light energy 6 CO 2 + Carbon dioxide 6 H 2 O Water C 6 H 12 O 6 PHOTOSYNTHESIS Glucose + 6 O 2 Oxygen gas

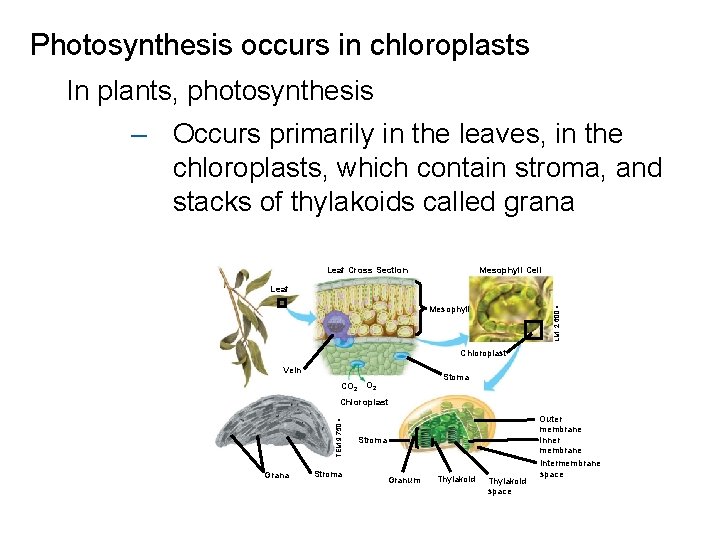
Photosynthesis occurs in chloroplasts In plants, photosynthesis – Occurs primarily in the leaves, in the chloroplasts, which contain stroma, and stacks of thylakoids called grana Mesophyll Cell Leaf Cross Section Leaf LM 2, 600 Mesophyll Chloroplast Vein Stoma CO 2 TEM 9, 750 Chloroplast Grana Stroma Granum Thylakoid space Outer membrane Inner membrane Intermembrane space

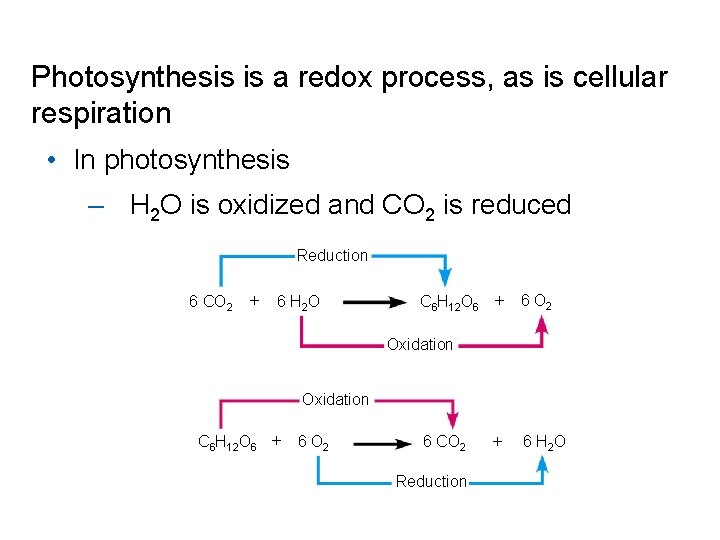
Photosynthesis is a redox process, as is cellular respiration • In photosynthesis – H 2 O is oxidized and CO 2 is reduced Reduction 6 CO 2 6 H 2 O C 6 H 12 O 6 6 O 2 6 H 2 O Oxidation C 6 H 12 O 6 6 O 2 6 CO 2 Reduction

Overview: Photosynthesis occurs in two stages linked by ATP and NADPH • The complete process of photosynthesis consists of two linked sets of reactions – The light reactions and – the Calvin cycle

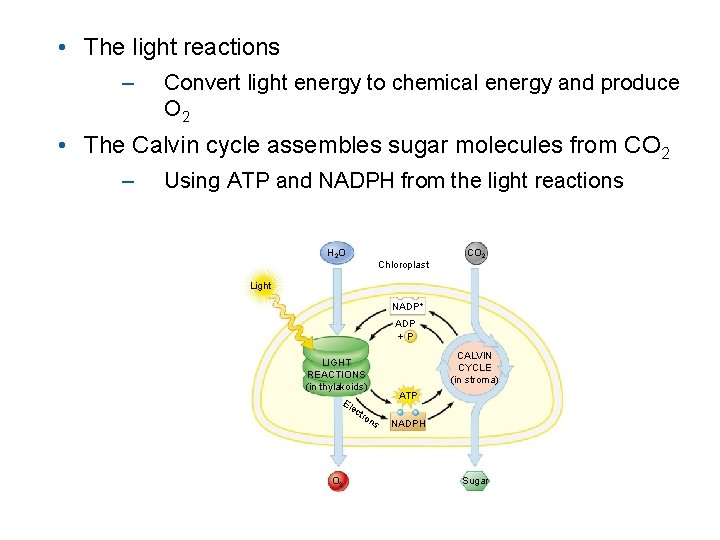
• The light reactions – Convert light energy to chemical energy and produce O 2 • The Calvin cycle assembles sugar molecules from CO 2 – Using ATP and NADPH from the light reactions H 2 O Chloroplast CO 2 Light NADP+ ADP +P CALVIN CYCLE (in stroma) LIGHT REACTIONS (in thylakoids) Ele ctr O 2 on ATP s NADPH Sugar

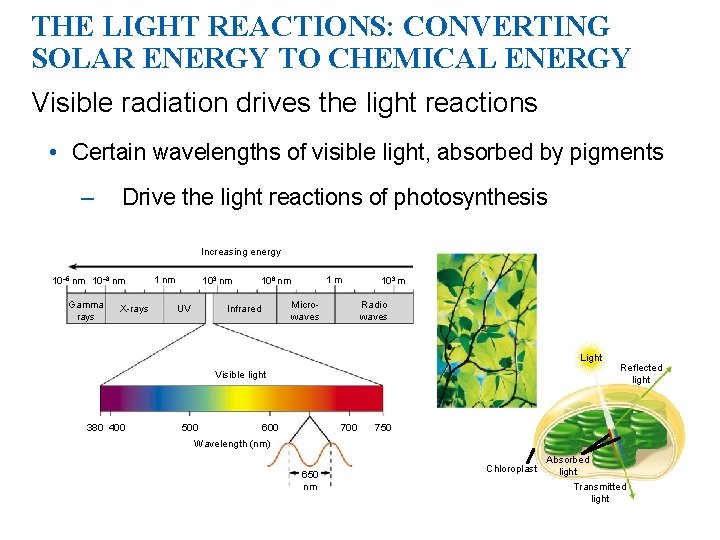
THE LIGHT REACTIONS: CONVERTING SOLAR ENERGY TO CHEMICAL ENERGY Visible radiation drives the light reactions • Certain wavelengths of visible light, absorbed by pigments – Drive the light reactions of photosynthesis Increasing energy 10– 5 nm 10– 3 nm Gamma rays X-rays 1 nm 103 nm UV 1 m 106 nm Microwaves Infrared 103 m Radio waves Light Visible light 380 400 500 600 700 Reflected light 750 Wavelength (nm) 650 nm Chloroplast Absorbed light Transmitted light

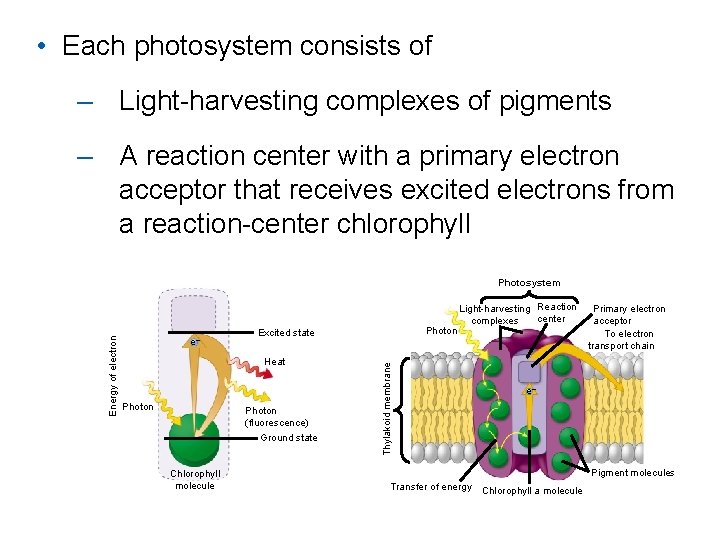
• Each photosystem consists of – Light-harvesting complexes of pigments – A reaction center with a primary electron acceptor that receives excited electrons from a reaction-center chlorophyll e– Heat Photon (fluorescence) Ground state Chlorophyll molecule Photon Excited state Thylakoid membrane Energy of electron Photosystem Light-harvesting Reaction center complexes Primary electron acceptor To electron transport chain e– Pigment molecules Transfer of energy Chlorophyll a molecule

In the light reactions, electron transport chains generate ATP and NADPH • Two connected photosystems absorb photons of light in pigments that transfer the energy to chlorophyll molecules in each of the photosystems {P 680 and P 700} • Photosystem regains electrons by removing electrons from water molecules, releasing O 2 gas

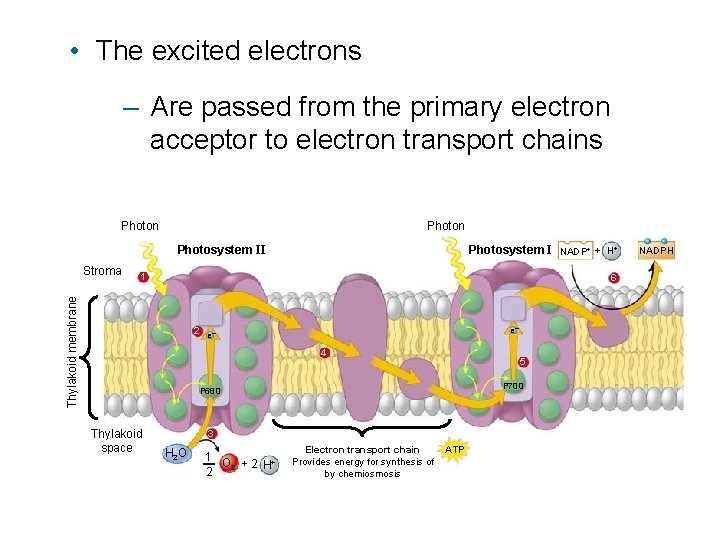
• The excited electrons – Are passed from the primary electron acceptor to electron transport chains Photon Photosystem II 1 6 Thylakoid membrane Stroma Photosystem I NADP+ + H+ e– 2 e– 4 5 P 700 P 680 Thylakoid space 3 H 2 O 1 O + 2 H 2 Electron transport chain Provides energy for synthesis of by chemiosmosis ATP NADPH

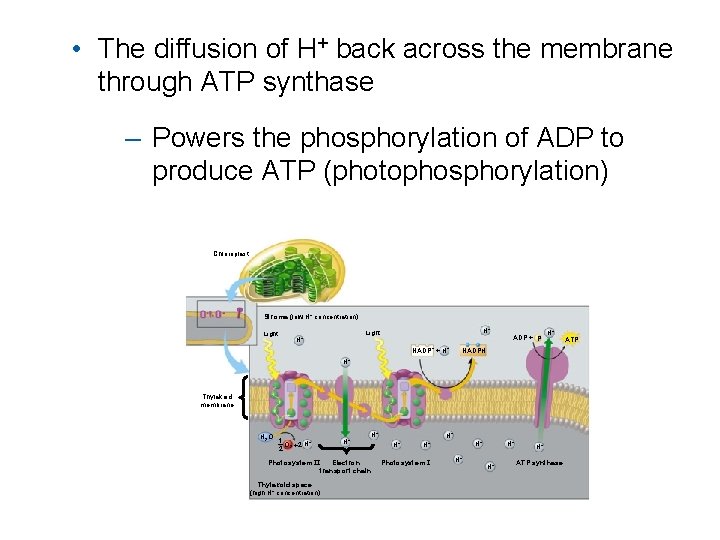
Chemiosmosis powers ATP synthesis in the light reactions • The electron transport chain – Pumps H+ into the thylakoid space

• The diffusion of H+ back across the membrane through ATP synthase – Powers the phosphorylation of ADP to produce ATP (photophosphorylation) Chloroplast Stroma (low H+ concentration) Light H+ NADP+ + H+ ADP + P H+ NADPH H+ Thylakoid membrane H 2 O 1 O +2 H+ 2 2 Photosystem II H+ H+ Electron transport chain Thylakoid space (high H+ concentration) H+ H+ Photosystem I H+ H+ ATP synthase ATP

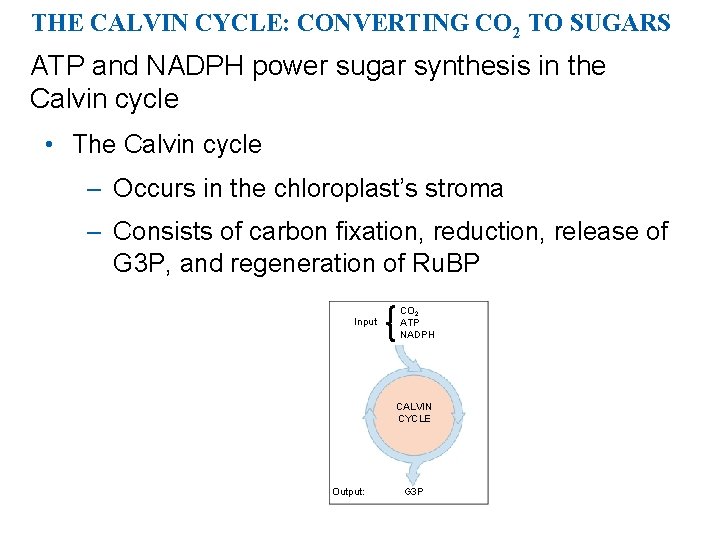
THE CALVIN CYCLE: CONVERTING CO 2 TO SUGARS ATP and NADPH power sugar synthesis in the Calvin cycle • The Calvin cycle – Occurs in the chloroplast’s stroma – Consists of carbon fixation, reduction, release of G 3 P, and regeneration of Ru. BP Input CO 2 ATP NADPH CALVIN CYCLE Output: G 3 P

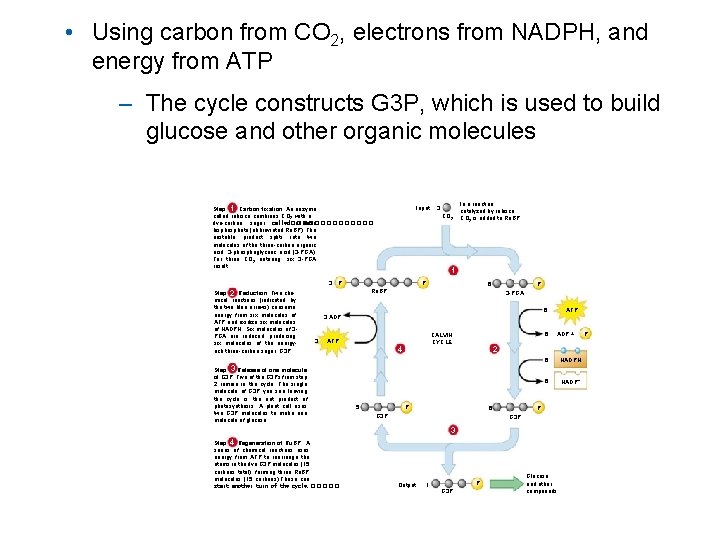
• Using carbon from CO 2, electrons from NADPH, and energy from ATP – The cycle constructs G 3 P, which is used to build glucose and other organic molecules Step 1 Carbon fixation. An enzyme called rubisco combines CO 2 with a five-carbon sugar called������� ribulose bisphosphate (abbreviated Ru. BP). The unstable product splits into two molecules of the three-carbon organic acid, 3 -phosphoglyceric acid (3 -PGA). For three CO 2 entering, six 3 -PGA result. 3 Step 2 Reduction. Two chemical reactions (indicated by the two blue arrows) consume energy from six molecules of ATP and oxidize six molecules of NADPH. Six molecules of 3 PGA are reduced, producing six molecules of the energyrich three-carbon sugar, G 3 P Input: 3 CO 2 In a reaction catalyzed by rubisco, CO 2 is added to Ru. BP. 1 P P P 6 Ru. BP 3 -PGA 6 ATP 3 ADP 3 ATP Step 3 Release of one molecule of G 3 P. Five of the G 3 Ps from step 2 remain in the cycle. The single molecule of G 3 P you see leaving the cycle is the net product of photosynthesis. A plant cell uses two G 3 P molecules to make one molecule of glucose. 4 5 6 CALVIN CYCLE ADP + 2 P 6 G 3 P 6 NADPH 6 NADP+ P G 3 P 3 Step 4 Regeneration of Ru. BP. A series of chemical reactions uses energy from ATP to rearrange the atoms in the five G 3 P molecules (15 carbons total), forming three Ru. BP molecules (15 carbons). These can start another turn of the cycle. ����� Output: 1 P G 3 P Glucose and other compounds P

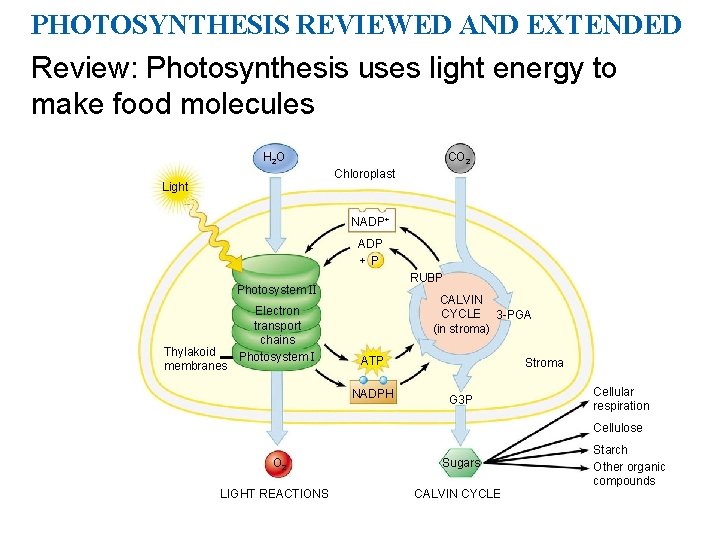
PHOTOSYNTHESIS REVIEWED AND EXTENDED Review: Photosynthesis uses light energy to make food molecules H 2 O CO 2 Chloroplast Light NADP+ ADP +P RUBP Photosystem II Thylakoid membranes Electron transport chains Photosystem I CALVIN CYCLE 3 -PGA (in stroma) ATP NADPH Stroma G 3 P Cellular respiration Cellulose O 2 LIGHT REACTIONS Sugars CALVIN CYCLE Starch Other organic compounds

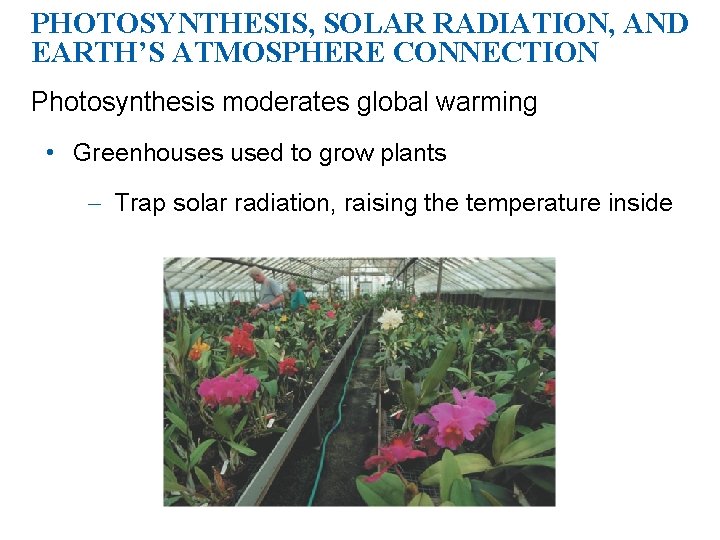
PHOTOSYNTHESIS, SOLAR RADIATION, AND EARTH’S ATMOSPHERE CONNECTION Photosynthesis moderates global warming • Greenhouses used to grow plants – Trap solar radiation, raising the temperature inside

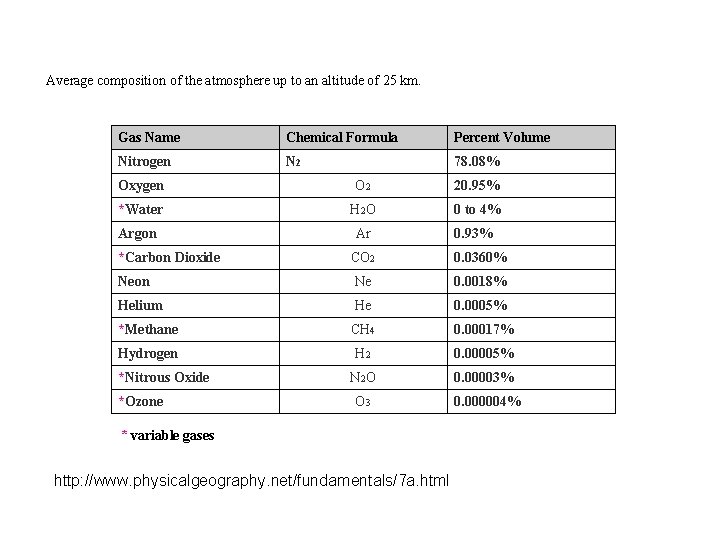
Average composition of the atmosphere up to an altitude of 25 km. Gas Name Chemical Formula Percent Volume Nitrogen N 2 78. 08% Oxygen O 2 20. 95% *Water H 2 O 0 to 4% Argon Ar 0. 93% *Carbon Dioxide CO 2 0. 0360% Neon Ne 0. 0018% Helium He 0. 0005% *Methane CH 4 0. 00017% Hydrogen H 2 0. 00005% N 2 O 0. 00003% O 3 0. 000004% *Nitrous Oxide *Ozone * variable gases http: //www. physicalgeography. net/fundamentals/7 a. html

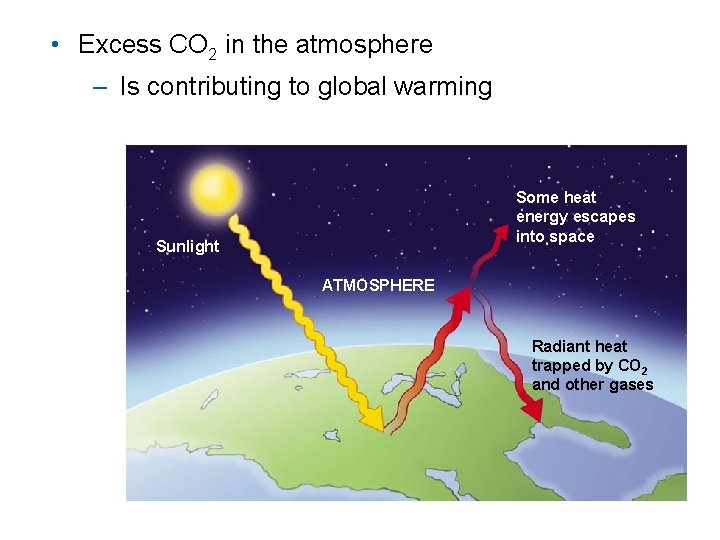
• Excess CO 2 in the atmosphere – Is contributing to global warming Some heat energy escapes into space Sunlight ATMOSPHERE Radiant heat trapped by CO 2 and other gases