An Introduction to Transition Metals complexes Written by

An Introduction to Transition Metals - complexes Written by A Bourne (MChem), 2014

Aims of this Lesson • Increase your understanding of transition metal properties and reactions, particularly complex ion formation • Increase your spatial skills and awareness using software designed by the CCDC
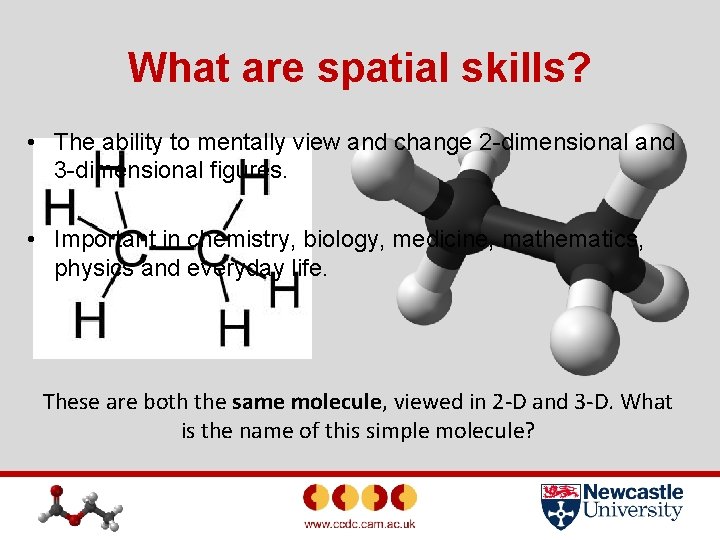
What are spatial skills? • The ability to mentally view and change 2 -dimensional and 3 -dimensional figures. • Important in chemistry, biology, medicine, mathematics, physics and everyday life. These are both the same molecule, viewed in 2 -D and 3 -D. What is the name of this simple molecule?
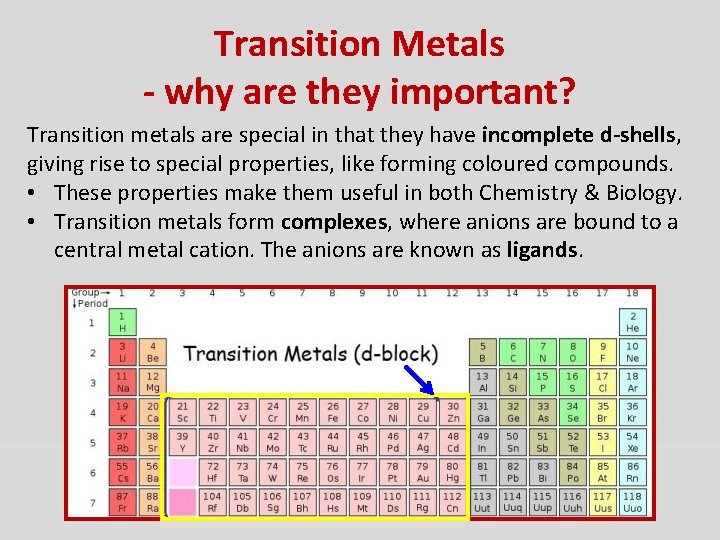
Transition Metals - why are they important? Transition metals are special in that they have incomplete d-shells, giving rise to special properties, like forming coloured compounds. • These properties make them useful in both Chemistry & Biology. • Transition metals form complexes, where anions are bound to a central metal cation. The anions are known as ligands.

Transition Metals - why are they important? In transition metal complexes, the ligands attach by co-ordinate bonding, also known as dative covalent bonding. Here, both electrons come from a lone pair of electrons on the ligand donated to an empty orbital on the metal ion.
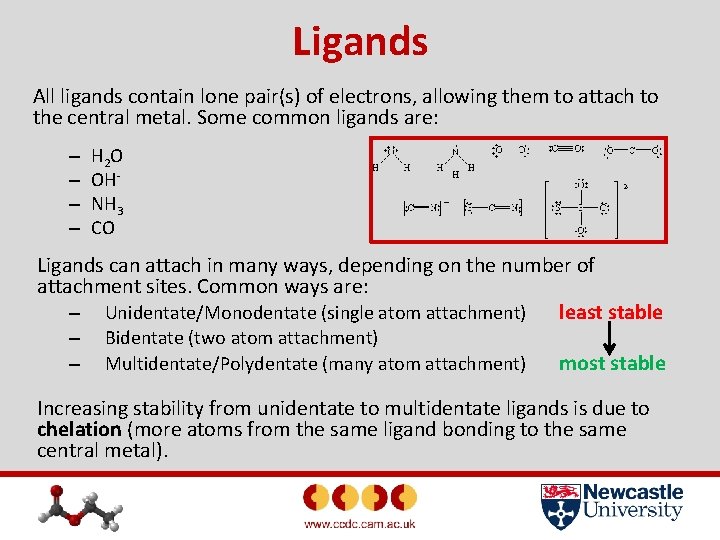
Ligands All ligands contain lone pair(s) of electrons, allowing them to attach to the central metal. Some common ligands are: – – H 2 O OHNH 3 CO Ligands can attach in many ways, depending on the number of attachment sites. Common ways are: – Unidentate/Monodentate (single atom attachment) least stable – – Bidentate (two atom attachment) Multidentate/Polydentate (many atom attachment) most stable Increasing stability from unidentate to multidentate ligands is due to chelation (more atoms from the same ligand bonding to the same central metal).
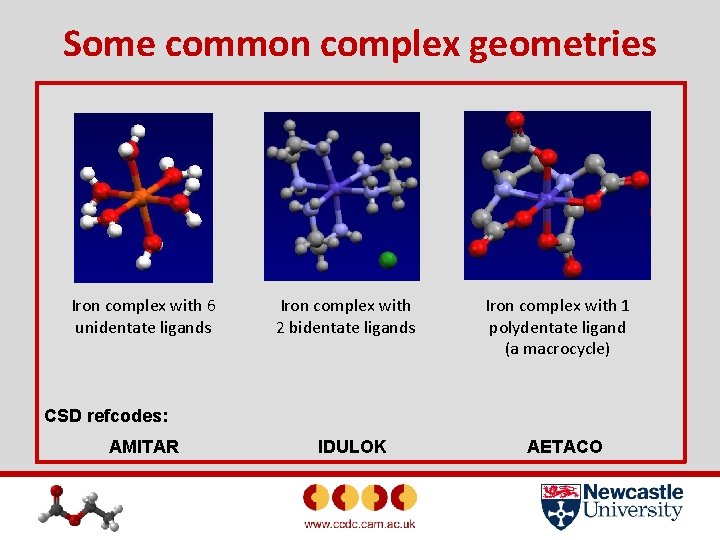
Some common complex geometries Iron complex with 6 unidentate ligands Iron complex with 2 bidentate ligands Iron complex with 1 polydentate ligand (a macrocycle) CSD refcodes: AMITAR IDULOK AETACO
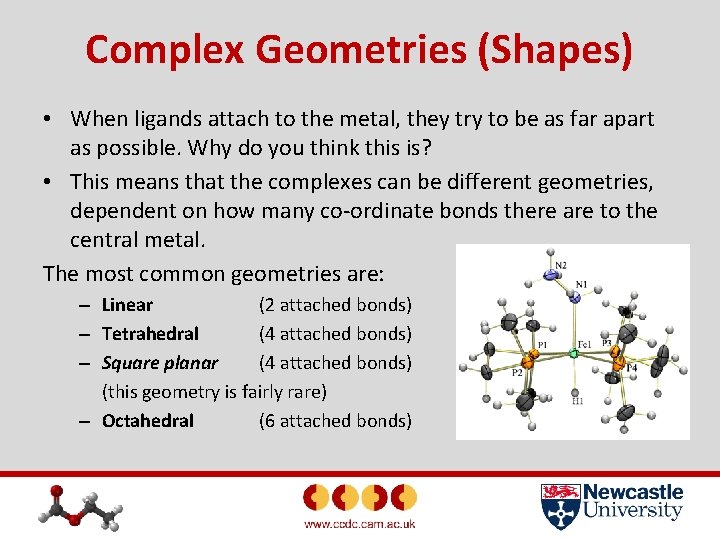
Complex Geometries (Shapes) • When ligands attach to the metal, they try to be as far apart as possible. Why do you think this is? • This means that the complexes can be different geometries, dependent on how many co-ordinate bonds there are to the central metal. The most common geometries are: – Linear (2 attached bonds) – Tetrahedral (4 attached bonds) – Square planar (4 attached bonds) (this geometry is fairly rare) – Octahedral (6 attached bonds)
Complex Geometries (Shapes) The coordination number of ligands (and hence the geometry) depends on three things: – Electronic factors (such as metal electron configuration, ligand charge & type, etc) – Metal ion size – Ligand size
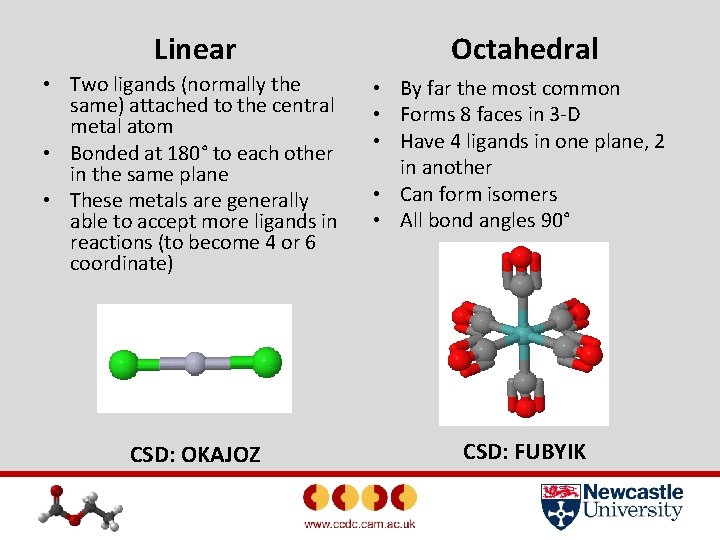
Linear Octahedral • Two ligands (normally the same) attached to the central metal atom • Bonded at 180° to each other in the same plane • These metals are generally able to accept more ligands in reactions (to become 4 or 6 coordinate) • By far the most common • Forms 8 faces in 3 -D • Have 4 ligands in one plane, 2 in another • Can form isomers • All bond angles 90° CSD: OKAJOZ CSD: FUBYIK
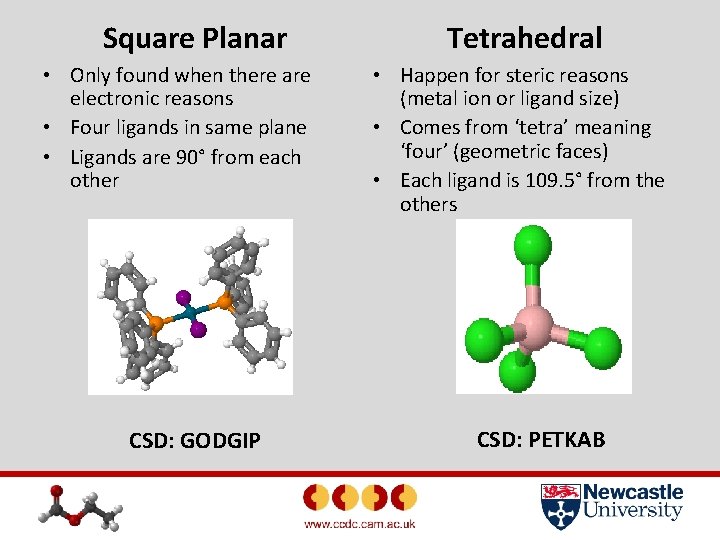
Square Planar • Only found when there are electronic reasons • Four ligands in same plane • Ligands are 90° from each other CSD: GODGIP Tetrahedral • Happen for steric reasons (metal ion or ligand size) • Comes from ‘tetra’ meaning ‘four’ (geometric faces) • Each ligand is 109. 5° from the others CSD: PETKAB
Isomerisation in Complexes Can form three types of isomers. First two are just like those found in organic compounds. These are: – Geometric – Optical – Meridional/Facial (cis/trans or E/Z) (enantiomers) (mer/fac)
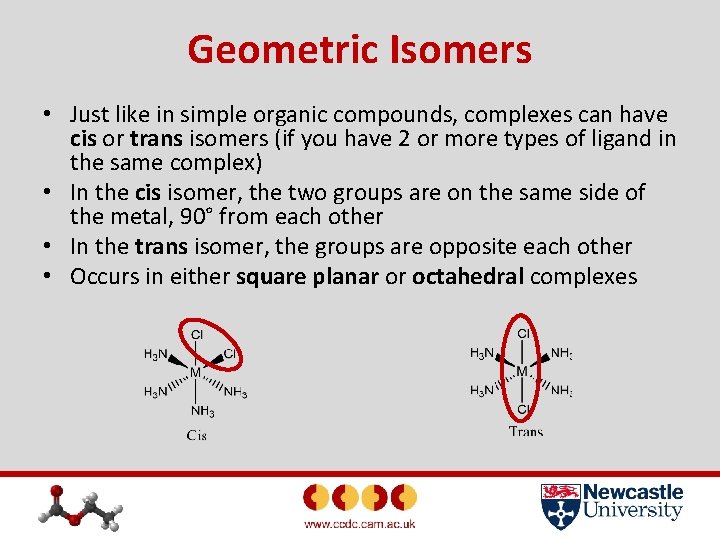
Geometric Isomers • Just like in simple organic compounds, complexes can have cis or trans isomers (if you have 2 or more types of ligand in the same complex) • In the cis isomer, the two groups are on the same side of the metal, 90° from each other • In the trans isomer, the groups are opposite each other • Occurs in either square planar or octahedral complexes
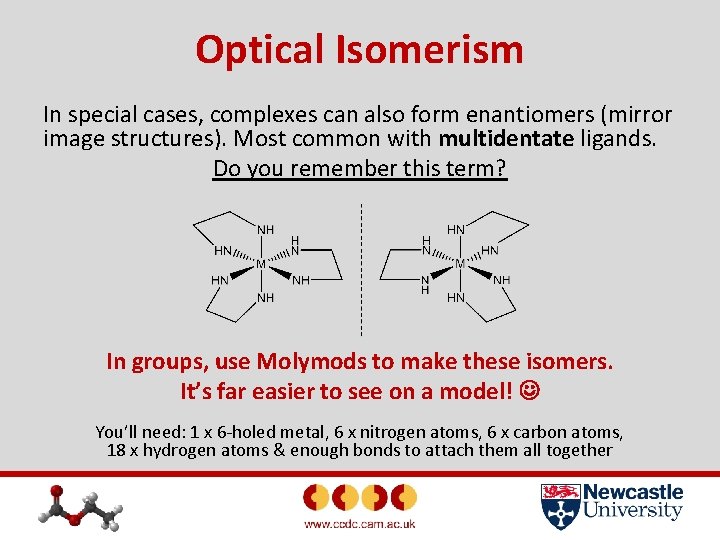
Optical Isomerism In special cases, complexes can also form enantiomers (mirror image structures). Most common with multidentate ligands. Do you remember this term? In groups, use Molymods to make these isomers. It’s far easier to see on a model! You’ll need: 1 x 6 -holed metal, 6 x nitrogen atoms, 6 x carbon atoms, 18 x hydrogen atoms & enough bonds to attach them all together
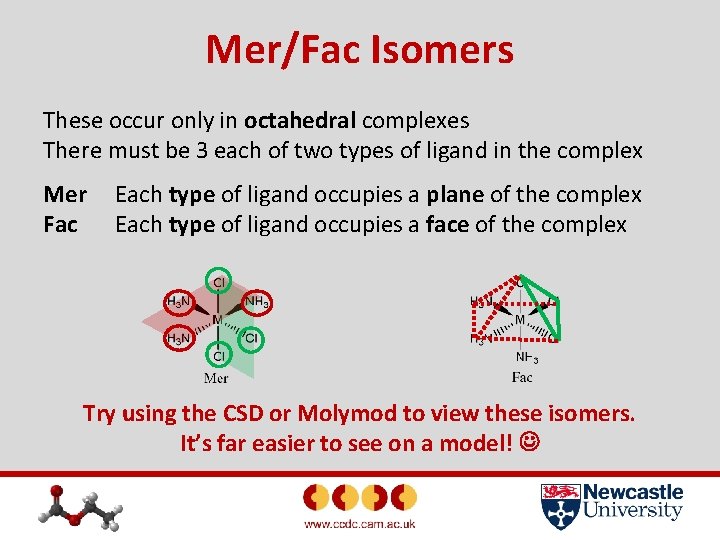
Mer/Fac Isomers These occur only in octahedral complexes There must be 3 each of two types of ligand in the complex Mer Fac Each type of ligand occupies a plane of the complex Each type of ligand occupies a face of the complex Try using the CSD or Molymod to view these isomers. It’s far easier to see on a model!

Re-cap • Transition metals contain partially filled d-orbitals. • They generally form coloured complexes. • Ligands are species with (at least) one lone pair of electrons that attach to the central metal ion via co -ordinate (dative covalent) bonding. • TM complexes can sometimes form isomers, just like organic compounds. Next: Macrocycles
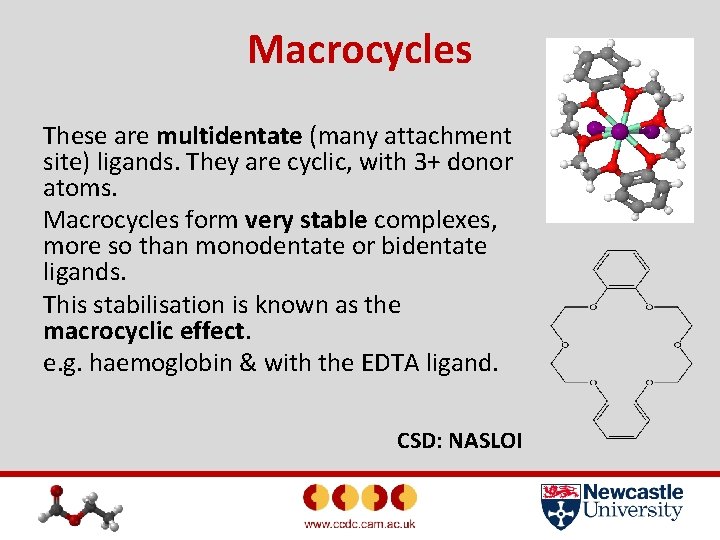
Macrocycles These are multidentate (many attachment site) ligands. They are cyclic, with 3+ donor atoms. Macrocycles form very stable complexes, more so than monodentate or bidentate ligands. This stabilisation is known as the macrocyclic effect. e. g. haemoglobin & with the EDTA ligand. CSD: NASLOI
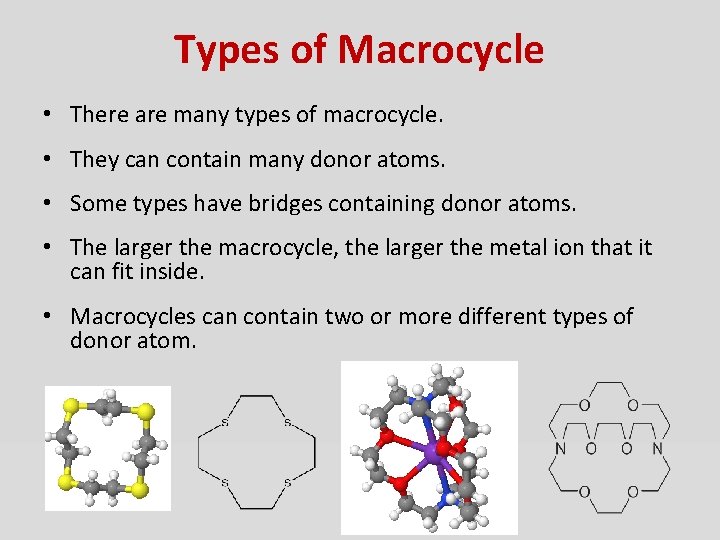
Types of Macrocycle • There are many types of macrocycle. • They can contain many donor atoms. • Some types have bridges containing donor atoms. • The larger the macrocycle, the larger the metal ion that it can fit inside. • Macrocycles can contain two or more different types of donor atom.
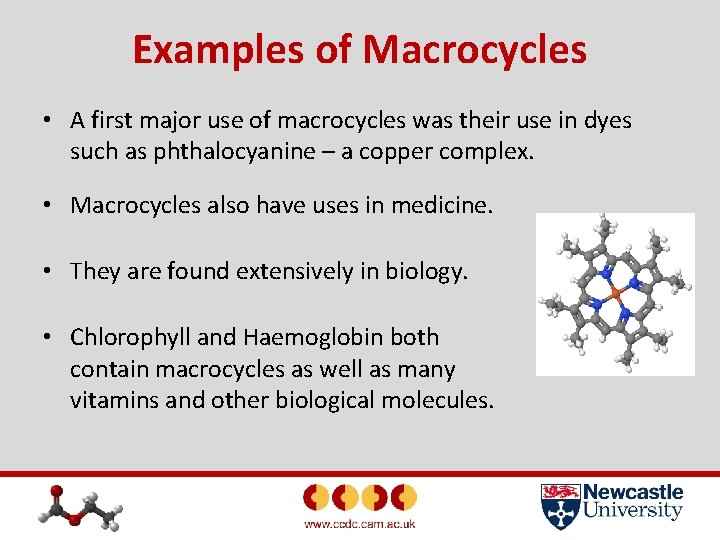
Examples of Macrocycles • A first major use of macrocycles was their use in dyes such as phthalocyanine – a copper complex. • Macrocycles also have uses in medicine. • They are found extensively in biology. • Chlorophyll and Haemoglobin both contain macrocycles as well as many vitamins and other biological molecules.
- Slides: 19