An Introduction to Organic Reactions Acids and Bases

An Introduction to Organic Reactions Acids and Bases © E. V. Blackburn, 2005
Reaction types Organic reactions fall into one of four general reaction types: • Substitutions • Additions • Eliminations • Rearrangements © E. V. Blackburn, 2005
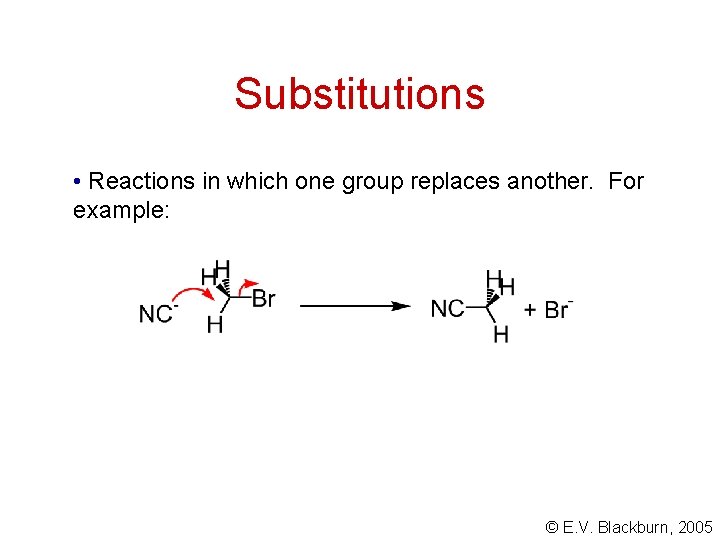
Substitutions • Reactions in which one group replaces another. For example: © E. V. Blackburn, 2005
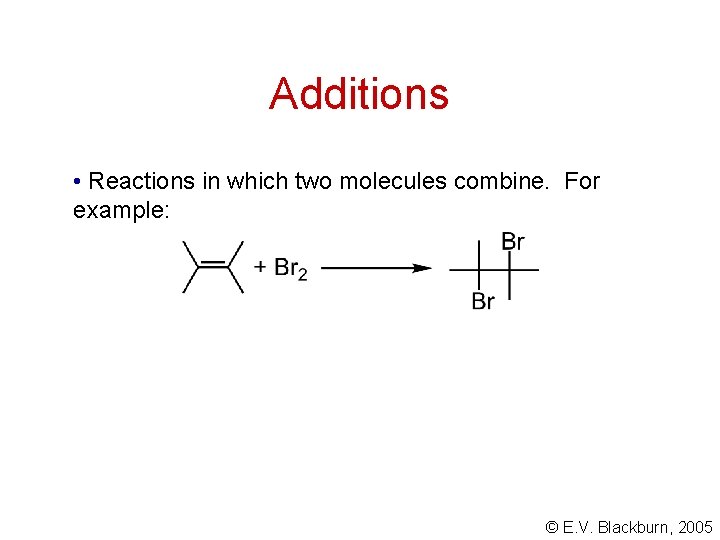
Additions • Reactions in which two molecules combine. For example: © E. V. Blackburn, 2005
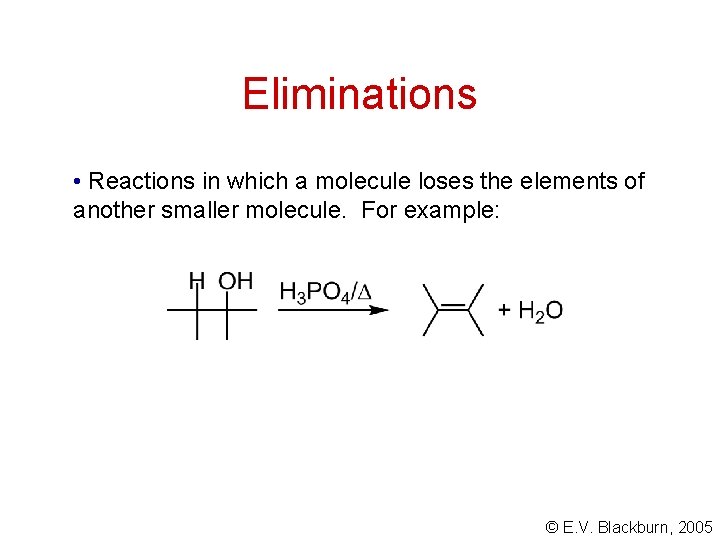
Eliminations • Reactions in which a molecule loses the elements of another smaller molecule. For example: © E. V. Blackburn, 2005
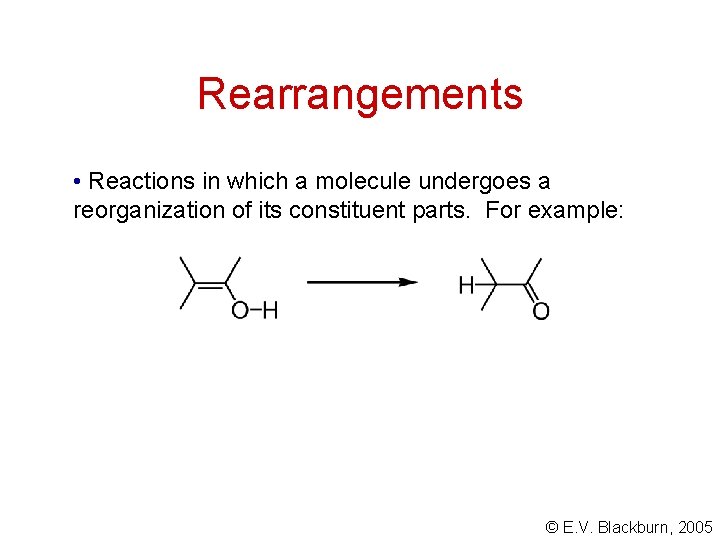
Rearrangements • Reactions in which a molecule undergoes a reorganization of its constituent parts. For example: © E. V. Blackburn, 2005
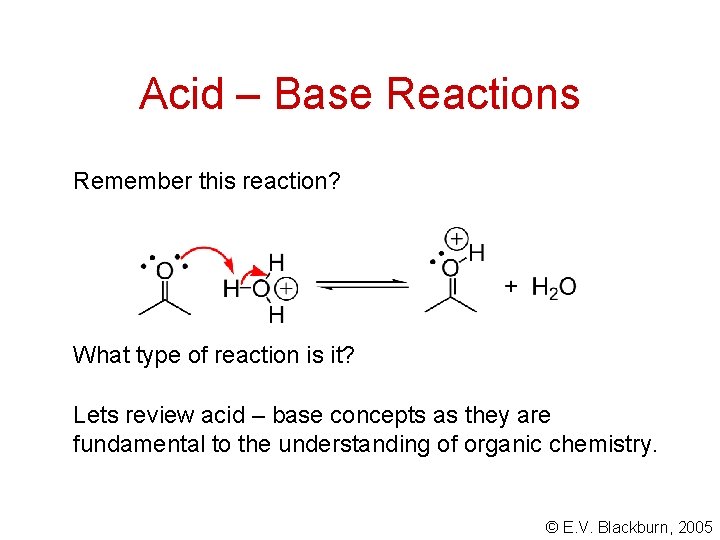
Acid – Base Reactions Remember this reaction? What type of reaction is it? Lets review acid – base concepts as they are fundamental to the understanding of organic chemistry. © E. V. Blackburn, 2005
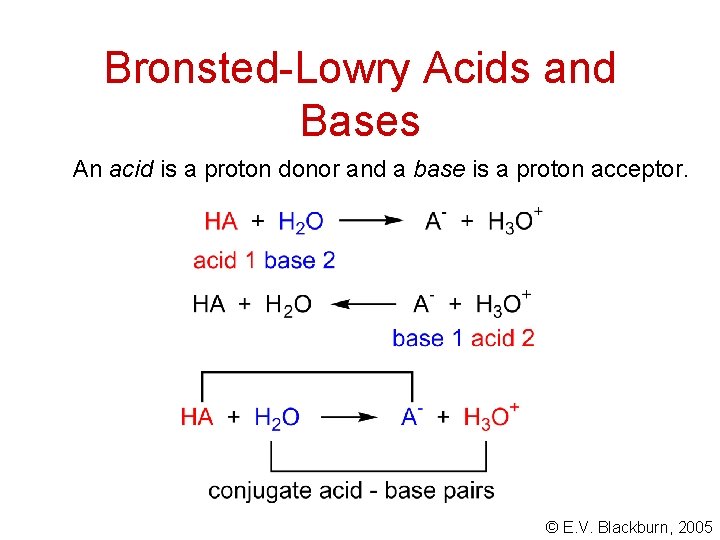
Bronsted-Lowry Acids and Bases An acid is a proton donor and a base is a proton acceptor. © E. V. Blackburn, 2005
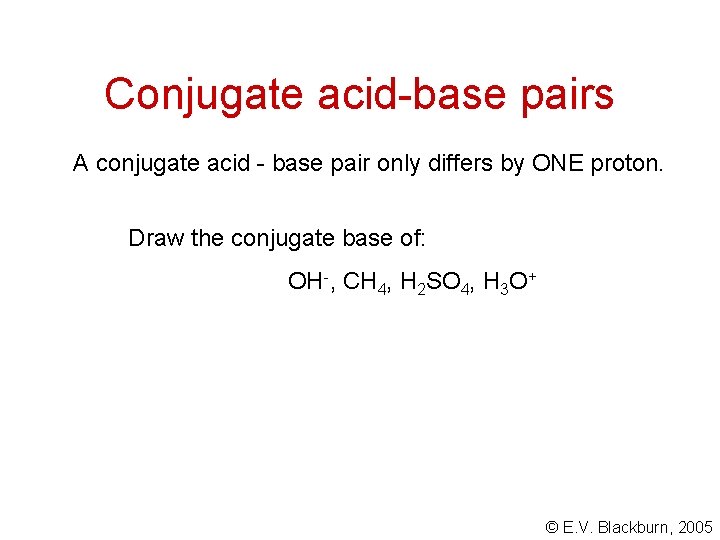
Conjugate acid-base pairs A conjugate acid - base pair only differs by ONE proton. Draw the conjugate base of: OH-, CH 4, H 2 SO 4, H 3 O+ © E. V. Blackburn, 2005
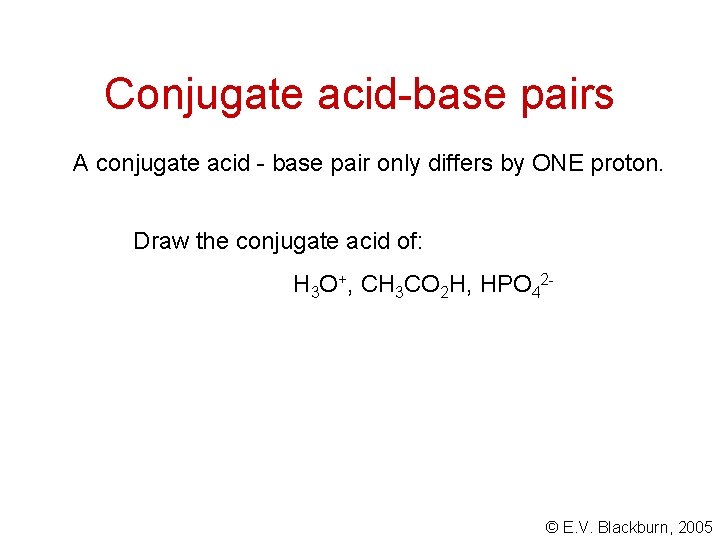
Conjugate acid-base pairs A conjugate acid - base pair only differs by ONE proton. Draw the conjugate acid of: H 3 O+, CH 3 CO 2 H, HPO 42 - © E. V. Blackburn, 2005
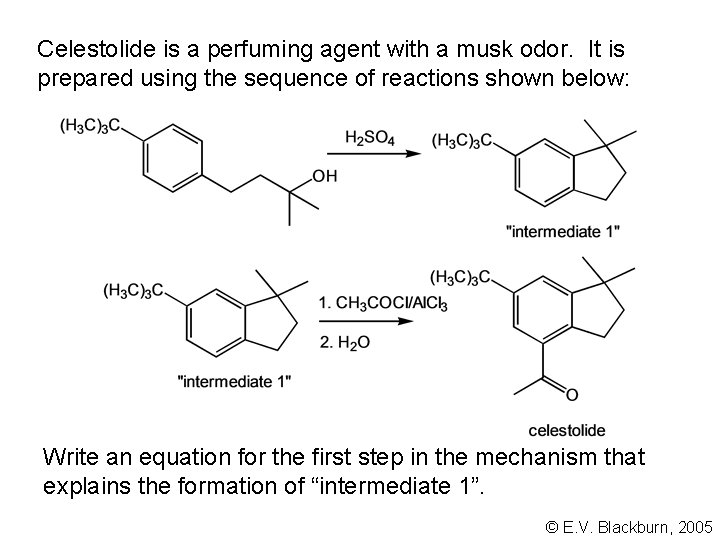
Celestolide is a perfuming agent with a musk odor. It is prepared using the sequence of reactions shown below: Write an equation for the first step in the mechanism that explains the formation of “intermediate 1”. © E. V. Blackburn, 2005
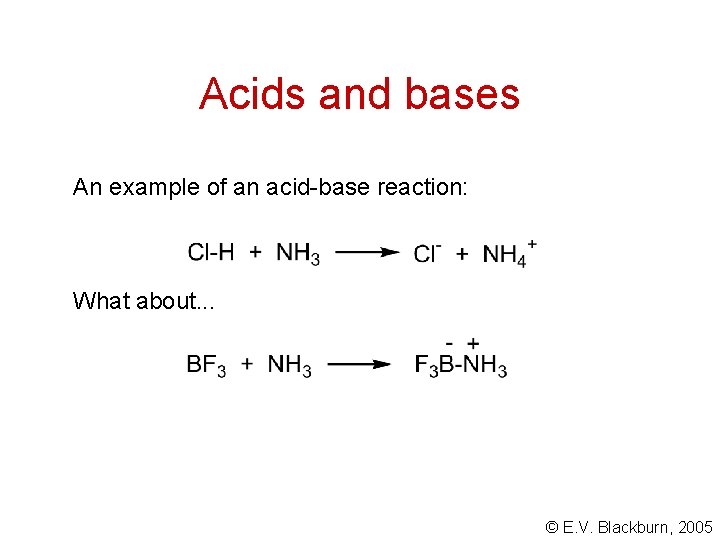
Acids and bases An example of an acid-base reaction: What about. . . © E. V. Blackburn, 2005
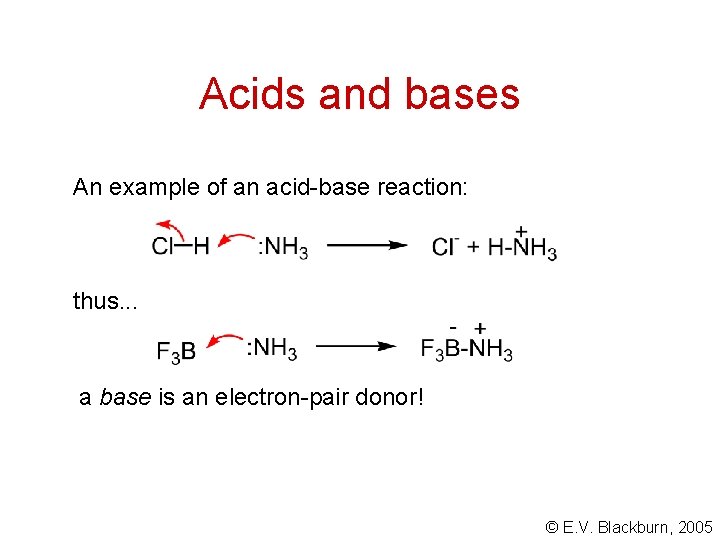
Acids and bases An example of an acid-base reaction: thus. . . a base is an electron-pair donor! © E. V. Blackburn, 2005
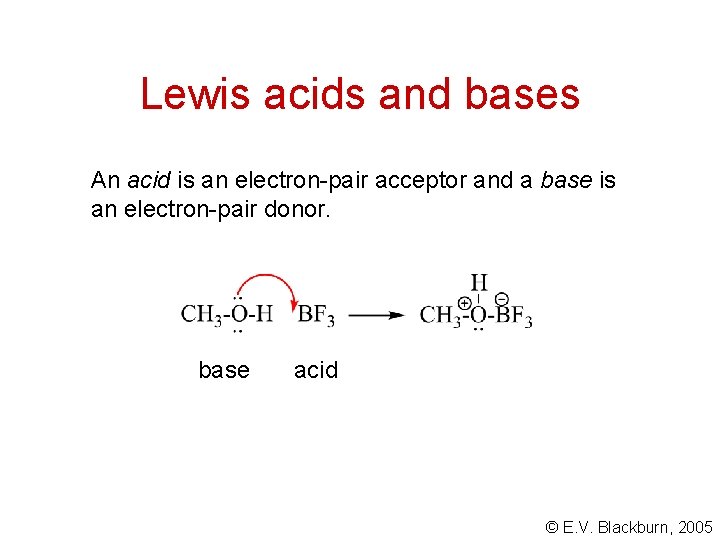
Lewis acids and bases An acid is an electron-pair acceptor and a base is an electron-pair donor. base acid © E. V. Blackburn, 2005
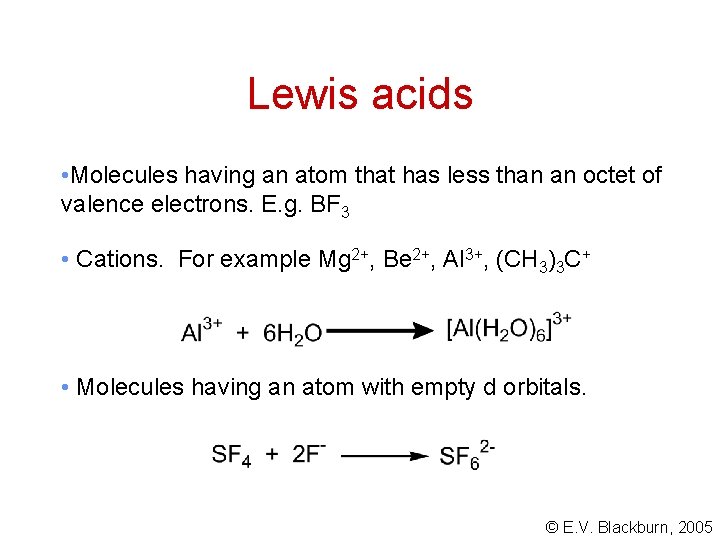
Lewis acids • Molecules having an atom that has less than an octet of valence electrons. E. g. BF 3 • Cations. For example Mg 2+, Be 2+, Al 3+, (CH 3)3 C+ • Molecules having an atom with empty d orbitals. © E. V. Blackburn, 2005
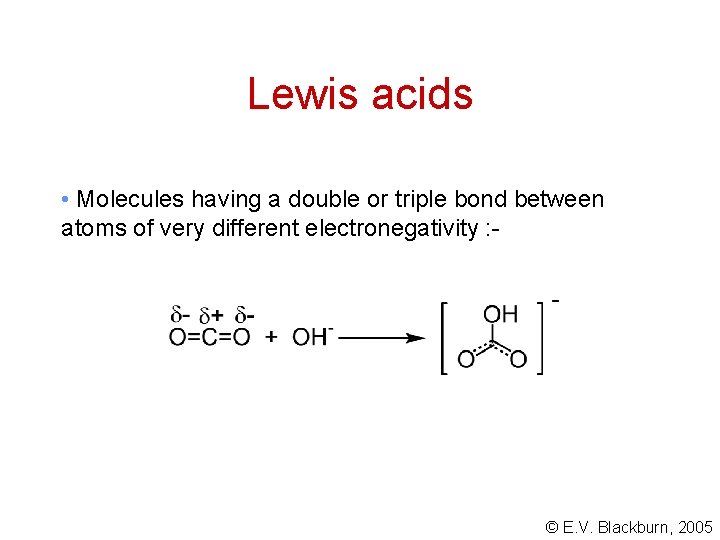
Lewis acids • Molecules having a double or triple bond between atoms of very different electronegativity : - © E. V. Blackburn, 2005
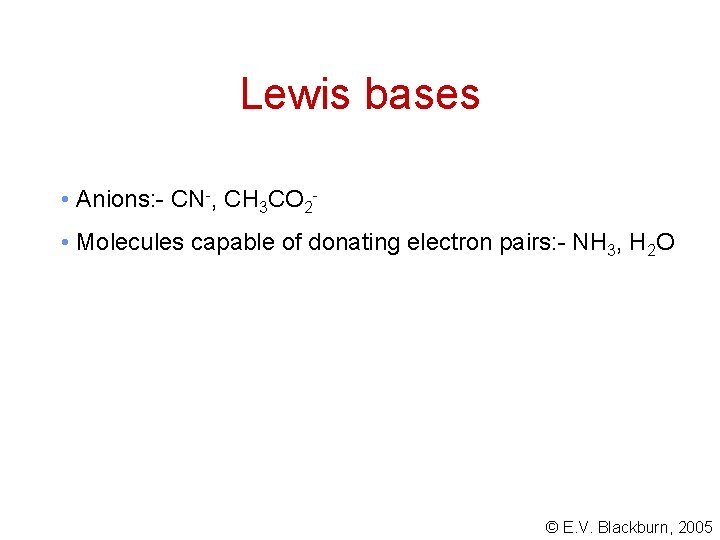
Lewis bases • Anions: - CN-, CH 3 CO 2 • Molecules capable of donating electron pairs: - NH 3, H 2 O © E. V. Blackburn, 2005
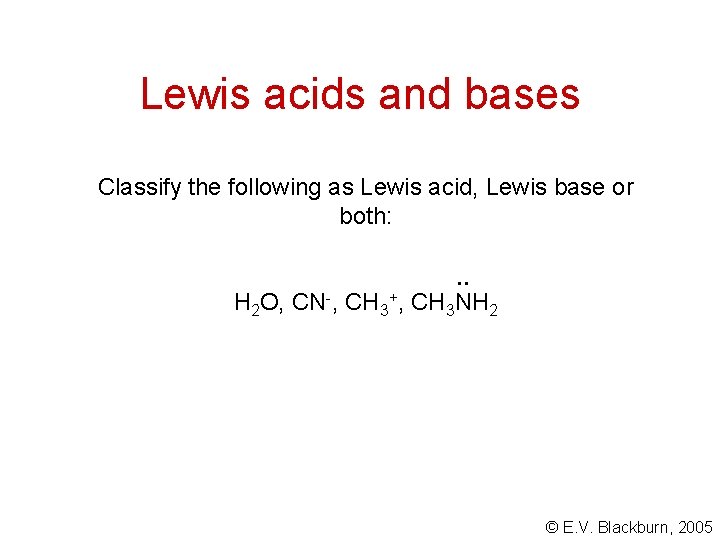
Lewis acids and bases Classify the following as Lewis acid, Lewis base or both: . . H 2 O, CN-, CH 3+, CH 3 NH 2 © E. V. Blackburn, 2005
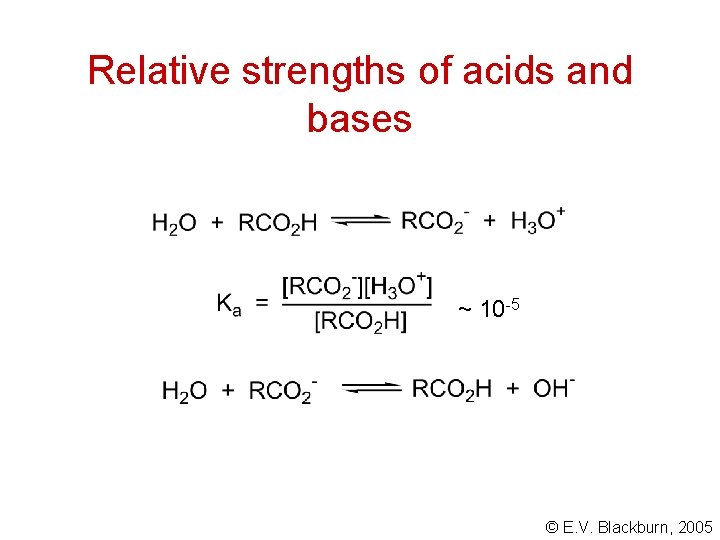
Relative strengths of acids and bases ~ 10 -5 © E. V. Blackburn, 2005
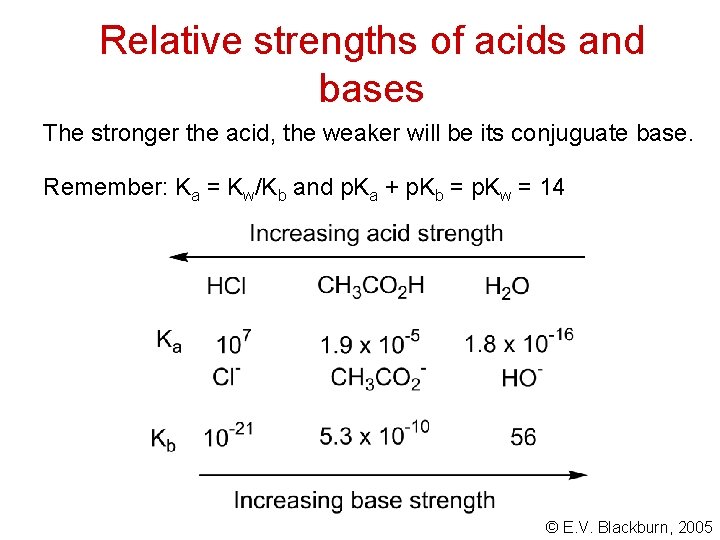
Relative strengths of acids and bases The stronger the acid, the weaker will be its conjuguate base. Remember: Ka = Kw/Kb and p. Ka + p. Kb = p. Kw = 14 © E. V. Blackburn, 2005
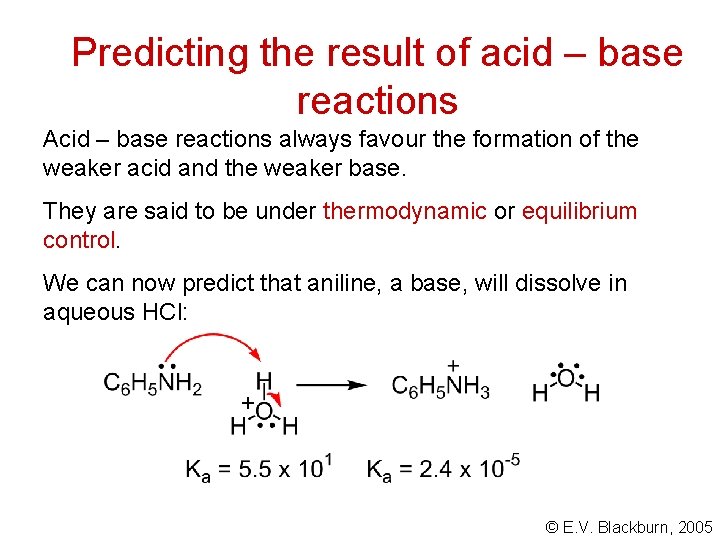
Predicting the result of acid – base reactions Acid – base reactions always favour the formation of the weaker acid and the weaker base. They are said to be under thermodynamic or equilibrium control. We can now predict that aniline, a base, will dissolve in aqueous HCl: + © E. V. Blackburn, 2005
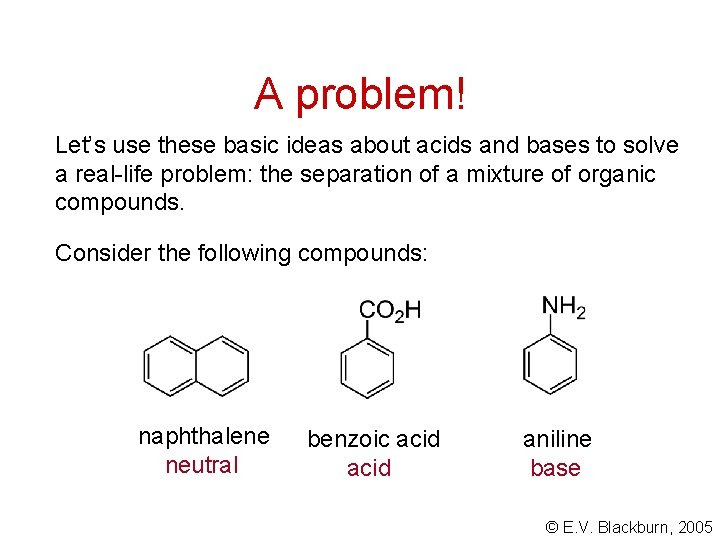
A problem! Let’s use these basic ideas about acids and bases to solve a real-life problem: the separation of a mixture of organic compounds. Consider the following compounds: naphthalene neutral benzoic acid aniline base © E. V. Blackburn, 2005
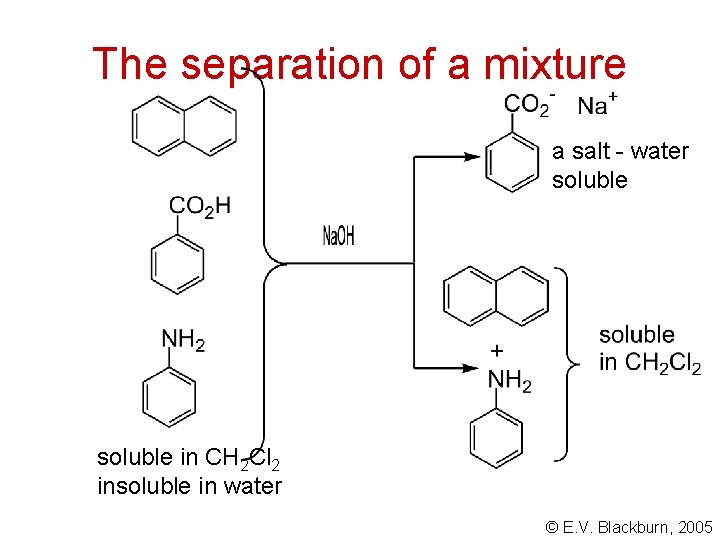
The separation of a mixture a salt - water soluble in CH 2 Cl 2 insoluble in water © E. V. Blackburn, 2005
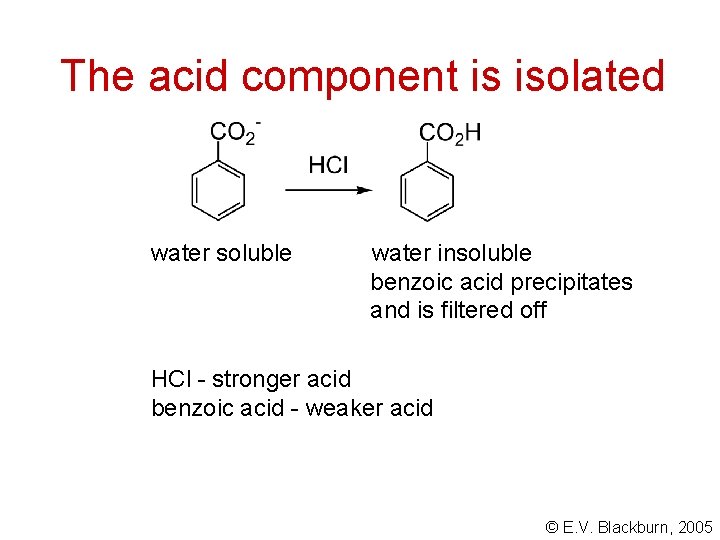
The acid component is isolated water soluble water insoluble benzoic acid precipitates and is filtered off HCl - stronger acid benzoic acid - weaker acid © E. V. Blackburn, 2005
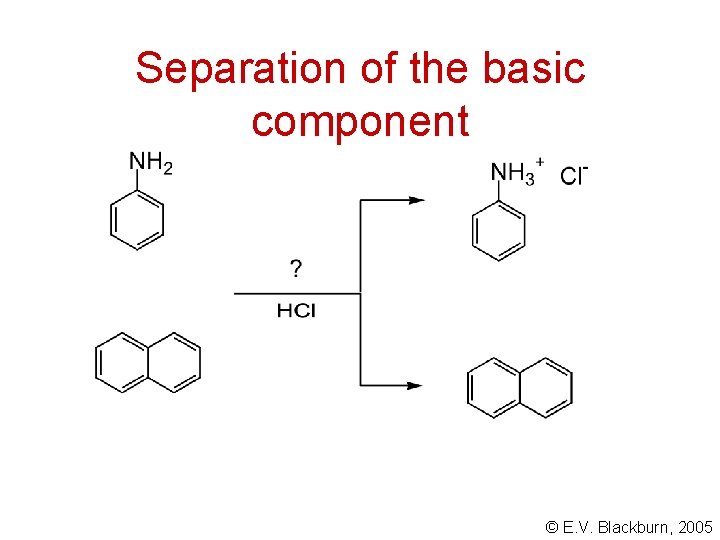
Separation of the basic component © E. V. Blackburn, 2005
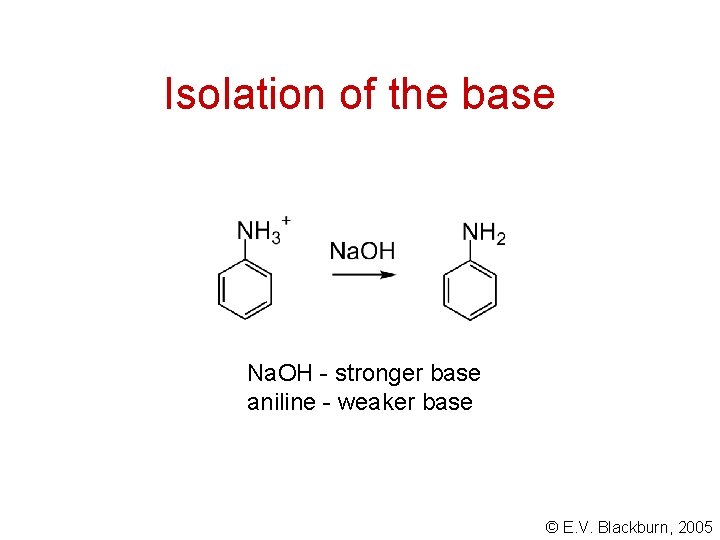
Isolation of the base Na. OH - stronger base aniline - weaker base © E. V. Blackburn, 2005
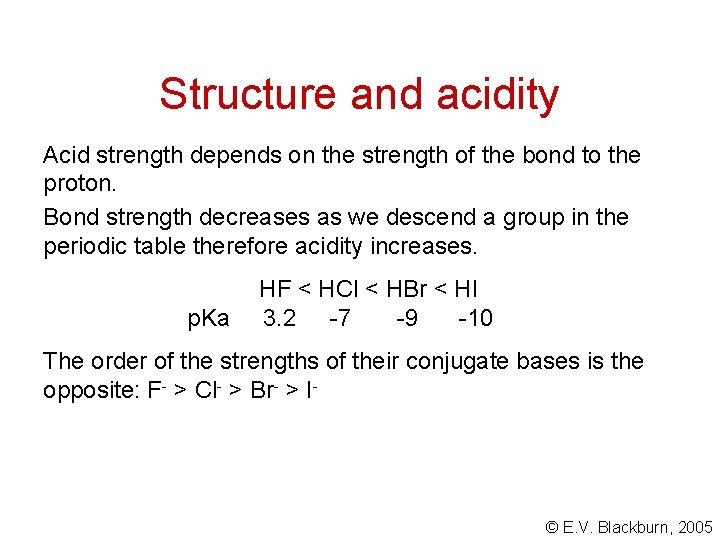
Structure and acidity Acid strength depends on the strength of the bond to the proton. Bond strength decreases as we descend a group in the periodic table therefore acidity increases. p. Ka HF < HCl < HBr < HI 3. 2 -7 -9 -10 The order of the strengths of their conjugate bases is the opposite: F- > Cl- > Br- > I- © E. V. Blackburn, 2005
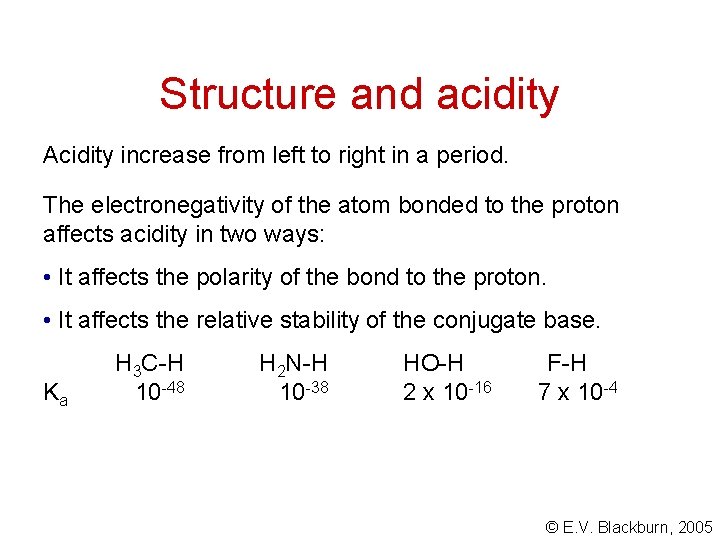
Structure and acidity Acidity increase from left to right in a period. The electronegativity of the atom bonded to the proton affects acidity in two ways: • It affects the polarity of the bond to the proton. • It affects the relative stability of the conjugate base. Ka H 3 C-H 10 -48 H 2 N-H 10 -38 HO-H 2 x 10 -16 F-H 7 x 10 -4 © E. V. Blackburn, 2005
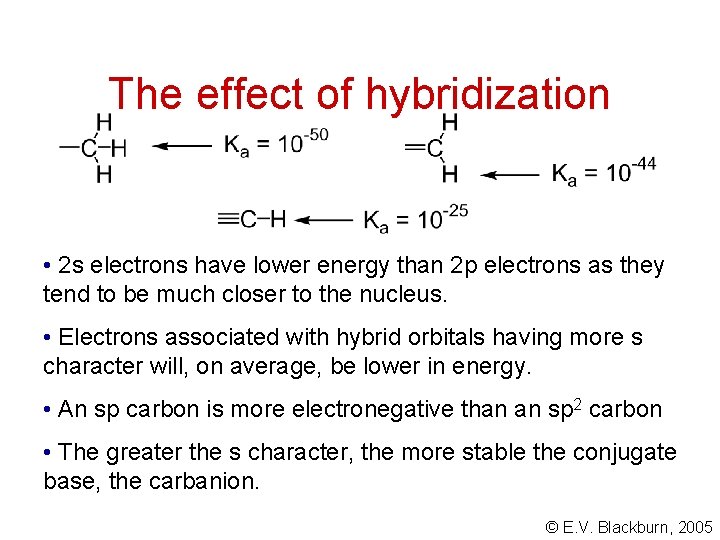
The effect of hybridization • 2 s electrons have lower energy than 2 p electrons as they tend to be much closer to the nucleus. • Electrons associated with hybrid orbitals having more s character will, on average, be lower in energy. • An sp carbon is more electronegative than an sp 2 carbon • The greater the s character, the more stable the conjugate base, the carbanion. © E. V. Blackburn, 2005
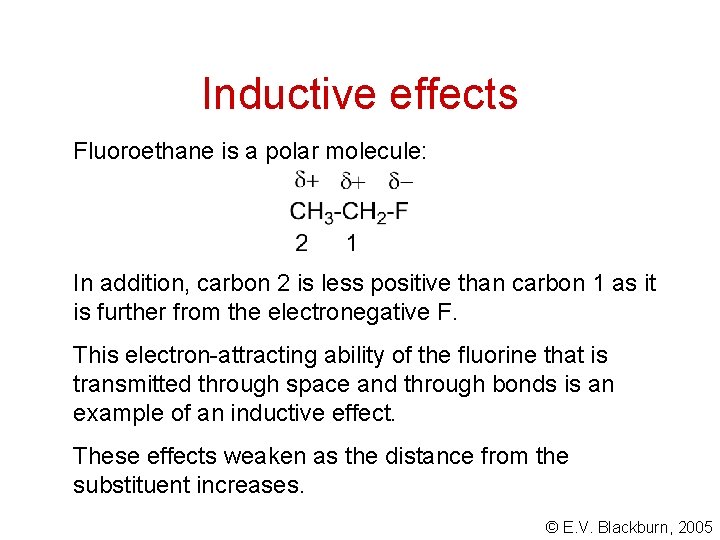
Inductive effects Fluoroethane is a polar molecule: In addition, carbon 2 is less positive than carbon 1 as it is further from the electronegative F. This electron-attracting ability of the fluorine that is transmitted through space and through bonds is an example of an inductive effect. These effects weaken as the distance from the substituent increases. © E. V. Blackburn, 2005
Stability and potential energy Inductive effects can stabilize or destabilize systems. But what does the chemist mean by “stability”? Chemical energy is a form of potential energy. It exists due to the attractive and repulsive forces between the molecular particles. We compare systems in terms of their relative potential energies. The more potential energy a system has, the less stable it is. © E. V. Blackburn, 2005
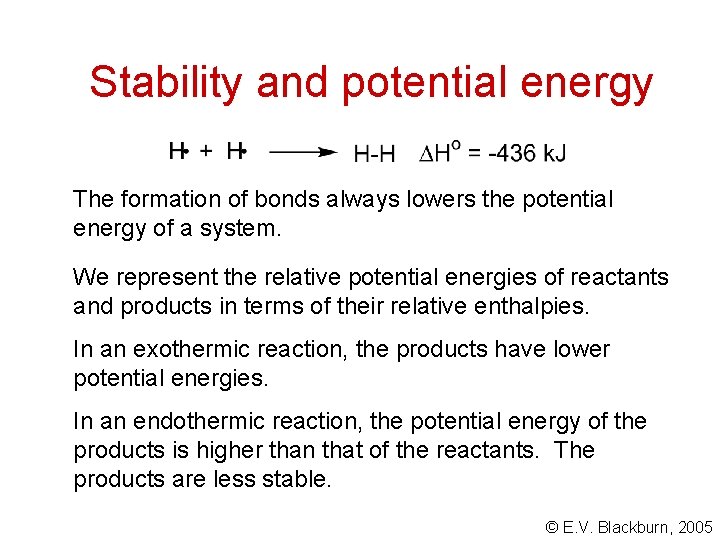
Stability and potential energy The formation of bonds always lowers the potential energy of a system. We represent the relative potential energies of reactants and products in terms of their relative enthalpies. In an exothermic reaction, the products have lower potential energies. In an endothermic reaction, the potential energy of the products is higher than that of the reactants. The products are less stable. © E. V. Blackburn, 2005
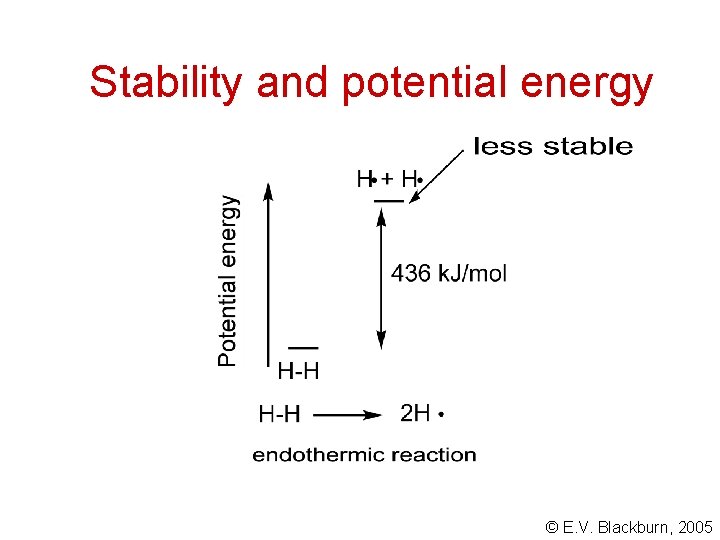
Stability and potential energy © E. V. Blackburn, 2005
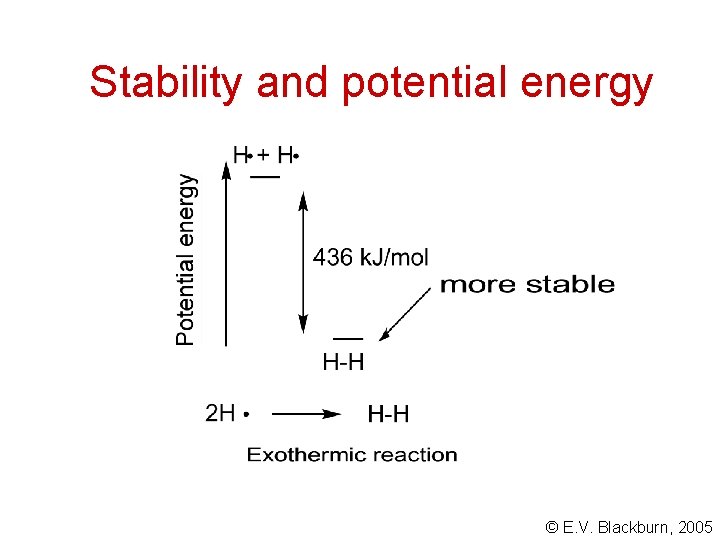
Stability and potential energy © E. V. Blackburn, 2005

Exothermicity When thermodynamics was a young science, it was believed that in order to be spontaneous, a reaction must be exothermic. However many spontaneous processes, such as the spontaneous melting of ice at temperatures above 0 o. C, are endothermic processes. Thus one cannot use “enthalpy” to predict spontaneity! © E. V. Blackburn, 2005
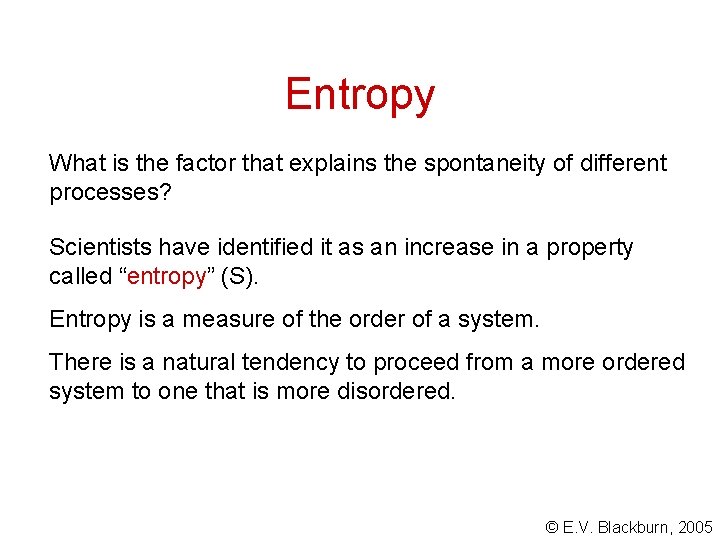
Entropy What is the factor that explains the spontaneity of different processes? Scientists have identified it as an increase in a property called “entropy” (S). Entropy is a measure of the order of a system. There is a natural tendency to proceed from a more ordered system to one that is more disordered. © E. V. Blackburn, 2005
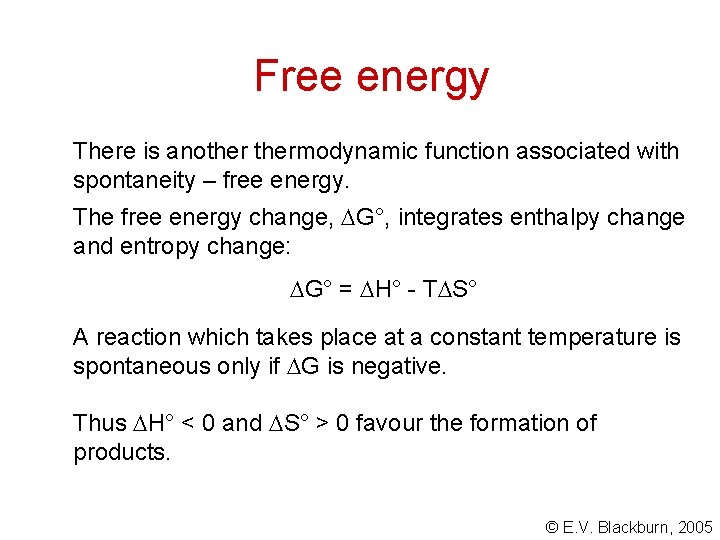
Free energy There is anothermodynamic function associated with spontaneity – free energy. The free energy change, G°, integrates enthalpy change and entropy change: G° = H° - T S° A reaction which takes place at a constant temperature is spontaneous only if G is negative. Thus H° < 0 and S° > 0 favour the formation of products. © E. V. Blackburn, 2005

Free energy There is a relationship between the equilibrium constant and the standard free energy change for a reaction: G° = -RTln(K) Thus a negative value of G° is associated with reactions that favour the formation of products when equilibrium is attained. Indeed reactions having G° less than -13 k. J/mol are said to be quantitative. © E. V. Blackburn, 2005

Problems Try problems 3. 8 and 3. 9 on page 115 of Solomons and Fryhle. © E. V. Blackburn, 2005
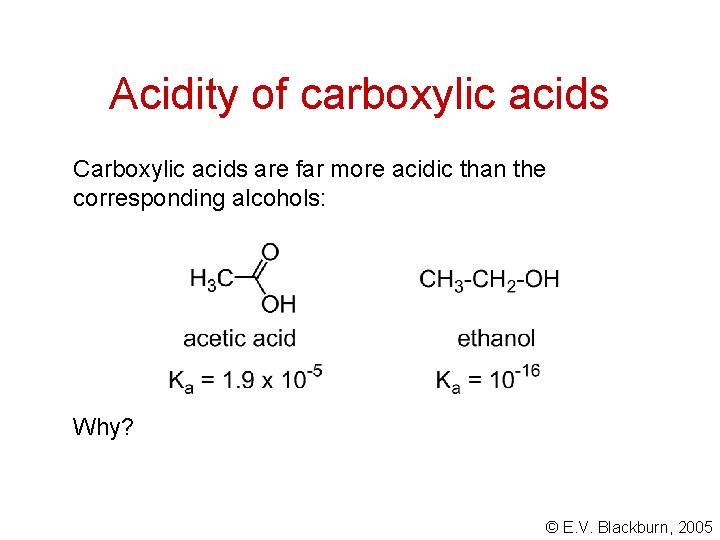
Acidity of carboxylic acids Carboxylic acids are far more acidic than the corresponding alcohols: Why? © E. V. Blackburn, 2005
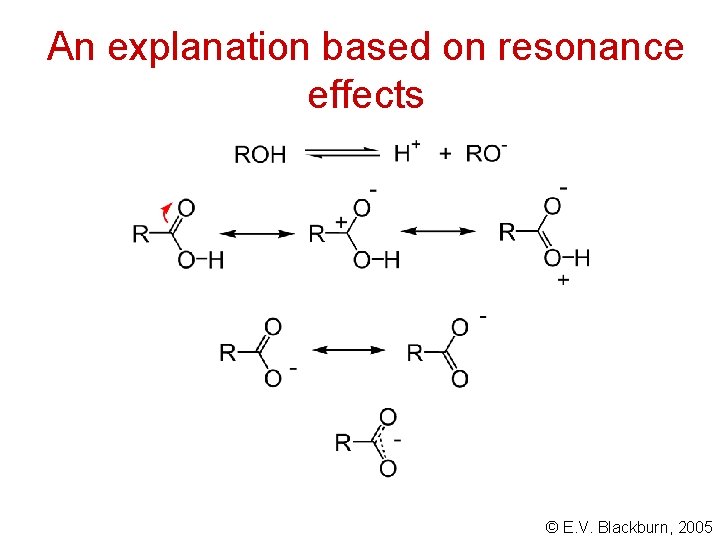
An explanation based on resonance effects © E. V. Blackburn, 2005
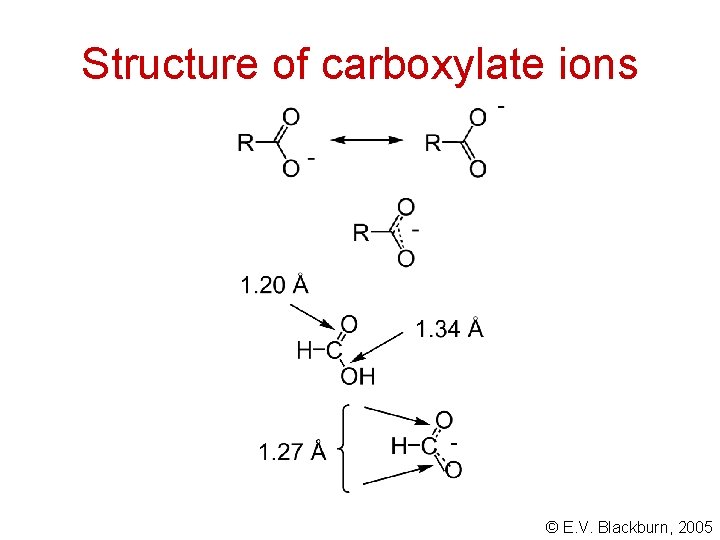
Structure of carboxylate ions © E. V. Blackburn, 2005
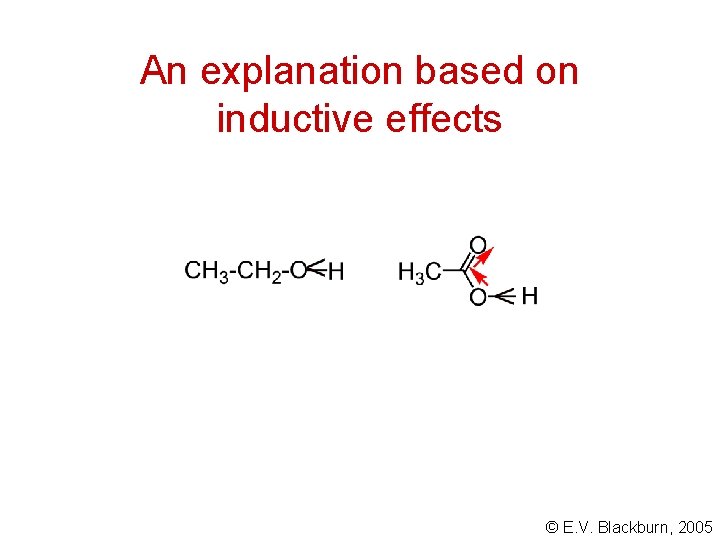
An explanation based on inductive effects © E. V. Blackburn, 2005
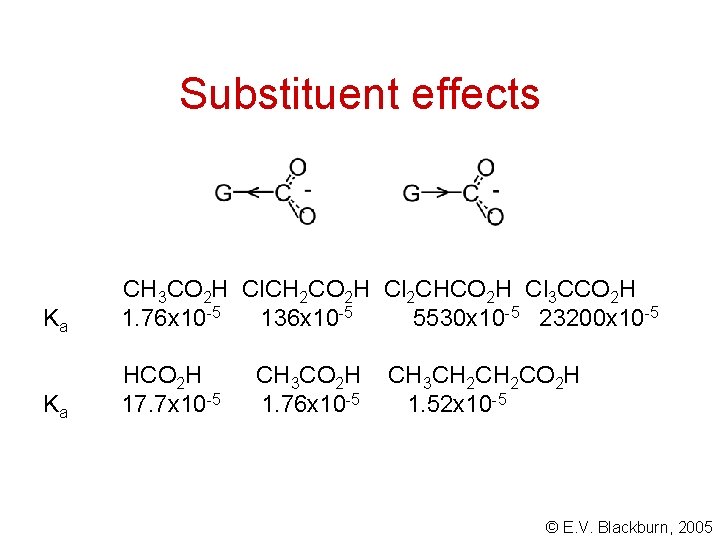
Substituent effects Ka CH 3 CO 2 H Cl. CH 2 CO 2 H Cl 2 CHCO 2 H Cl 3 CCO 2 H 1. 76 x 10 -5 136 x 10 -5 5530 x 10 -5 23200 x 10 -5 Ka HCO 2 H 17. 7 x 10 -5 CH 3 CO 2 H 1. 76 x 10 -5 CH 3 CH 2 CO 2 H 1. 52 x 10 -5 © E. V. Blackburn, 2005

Problems Try problems 3. 27 – 3. 31 on page 130 of Solomons and Fryhle. © E. V. Blackburn, 2005
- Slides: 45