An Introduction to Physics Honors Physics Wilmington HS




![S. I. Unit for length, [L] • The “meter” – Abbreviated m – The S. I. Unit for length, [L] • The “meter” – Abbreviated m – The](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-9.jpg)

![SI Unit for Mass, [M] • Mass: the kilogram – One kilogram is the SI Unit for Mass, [M] • Mass: the kilogram – One kilogram is the](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-11.jpg)

![S. I. Unit for time, [T] • The “second” – Abbreviated s – The S. I. Unit for time, [T] • The “second” – Abbreviated s – The](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-13.jpg)



- Slides: 30

An Introduction to Physics Honors Physics Wilmington HS 2011 -2012

What IS physics? • Physics is the most fundamental science – Physics describes the nature of things such as force, motion, energy, matter, heat, sound, light, and atomic and nuclei compositions. • Physics explains all phenomena found in the other sciences and is the foundation for all life and matter

Why study • Physics is the study of the fundamental laws of nature. It is physics? the science that regulates and describes all of the other sciences!!! • Pythagoras ancient Greece • Aristotle “The Natural Sciences” • Galileo 16 th century • Newton 17 th century Sir Isaac • Modern Physics 19 th century Newton, considered by many to be the “Father of Physics”.

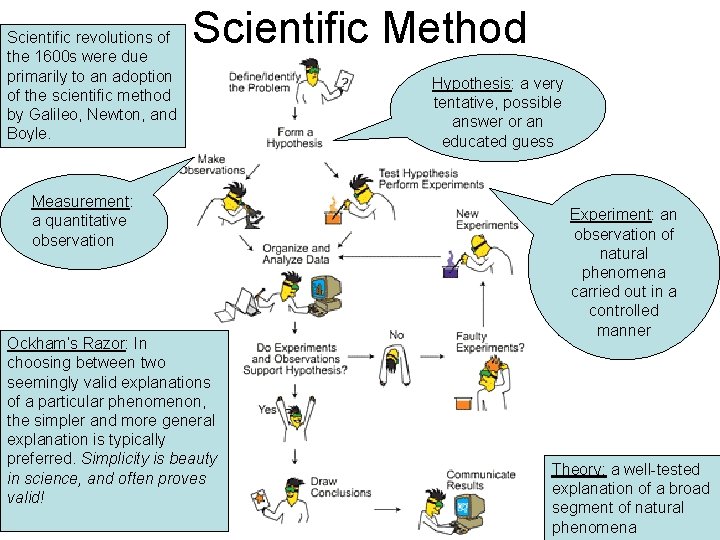
Scientific revolutions of the 1600 s were due primarily to an adoption of the scientific method by Galileo, Newton, and Boyle. Scientific Method Measurement: a quantitative observation Ockham’s Razor: In choosing between two seemingly valid explanations of a particular phenomenon, the simpler and more general explanation is typically preferred. Simplicity is beauty in science, and often proves valid! Hypothesis: a very tentative, possible answer or an educated guess Experiment: an observation of natural phenomena carried out in a controlled manner Theory: a well-tested explanation of a broad segment of natural phenomena

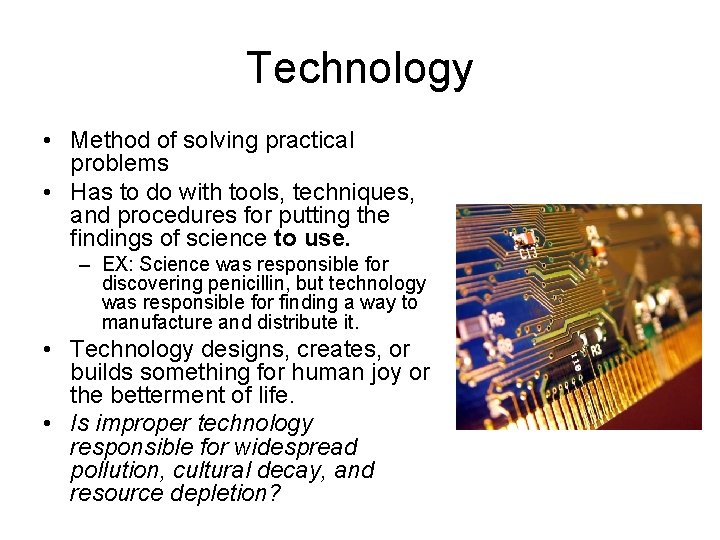
Science, Technology, and Society • Science – A method of answering THEORETICAL questions – Has to do with discovering facts and relationships between observable happenings in nature and with established theories – Usually driven simply by the urge to know and discover – In an ideal world, science is free of belief, values, and current pop trends • But is that always the case?

Technology • Method of solving practical problems • Has to do with tools, techniques, and procedures for putting the findings of science to use. – EX: Science was responsible for discovering penicillin, but technology was responsible for finding a way to manufacture and distribute it. • Technology designs, creates, or builds something for human joy or the betterment of life. • Is improper technology responsible for widespread pollution, cultural decay, and resource depletion?

Chapter 1: Introduction to Physics Standards of length, mass, and time, measurement, uncertainty, and mathematics

The three fundamental quantities • Length (L), mass (M), and time (T) – All other physical quantities can be constructed from these three • Example: the unit for acceleration is m/s 2
![S I Unit for length L The meter Abbreviated m The S. I. Unit for length, [L] • The “meter” – Abbreviated m – The](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-9.jpg)
S. I. Unit for length, [L] • The “meter” – Abbreviated m – The meter is defined as the distance traveled by light in a vacuum during a time interval of 1/299, 792, 458 th second – The speed of light is 299, 792, 458 m/s Saturn, as seen during last year’s lunar eclipse through telescope

Approximate values of some measured lengths Distance from earth to most remote known star 1 x 1026 meters Distance from Earth to Andromeda galaxy 2 x 1022 m One light year 9 x 1015 m Orbit radius of Earth about sun 2 x 1011 m Mean distance from Earth to moon 4 x 108 m Length of a football field 9 x 101 m Length of a housefly 5 x 10 -3 m Size of smallest dust particles 1 x 10 -4 m Size of most living cells 1 x 10 -5 m Diameter of hydrogen atom 1 x 10 -10 m Diameter of atomic nucleus 1 x 10 -14 m Diameter of a proton 1 x 10 -15 m
![SI Unit for Mass M Mass the kilogram One kilogram is the SI Unit for Mass, [M] • Mass: the kilogram – One kilogram is the](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-11.jpg)
SI Unit for Mass, [M] • Mass: the kilogram – One kilogram is the mass of a particular platinum-iridium cylinder kept at the International Bureau of Weights and Standards, Sèvres, France. – One kilogram is roughly 2. 2 lbs.

Approximate values of some masses Observable Universe 1 x 1052 kilograms Earth 6 x 1024 kilograms Shark 1 x 102 kilograms Human 7 x 101 kilograms Mosquito 1 x 10 -5 kilograms Bacterium 1 x 10 -15 kilograms Hydrogen Atom 2 x 10 -27 kilograms Electron 9 x 10 -31 kilograms
![S I Unit for time T The second Abbreviated s The S. I. Unit for time, [T] • The “second” – Abbreviated s – The](https://slidetodoc.com/presentation_image_h2/b61d92bd773cf2b34f3acdc33ea8b8dc/image-13.jpg)
S. I. Unit for time, [T] • The “second” – Abbreviated s – The second is now defined as 9, 192, 631, 700 times the period of oscillation of radiation from the cesium atom

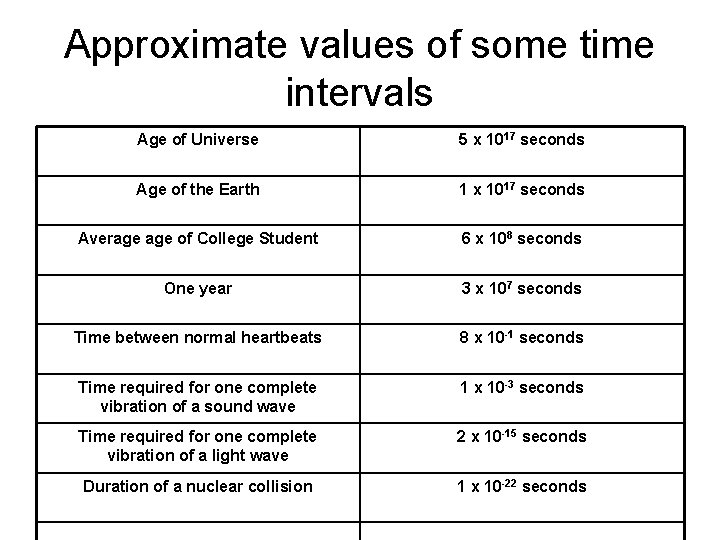
Approximate values of some time intervals Age of Universe 5 x 1017 seconds Age of the Earth 1 x 1017 seconds Average of College Student 6 x 108 seconds One year 3 x 107 seconds Time between normal heartbeats 8 x 10 -1 seconds Time required for one complete vibration of a sound wave 1 x 10 -3 seconds Time required for one complete vibration of a light wave 2 x 10 -15 seconds Duration of a nuclear collision 1 x 10 -22 seconds

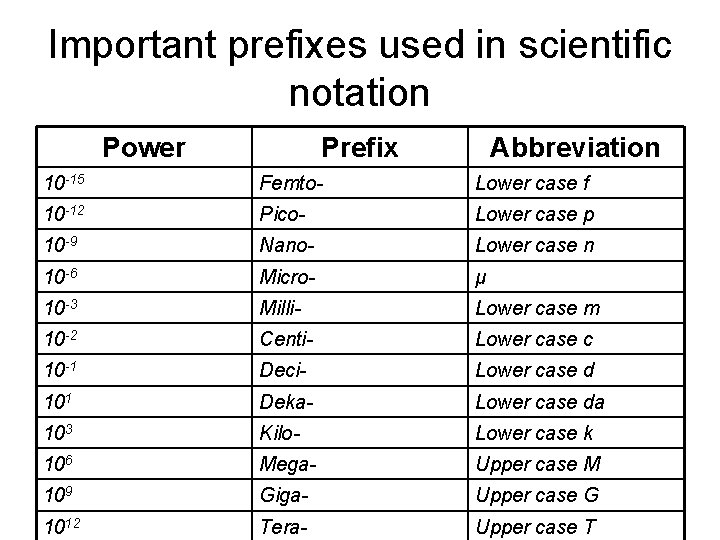
Important prefixes used in scientific notation Power Prefix Abbreviation 10 -15 Femto- Lower case f 10 -12 Pico- Lower case p 10 -9 Nano- Lower case n 10 -6 Micro- μ 10 -3 Milli- Lower case m 10 -2 Centi- Lower case c 10 -1 Deci- Lower case d 101 Deka- Lower case da 103 Kilo- Lower case k 106 Mega- Upper case M 109 Giga- Upper case G 1012 Tera- Upper case T

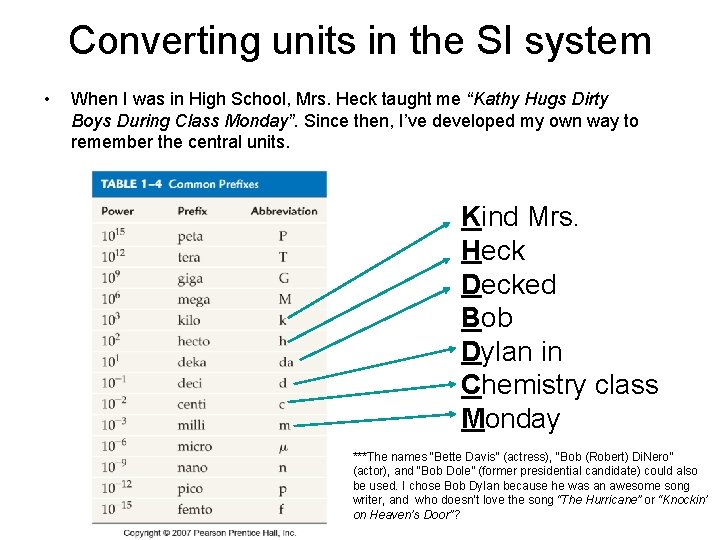
Converting units in the SI system • When I was in High School, Mrs. Heck taught me “Kathy Hugs Dirty Boys During Class Monday”. Since then, I’ve developed my own way to remember the central units. Kind Mrs. Heck Decked Bob Dylan in Chemistry class Monday ***The names “Bette Davis” (actress), “Bob (Robert) Di. Nero” (actor), and “Bob Dole” (former presidential candidate) could also be used. I chose Bob Dylan because he was an awesome song writer, and who doesn’t love the song “The Hurricane” or “Knockin’ on Heaven’s Door”?

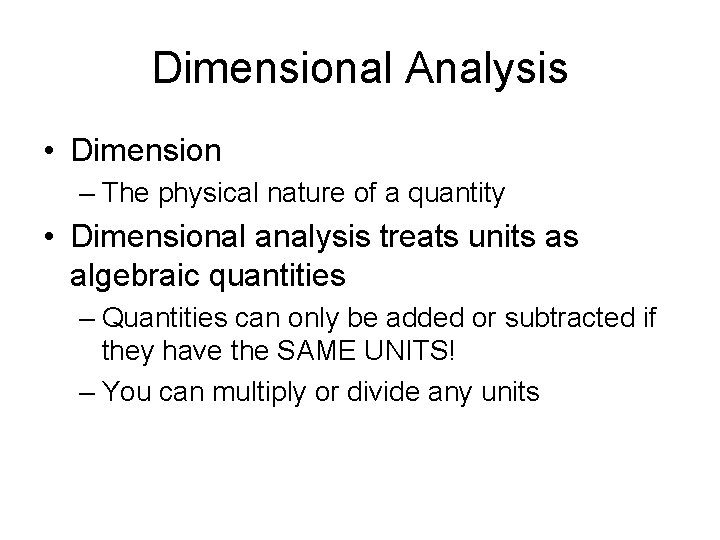
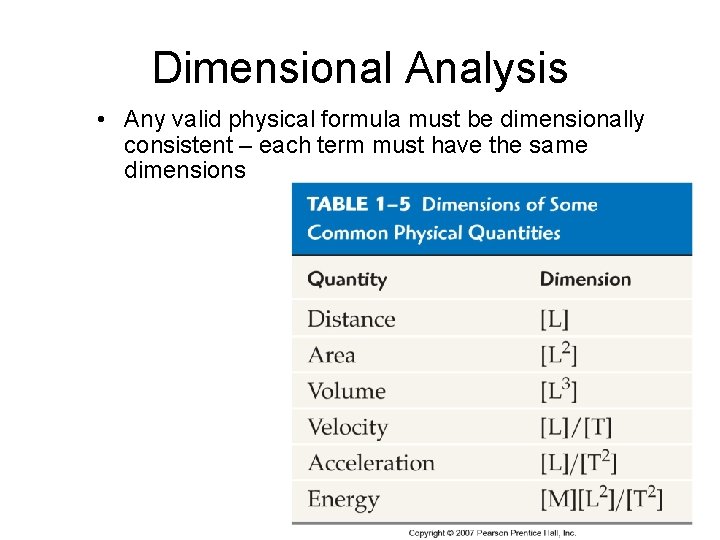
Dimensional Analysis • Dimension – The physical nature of a quantity • Dimensional analysis treats units as algebraic quantities – Quantities can only be added or subtracted if they have the SAME UNITS! – You can multiply or divide any units

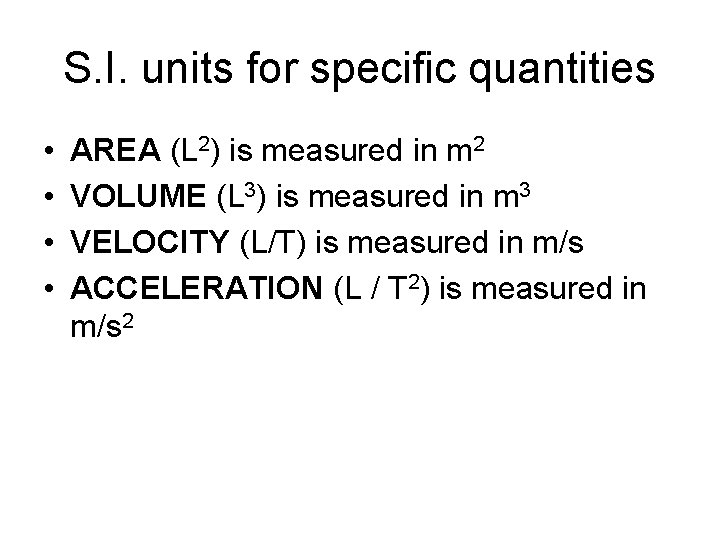
S. I. units for specific quantities • • AREA (L 2) is measured in m 2 VOLUME (L 3) is measured in m 3 VELOCITY (L/T) is measured in m/s ACCELERATION (L / T 2) is measured in m/s 2

Dimensional Analysis • Any valid physical formula must be dimensionally consistent – each term must have the same dimensions

Uncertainty and Significant Figures • No physical quantity can be determined with perfect accuracy because our physical senses are limited • Accuracy of measurement depends on the sensitivity of the apparatus, skill of the measurer, and the number of times the measurement is repeated • A measurement is MEANINGLESS without consideration to the error involved

Significant figures • A reliably known digit – In this class, you will ALWAYS use significant figures. You will lose half of your total points per problem if the incorrect significant figures are used • Zeroes can be tricky… – Examples

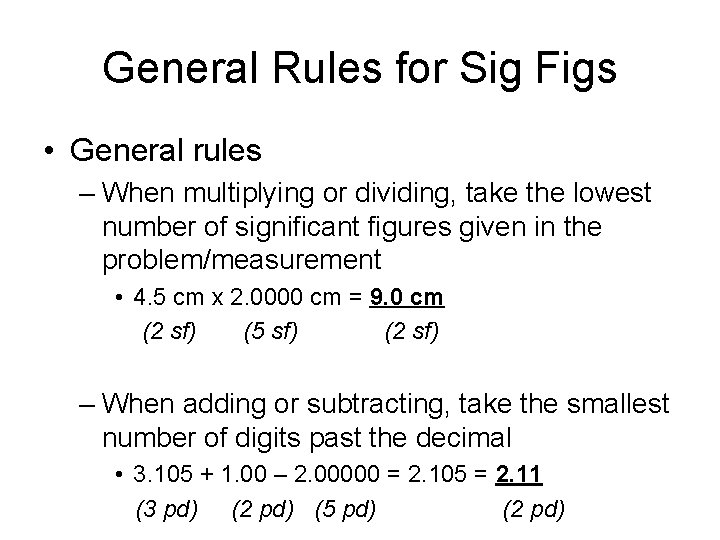
General Rules for Sig Figs • General rules – When multiplying or dividing, take the lowest number of significant figures given in the problem/measurement • 4. 5 cm x 2. 0000 cm = 9. 0 cm (2 sf) (5 sf) (2 sf) – When adding or subtracting, take the smallest number of digits past the decimal • 3. 105 + 1. 00 – 2. 00000 = 2. 105 = 2. 11 (3 pd) (2 pd) (5 pd) (2 pd)

Unit conversions • Sometimes it is necessary to convert units from one to another (trust me on this…you do these conversions all of your life) • Helpful units • • One mile = 1609 m = 1. 609 km 1 meter = 39. 37 in = 3. 281 ft 1 foot = 0. 3048 m = 30. 48 cm 1 inch = 0. 0254 m = 2. 54 cm

Estimates and orders of magnitude • When getting an exact answer is impossible, we use approximations – You may not know a car is going 70 mph, but you may be able to approximate it to 65 based on the speed limit, for instance. • Simply find the power of 10 that is closest to the value

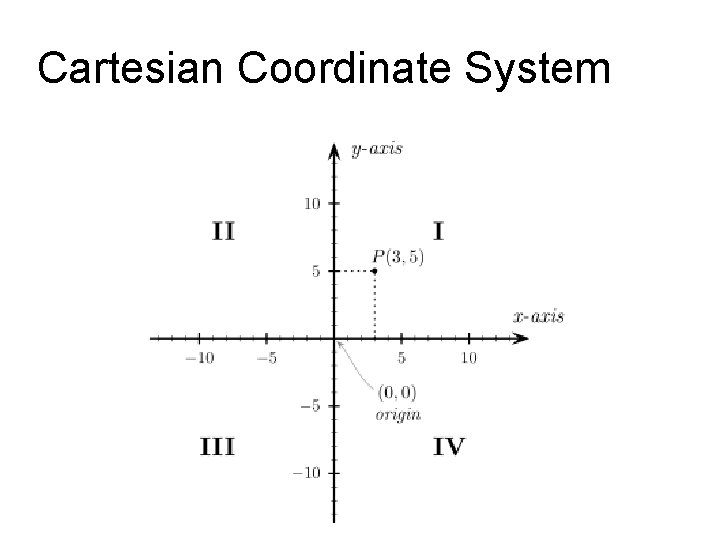
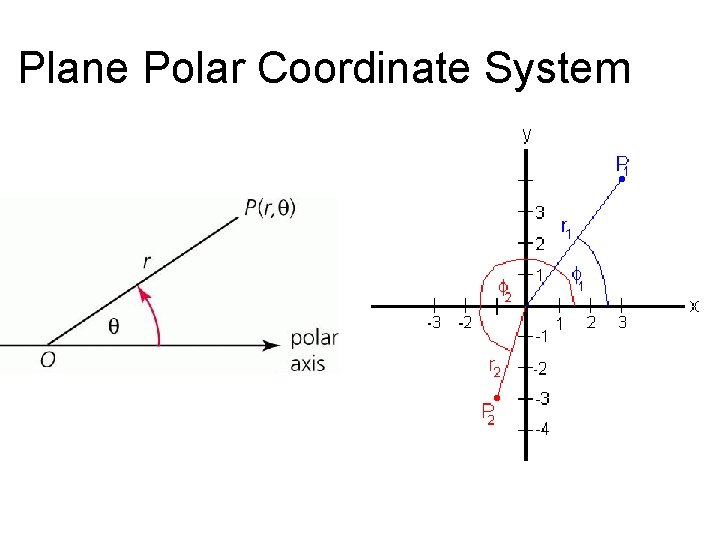
Coordinate systems • Many aspects of physics deal with location in space • Coordinate systems – Reference point “O” is the origin – Specified axes, or directions, with proper units – We will normally use Cartesian coordinate systems (2 -d rectangles) – We will also use plane polar coordinates that reference a point by the ordered pair (r, θ)

Cartesian Coordinate System

Plane Polar Coordinate System

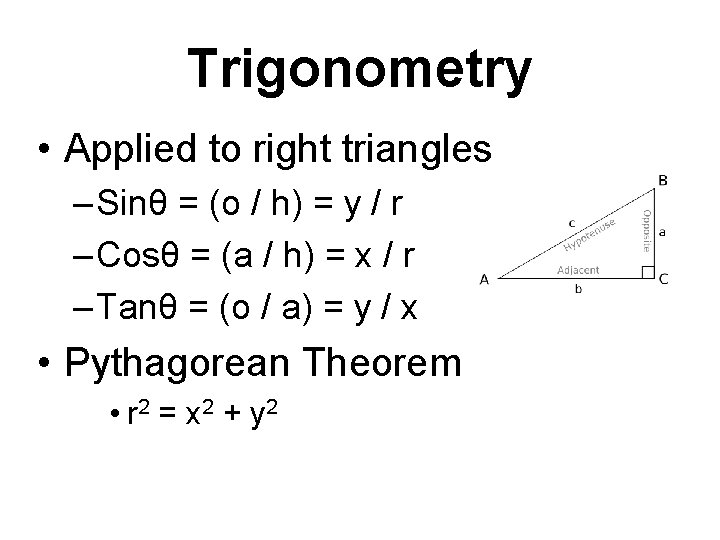
Trigonometry • Applied to right triangles – Sinθ = (o / h) = y / r – Cosθ = (a / h) = x / r – Tanθ = (o / a) = y / x • Pythagorean Theorem • r 2 = x 2 + y 2

Scalars and Vectors • Scalar – a numerical value that is directionless. • May be positive or negative. • Examples: distance, temperature, speed, height, mass • Vector – a quantity with both magnitude and direction. • Examples: displacement (e. g. , 10 feet north), velocity, acceleration, force, magnetic field, weight • The difference between these two quantities will be important as the course progresses!

The Problem-Solving Strategy • 1) Conceptual Grasp • What knowledge is relevant to the situation? – What are the given conditions and assumptions that must be made? • 2) Devise a plan • Draw a picture if you haven’t already done so!! • What steps are necessary to solve the problem? • Can you imagine or visualize the conditions of the problem? • 3) Solve the problem • Mathematically manipulate your problem-solving plan • Keep your work neat and organized so that you don’t get lost • 4) Check your answer for plausibility • Comparison analysis? • Dimensional analysis? • Does your answer make sense given the circumstances?