AN INTRODUCTION TO SYNTHETIC ORGANIC CHEMISTRY KNOCKHARDY PUBLISHING

AN INTRODUCTION TO SYNTHETIC ORGANIC CHEMISTRY KNOCKHARDY PUBLISHING
KNOCKHARDY PUBLISHING ORGANIC REACTION SEQUENCES INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A 2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A 2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at. . . www. argonet. co. uk/users/hoptonj/sci. htm Navigation is achieved by. . . either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard CONVERSIONS
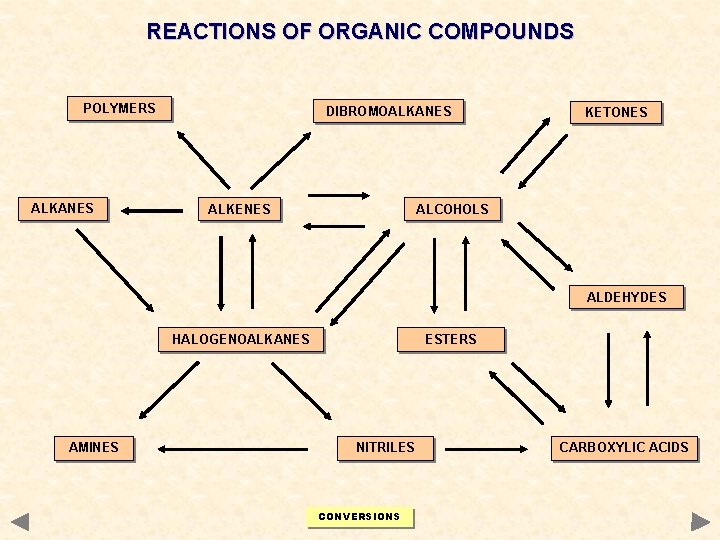
REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKANES DIBROMOALKANES ALKENES KETONES ALCOHOLS ALDEHYDES HALOGENOALKANES AMINES ESTERS NITRILES CONVERSIONS CARBOXYLIC ACIDS
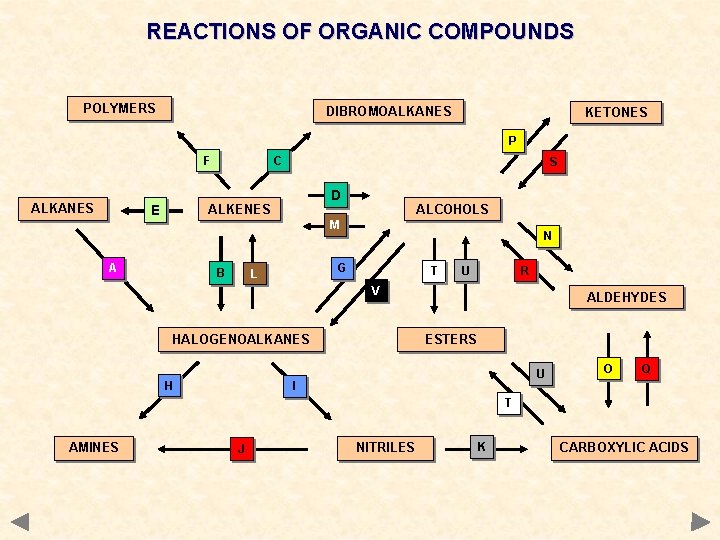
REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES KETONES P F ALKANES C S D ALKENES E ALCOHOLS M A B N G L T U R V HALOGENOALKANES H ALDEHYDES ESTERS U I O Q T AMINES J NITRILES K CARBOXYLIC ACIDS
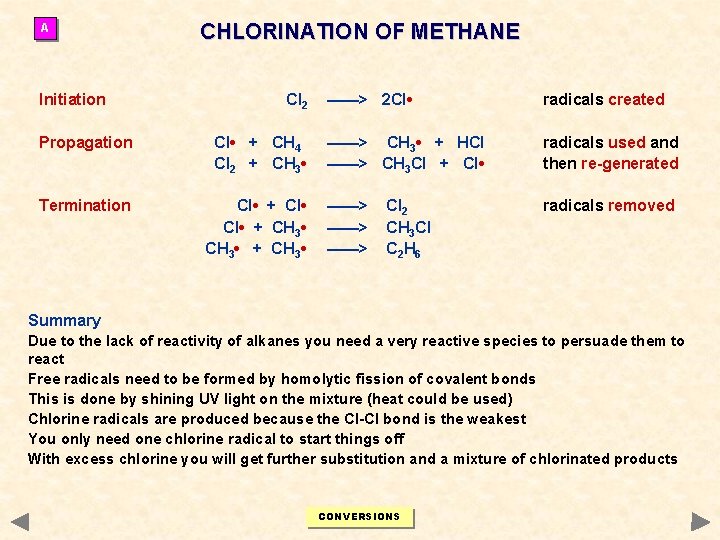
A Initiation CHLORINATION OF METHANE Cl 2 Propagation Cl • + CH 4 Cl 2 + CH 3 • Termination Cl • + CH 3 • + CH 3 • ——> 2 Cl • radicals created ——> CH 3 • + HCl ——> CH 3 Cl + Cl • radicals used and then re-generated ——> ——> radicals removed Cl 2 CH 3 Cl C 2 H 6 Summary Due to the lack of reactivity of alkanes you need a very reactive species to persuade them to react Free radicals need to be formed by homolytic fission of covalent bonds This is done by shining UV light on the mixture (heat could be used) Chlorine radicals are produced because the Cl-Cl bond is the weakest You only need one chlorine radical to start things off With excess chlorine you will get further substitution and a mixture of chlorinated products CONVERSIONS
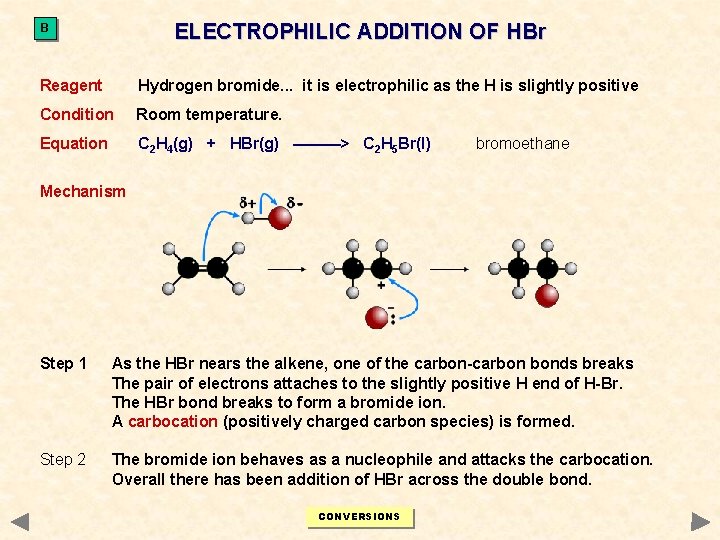
ELECTROPHILIC ADDITION OF HBr B Reagent Hydrogen bromide. . . it is electrophilic as the H is slightly positive Condition Room temperature. Equation C 2 H 4(g) + HBr(g) ———> C 2 H 5 Br(l) bromoethane Mechanism Step 1 As the HBr nears the alkene, one of the carbon-carbon bonds breaks The pair of electrons attaches to the slightly positive H end of H-Br. The HBr bond breaks to form a bromide ion. A carbocation (positively charged carbon species) is formed. Step 2 The bromide ion behaves as a nucleophile and attacks the carbocation. Overall there has been addition of HBr across the double bond. CONVERSIONS
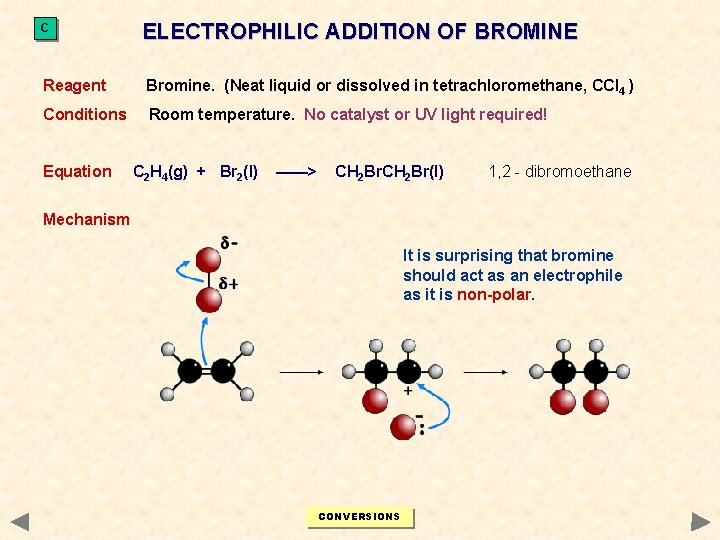
C ELECTROPHILIC ADDITION OF BROMINE Reagent Bromine. (Neat liquid or dissolved in tetrachloromethane, CCl 4 ) Conditions Room temperature. No catalyst or UV light required! Equation C 2 H 4(g) + Br 2(l) ——> CH 2 Br(l) 1, 2 - dibromoethane Mechanism It is surprising that bromine should act as an electrophile as it is non-polar. CONVERSIONS

D Reagent DIRECT HYDRATION OF ALKENES steam Conditions high pressure Catalyst phosphoric acid Product alcohol Equation C 2 H 4(g) + Use ethanol manufacture Comments It may be surprising that water needs such vigorous conditions to react with ethene. It is a highly polar molecule and you would expect it to be a good electrophile. H 2 O(g) C 2 H 5 OH(g) ethanol However, the O-H bonds are very strong so require a great deal of energy to be broken. This necessitates the need for a catalyst. CONVERSIONS

HYDROGENATION E Reagent Conditions hydrogen nickel catalyst - finely divided Product alkanes Equation C 2 H 4(g) + H 2(g) Use margarine manufacture ———> C 2 H 6(g) CONVERSIONS ethane
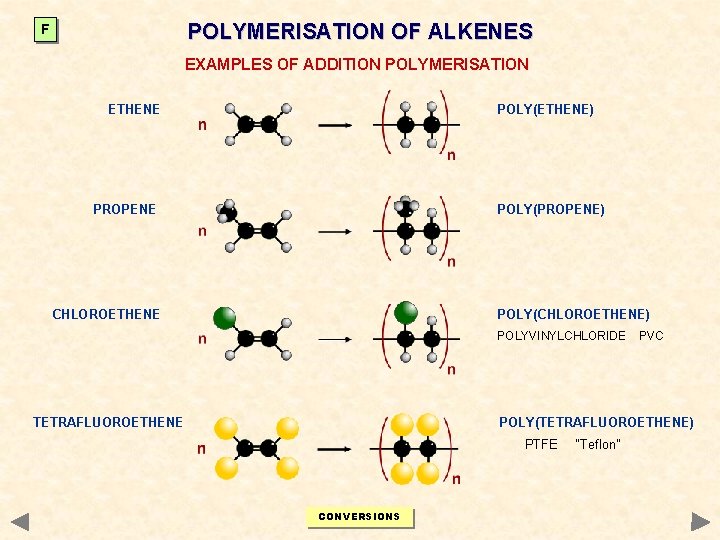
POLYMERISATION OF ALKENES F EXAMPLES OF ADDITION POLYMERISATION ETHENE POLY(ETHENE) PROPENE POLY(PROPENE) CHLOROETHENE POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE CONVERSIONS “Teflon”
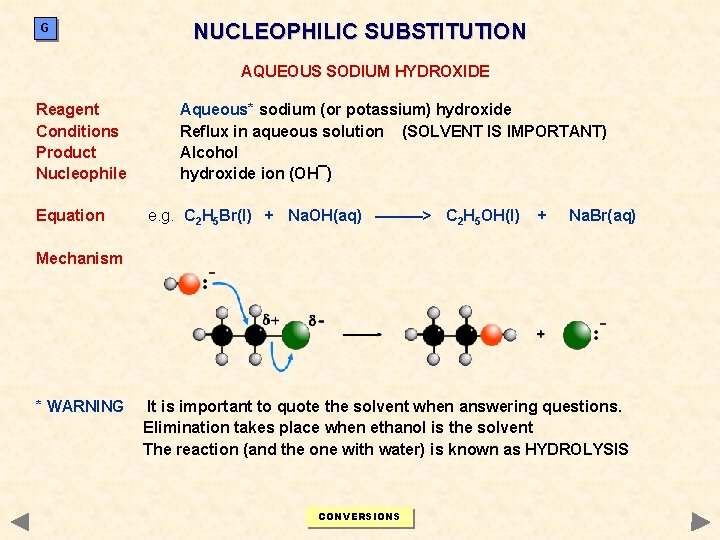
G NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE Reagent Conditions Product Nucleophile Equation Aqueous* sodium (or potassium) hydroxide Reflux in aqueous solution (SOLVENT IS IMPORTANT) Alcohol hydroxide ion (OH¯) e. g. C 2 H 5 Br(l) + Na. OH(aq) ———> C 2 H 5 OH(l) + Na. Br(aq) Mechanism * WARNING It is important to quote the solvent when answering questions. Elimination takes place when ethanol is the solvent The reaction (and the one with water) is known as HYDROLYSIS CONVERSIONS
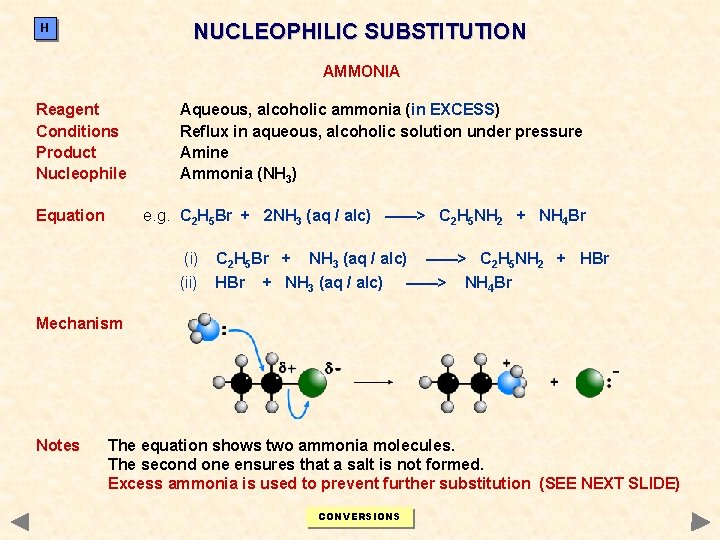
NUCLEOPHILIC SUBSTITUTION H AMMONIA Reagent Conditions Product Nucleophile Equation Aqueous, alcoholic ammonia (in EXCESS) Reflux in aqueous, alcoholic solution under pressure Amine Ammonia (NH 3) e. g. C 2 H 5 Br + 2 NH 3 (aq / alc) ——> C 2 H 5 NH 2 + NH 4 Br (i) C 2 H 5 Br + (ii) HBr NH 3 (aq / alc) + NH 3 (aq / alc) ——> C 2 H 5 NH 2 + HBr ——> NH 4 Br Mechanism Notes The equation shows two ammonia molecules. The second one ensures that a salt is not formed. Excess ammonia is used to prevent further substitution (SEE NEXT SLIDE) CONVERSIONS
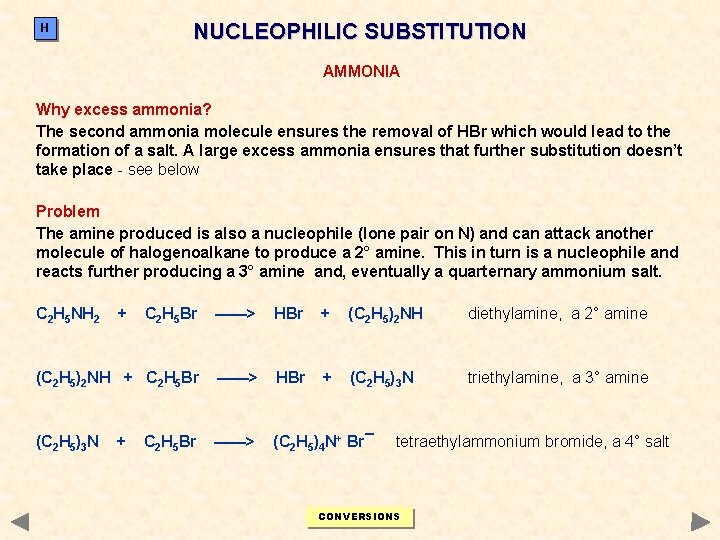
NUCLEOPHILIC SUBSTITUTION H AMMONIA Why excess ammonia? The second ammonia molecule ensures the removal of HBr which would lead to the formation of a salt. A large excess ammonia ensures that further substitution doesn’t take place - see below Problem The amine produced is also a nucleophile (lone pair on N) and can attack another molecule of halogenoalkane to produce a 2° amine. This in turn is a nucleophile and reacts further producing a 3° amine and, eventually a quarternary ammonium salt. C 2 H 5 NH 2 + C 2 H 5 Br ——> HBr + (C 2 H 5)2 NH diethylamine, a 2° amine (C 2 H 5)2 NH + C 2 H 5 Br ——> HBr + (C 2 H 5)3 N triethylamine, a 3° amine (C 2 H 5)3 N ——> (C 2 H 5)4 N+ Br¯ + C 2 H 5 Br tetraethylammonium bromide, a 4° salt CONVERSIONS
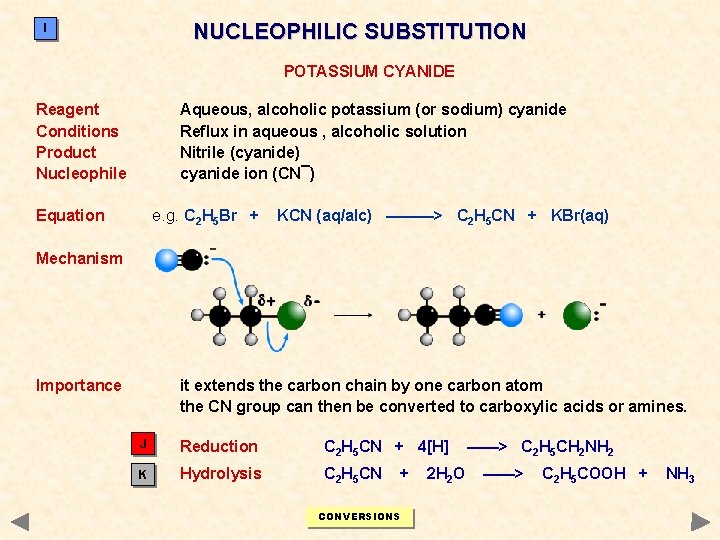
NUCLEOPHILIC SUBSTITUTION I POTASSIUM CYANIDE Reagent Conditions Product Nucleophile Aqueous, alcoholic potassium (or sodium) cyanide Reflux in aqueous , alcoholic solution Nitrile (cyanide) cyanide ion (CN¯) Equation e. g. C 2 H 5 Br + KCN (aq/alc) ———> C 2 H 5 CN + KBr(aq) Mechanism Importance it extends the carbon chain by one carbon atom the CN group can then be converted to carboxylic acids or amines. J Reduction C 2 H 5 CN + 4[H] K Hydrolysis C 2 H 5 CN + CONVERSIONS 2 H 2 O ——> C 2 H 5 CH 2 NH 2 ——> C 2 H 5 COOH + NH 3
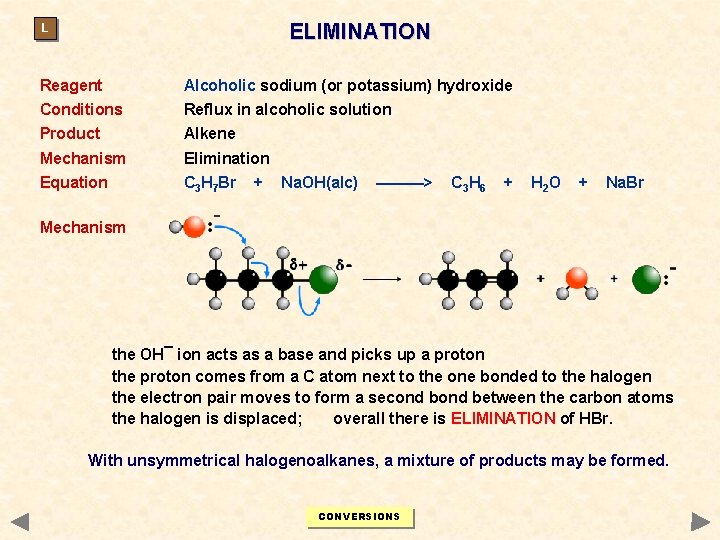
ELIMINATION L Reagent Alcoholic sodium (or potassium) hydroxide Conditions Reflux in alcoholic solution Product Alkene Mechanism Elimination Equation C 3 H 7 Br + Na. OH(alc) ———> C 3 H 6 + H 2 O + Na. Br Mechanism the OH¯ ion acts as a base and picks up a proton the proton comes from a C atom next to the one bonded to the halogen the electron pair moves to form a second between the carbon atoms the halogen is displaced; overall there is ELIMINATION of HBr. With unsymmetrical halogenoalkanes, a mixture of products may be formed. CONVERSIONS
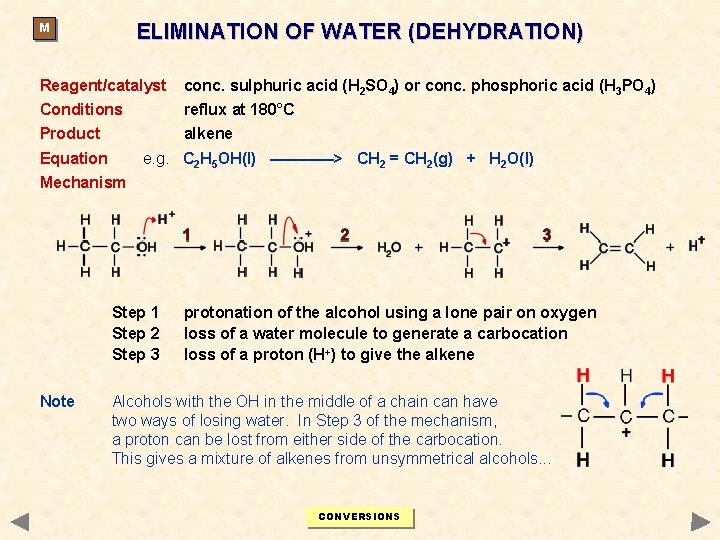
ELIMINATION OF WATER (DEHYDRATION) M Reagent/catalyst conc. sulphuric acid (H 2 SO 4) or conc. phosphoric acid (H 3 PO 4) Conditions reflux at 180°C Product alkene Equation e. g. C 2 H 5 OH(l) ————> CH 2 = CH 2(g) + H 2 O(l) Mechanism Step 1 Step 2 Step 3 Note protonation of the alcohol using a lone pair on oxygen loss of a water molecule to generate a carbocation loss of a proton (H+) to give the alkene Alcohols with the OH in the middle of a chain can have two ways of losing water. In Step 3 of the mechanism, a proton can be lost from either side of the carbocation. This gives a mixture of alkenes from unsymmetrical alcohols. . . CONVERSIONS
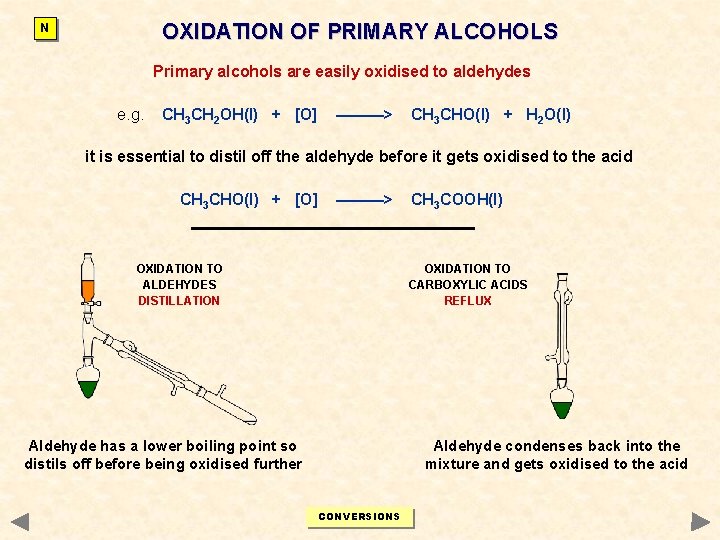
OXIDATION OF PRIMARY ALCOHOLS N Primary alcohols are easily oxidised to aldehydes e. g. CH 3 CH 2 OH(l) + [O] ———> CH 3 CHO(l) + H 2 O(l) it is essential to distil off the aldehyde before it gets oxidised to the acid CH 3 CHO(l) + [O] ———> OXIDATION TO ALDEHYDES DISTILLATION CH 3 COOH(l) OXIDATION TO CARBOXYLIC ACIDS REFLUX Aldehyde has a lower boiling point so distils off before being oxidised further Aldehyde condenses back into the mixture and gets oxidised to the acid CONVERSIONS
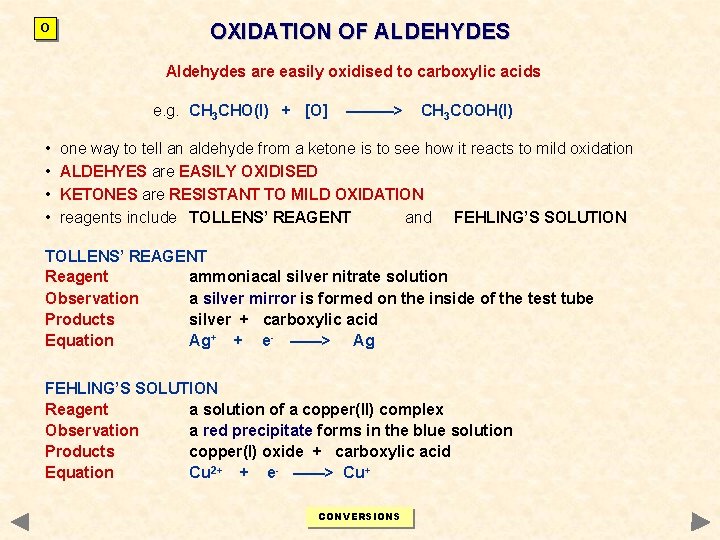
O OXIDATION OF ALDEHYDES Aldehydes are easily oxidised to carboxylic acids e. g. CH 3 CHO(l) + [O] • • ———> CH 3 COOH(l) one way to tell an aldehyde from a ketone is to see how it reacts to mild oxidation ALDEHYES are EASILY OXIDISED KETONES are RESISTANT TO MILD OXIDATION reagents include TOLLENS’ REAGENT and FEHLING’S SOLUTION TOLLENS’ REAGENT Reagent ammoniacal silver nitrate solution Observation a silver mirror is formed on the inside of the test tube Products silver + carboxylic acid Equation Ag+ + e- ——> Ag FEHLING’S SOLUTION Reagent a solution of a copper(II) complex Observation a red precipitate forms in the blue solution Products copper(I) oxide + carboxylic acid Equation Cu 2+ + e- ——> Cu+ CONVERSIONS

P OXIDATION OF SECONDARY ALCOHOLS Secondary alcohols are easily oxidised to ketones e. g. CH 3 CHOHCH 3(l) + [O] ———> CH 3 COCH 3(l) + H 2 O(l) The alcohol is refluxed with acidified K 2 Cr 2 O 7. However, on prolonged treatment with a powerful oxidising agent they can be further oxidised to a mixture of acids with fewer carbon atoms than the original alcohol. CONVERSIONS
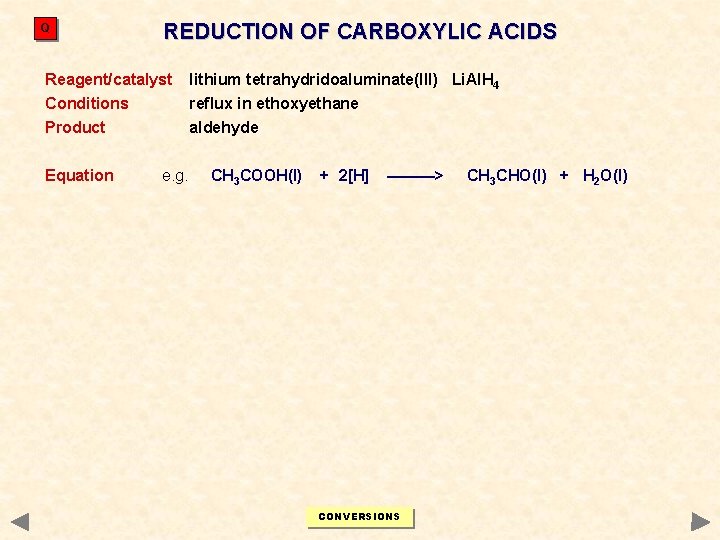
Q REDUCTION OF CARBOXYLIC ACIDS Reagent/catalyst lithium tetrahydridoaluminate(III) Li. Al. H 4 Conditions reflux in ethoxyethane Product aldehyde Equation e. g. CH 3 COOH(l) + 2[H] ———> CONVERSIONS CH 3 CHO(l) + H 2 O(l)
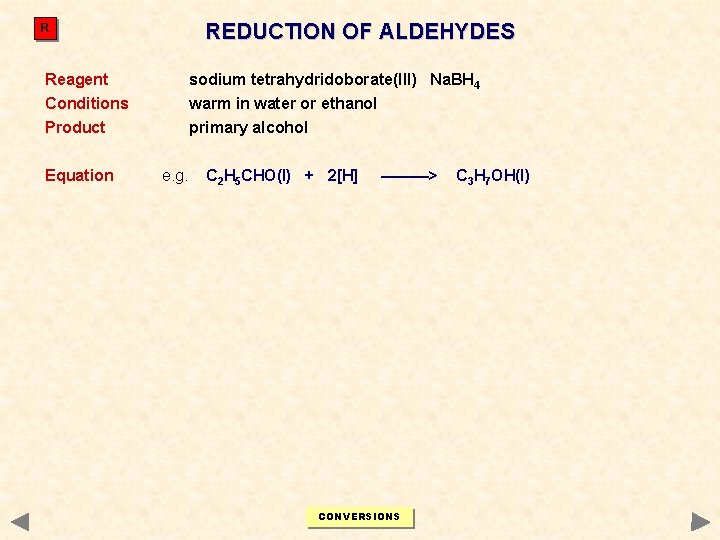
REDUCTION OF ALDEHYDES R Reagent sodium tetrahydridoborate(III) Na. BH 4 Conditions warm in water or ethanol Product primary alcohol Equation e. g. C 2 H 5 CHO(l) + 2[H] ———> CONVERSIONS C 3 H 7 OH(l)
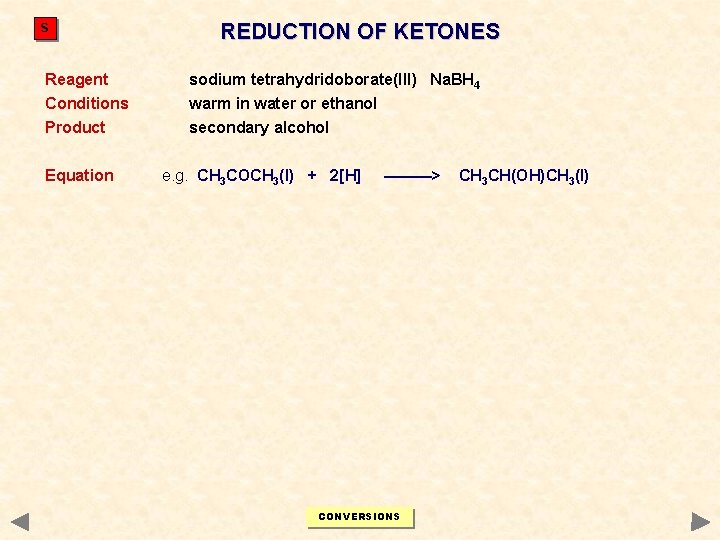
S REDUCTION OF KETONES Reagent sodium tetrahydridoborate(III) Na. BH 4 Conditions warm in water or ethanol Product secondary alcohol Equation e. g. CH 3 COCH 3(l) + 2[H] ———> CONVERSIONS CH 3 CH(OH)CH 3(l)

ESTERIFICATION T Reagent(s) carboxylic acid + strong acid catalyst (e. g conc. H 2 SO 4 ) Conditions reflux Product ester Equation e. g. CH 3 CH 2 OH(l) + CH 3 COOH(l) CH 3 COOC 2 H 5(l) + H 2 O(l) Notes Concentrated H 2 SO 4 is also a dehydrating agent, it removes water as it is formed causing the equilibrium to move to the right and thus increasing the yield of ester. Uses of esters Esters are fairly unreactive but that doesn’t make them useless Used as flavourings Naming esters Named from the alcohol and carboxylic acid which made them. . . CH 3 OH + CH 3 COOH from ethanoic acid CH 3 COOCH 3 + H 2 O CH 3 COOCH 3 METHYL ETHANOATE CONVERSIONS from methanol
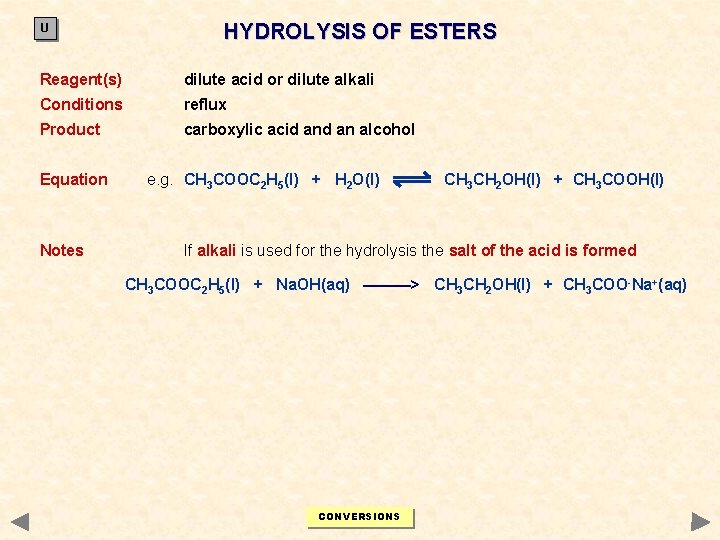
U HYDROLYSIS OF ESTERS Reagent(s) dilute acid or dilute alkali Conditions reflux Product carboxylic acid an alcohol Equation Notes e. g. CH 3 COOC 2 H 5(l) + H 2 O(l) CH 3 CH 2 OH(l) + CH 3 COOH(l) If alkali is used for the hydrolysis the salt of the acid is formed CH 3 COOC 2 H 5(l) + Na. OH(aq) ———> CH 3 CH 2 OH(l) + CH 3 COO-Na+(aq) CONVERSIONS
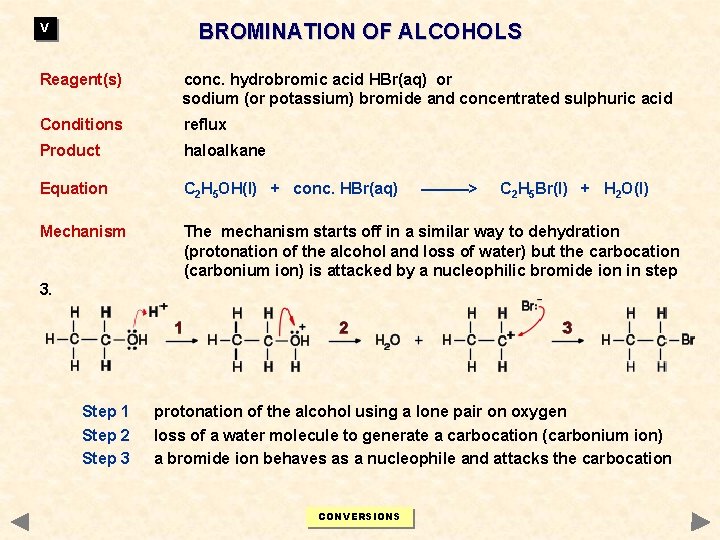
BROMINATION OF ALCOHOLS V Reagent(s) conc. hydrobromic acid HBr(aq) or sodium (or potassium) bromide and concentrated sulphuric acid Conditions reflux Product haloalkane Equation C 2 H 5 OH(l) + conc. HBr(aq) Mechanism The mechanism starts off in a similar way to dehydration (protonation of the alcohol and loss of water) but the carbocation (carbonium ion) is attacked by a nucleophilic bromide ion in step ———> C 2 H 5 Br(l) + H 2 O(l) 3. Step 1 Step 2 Step 3 protonation of the alcohol using a lone pair on oxygen loss of a water molecule to generate a carbocation (carbonium ion) a bromide ion behaves as a nucleophile and attacks the carbocation CONVERSIONS

AN INTRODUCTION TO SYNTHETIC ORGANIC CHEMISTRY THE END © 2003 JONATHAN HOPTON & KNOCKHARDY PUBLISHING
- Slides: 26