An overview of carbonyl group chemistry CARBONYL GROUP

An overview of carbonyl group chemistry
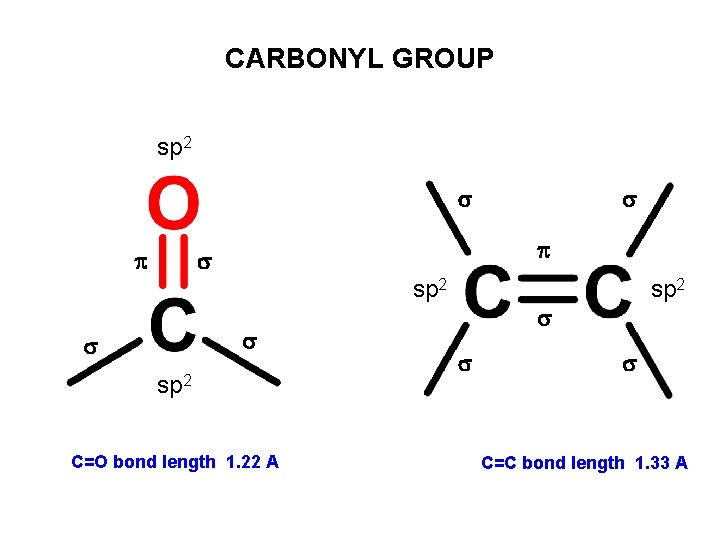
CARBONYL GROUP sp 2 C=O bond length 1. 22 A sp 2 C=C bond length 1. 33 A
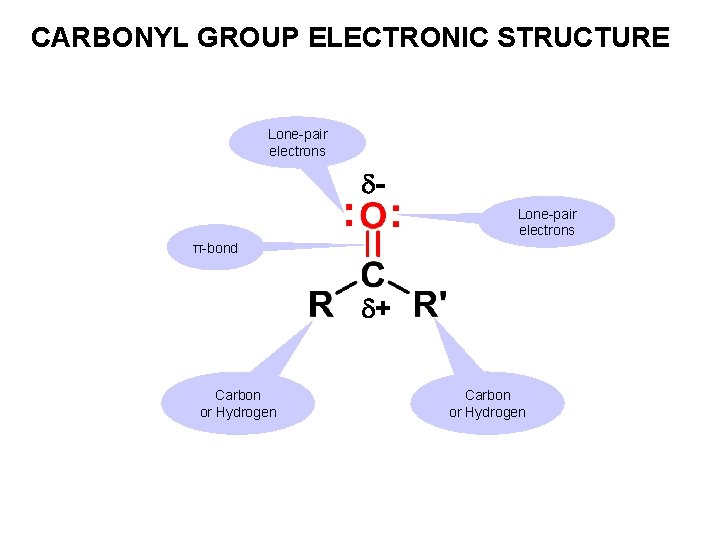
CARBONYL GROUP ELECTRONIC STRUCTURE Lone-pair electrons π-bond + Carbon or Hydrogen
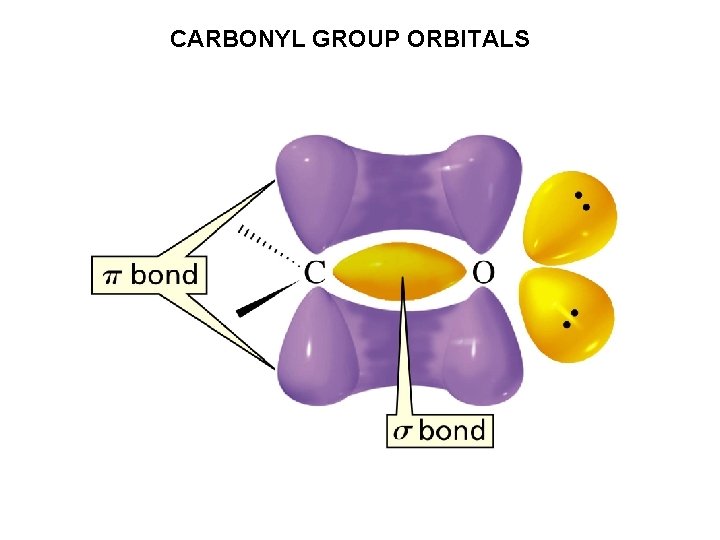
CARBONYL GROUP ORBITALS
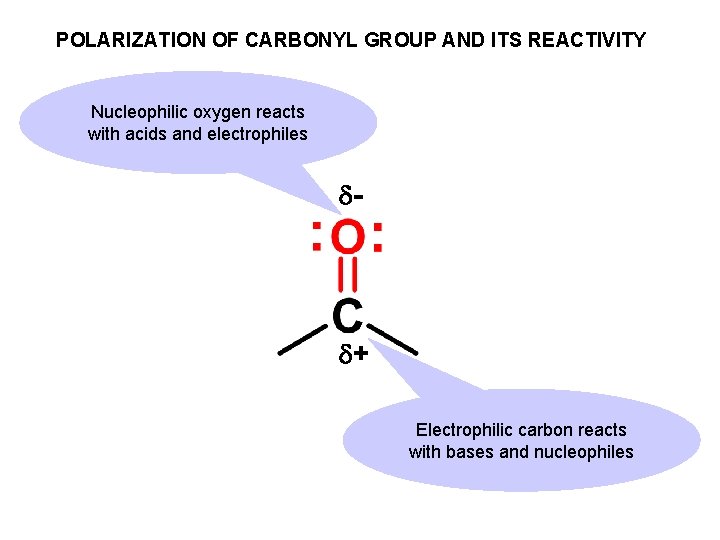
POLARIZATION OF CARBONYL GROUP AND ITS REACTIVITY Nucleophilic oxygen reacts with acids and electrophiles - + Electrophilic carbon reacts with bases and nucleophiles
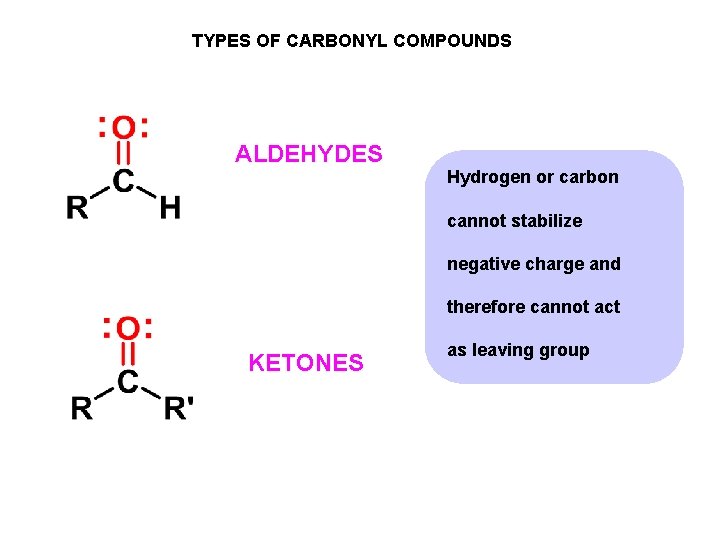
TYPES OF CARBONYL COMPOUNDS ALDEHYDES Hydrogen or carbon cannot stabilize negative charge and therefore cannot act KETONES as leaving group
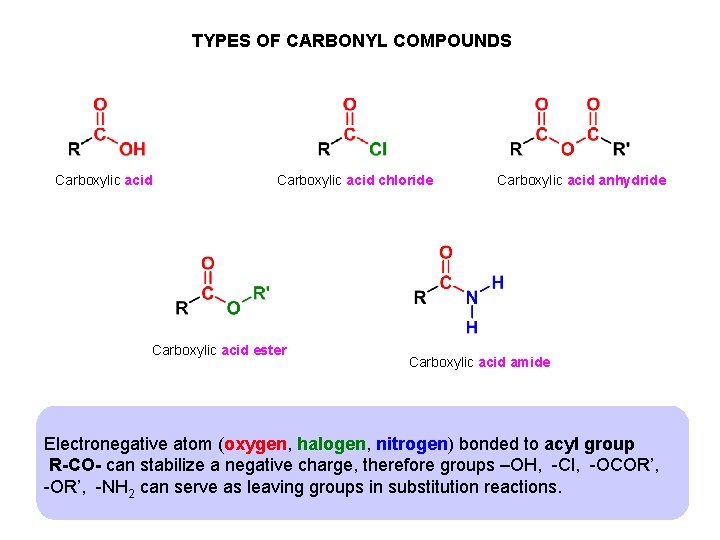
TYPES OF CARBONYL COMPOUNDS Carboxylic acid chloride Carboxylic acid ester Carboxylic acid anhydride Carboxylic acid amide Electronegative atom (oxygen, halogen, nitrogen) bonded to acyl group R-CO- can stabilize a negative charge, therefore groups –OH, -Cl, -OCOR’, -NH 2 can serve as leaving groups in substitution reactions.
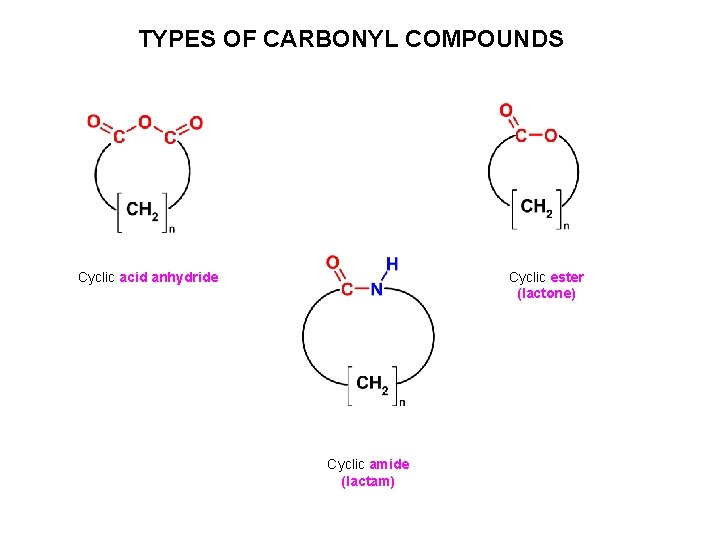
TYPES OF CARBONYL COMPOUNDS Cyclic acid anhydride Cyclic ester (lactone) Cyclic amide (lactam)
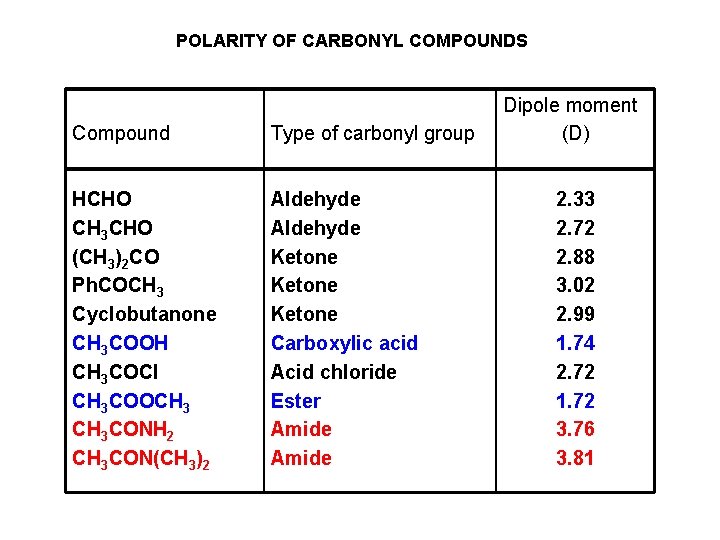
POLARITY OF CARBONYL COMPOUNDS Compound Type of carbonyl group HCHO CH 3 CHO (CH 3)2 CO Ph. COCH 3 Cyclobutanone CH 3 COOH CH 3 COCl CH 3 COOCH 3 CONH 2 CH 3 CON(CH 3)2 Aldehyde Ketone Carboxylic acid Acid chloride Ester Amide Dipole moment (D) 2. 33 2. 72 2. 88 3. 02 2. 99 1. 74 2. 72 1. 72 3. 76 3. 81
TYPES OF CARBONYL COMPOUNDS REACTIONS GENERAL MECHANISMS • Nucleophilic addition • Nucleophilic acyl substitution • -Substitution • Condensation reactions

Nucleophilic addition to carbonyl group 1 st step Carbonyl carbon rehybridizes from sp 2 to sp 3
Nucleophilic addition to carbonyl group 2 nd step Two ways of stabilization of tetrahedral intermediate
Nucleophilic addition to carbonyl group EXAMPLE 1 – addition of methylmagnesium bromide to cyclohexanone Synthesis of tertiary alcohol from ketone
Nucleophilic addition to carbonyl group EXAMPLE 2 – cyclohexanone imine formation Net effect – replacing C=O by C=NR
Nucleophilic acyl substitution Net effect – replacing of group Y with nuclephile. -Y is called leaving group Y = -OR (ester) -OCOR (anhydride) -Cl (acid chloride) -NH 2, -NHR, -NR 2 (amide)
Nucleophilic acyl substitution EXAMPLE – ester synthesis from acid chloride and alkoxide Net effect – replacing of Cl by OR
-Substitution reactions -carbon -substituted carbonyl compound Intermediates
-Substitution reactions Net effect – extending of carbonyl compound hydrocarbon framework by –CH 2 R fragment at -carbon
-Substitution reactions EXAMPLE – -methylation of cyclohexanone
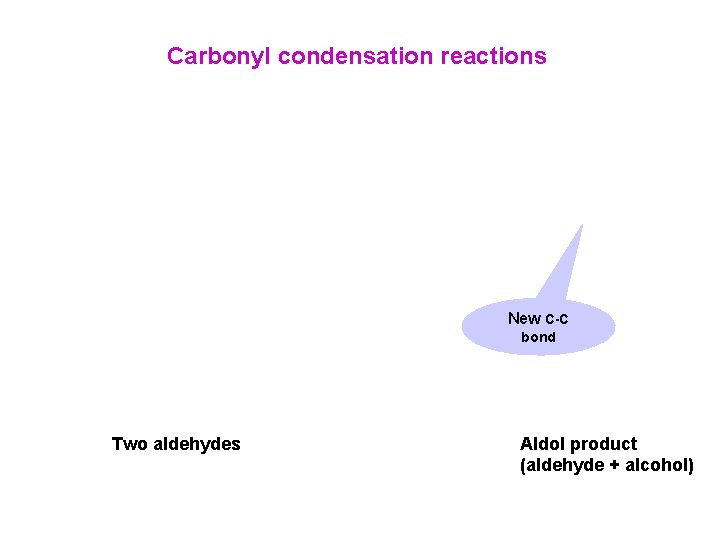
Carbonyl condensation reactions New C-C bond Two aldehydes Aldol product (aldehyde + alcohol)
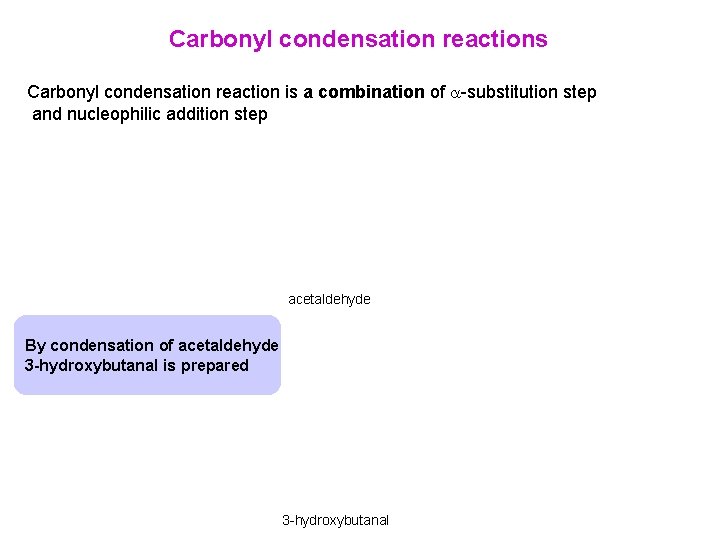
Carbonyl condensation reactions Carbonyl condensation reaction is a combination of -substitution step and nucleophilic addition step acetaldehyde By condensation of acetaldehyde 3 -hydroxybutanal is prepared 3 -hydroxybutanal
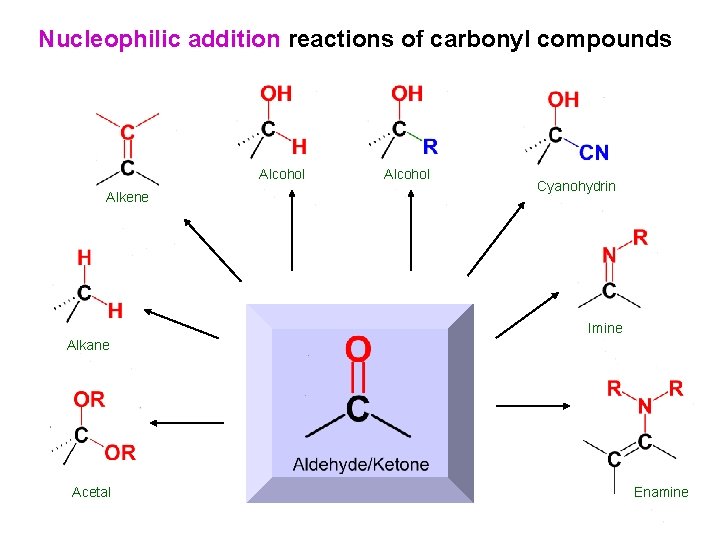
Nucleophilic addition reactions of carbonyl compounds Alcohol Alkene Alcohol Cyanohydrin Imine Alkane Acetal Enamine
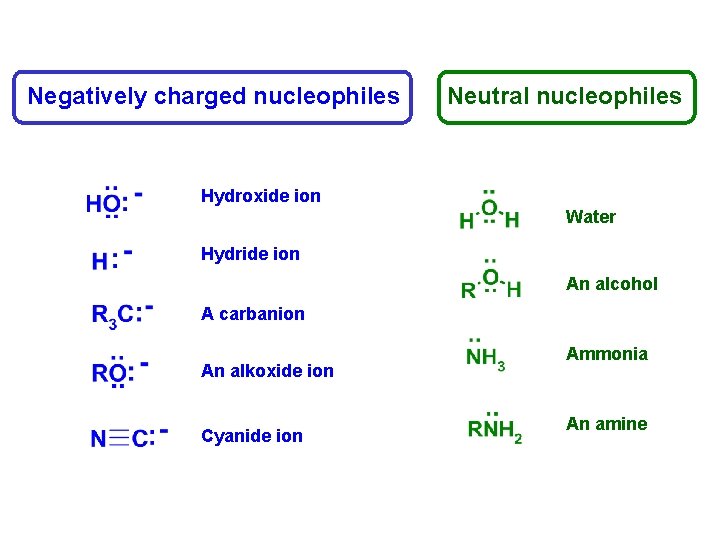
Negatively charged nucleophiles Neutral nucleophiles Hydroxide ion Water Hydride ion An alcohol A carbanion An alkoxide ion Cyanide ion Ammonia An amine
- Slides: 23