An Introduction to Organic Chemistry High School Level















- Slides: 84

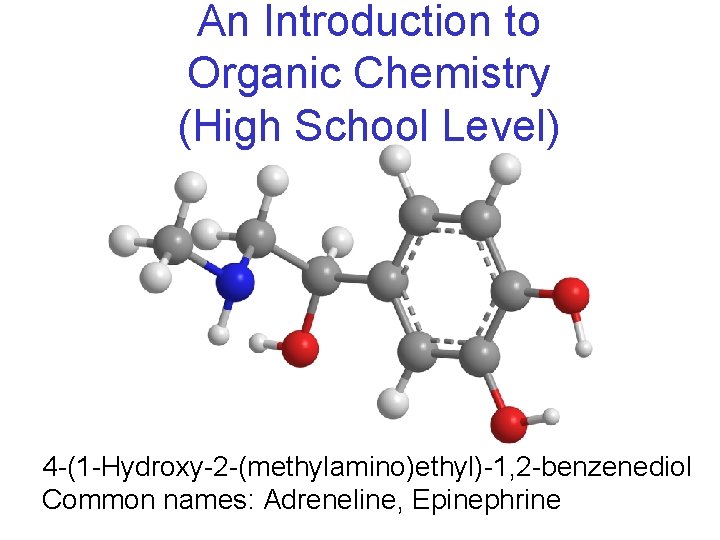
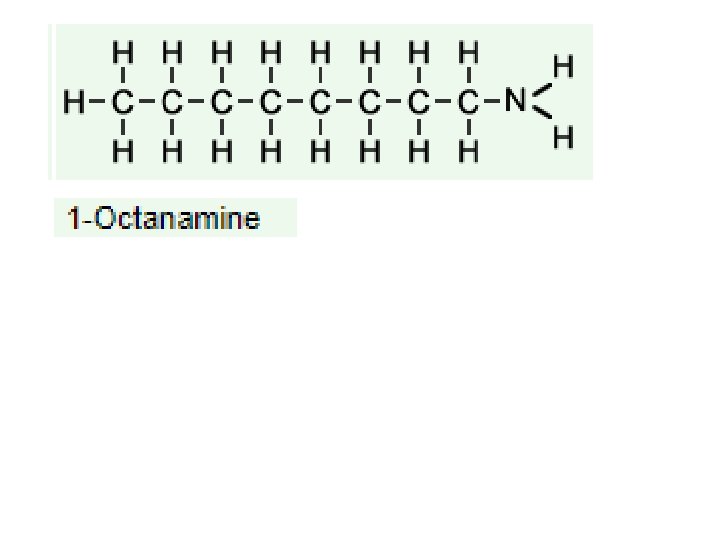
An Introduction to Organic Chemistry (High School Level) 4 -(1 -Hydroxy-2 -(methylamino)ethyl)-1, 2 -benzenediol Common names: Adreneline, Epinephrine

Classification of Hydrocarbons 24. 1

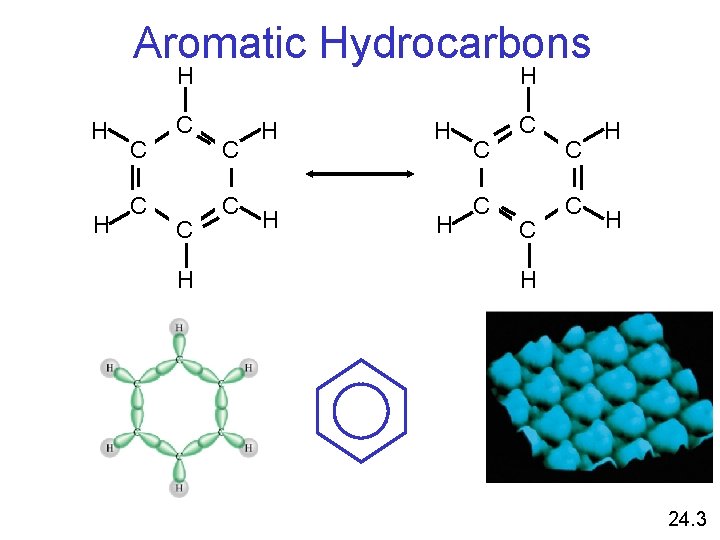
Classification of Hydrocarbons Aromatic: a hydrocarbon that contains one or more benzene rings. 24. 1

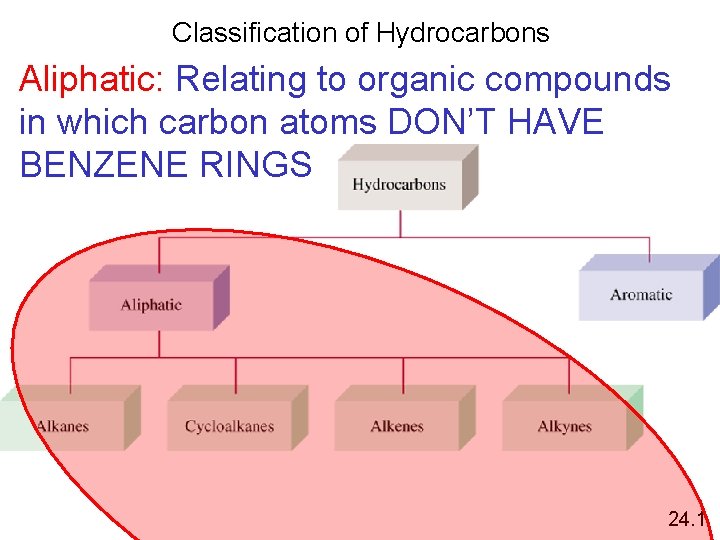
Classification of Hydrocarbons Aliphatic: Relating to organic compounds in which carbon atoms DON’T HAVE BENZENE RINGS 24. 1

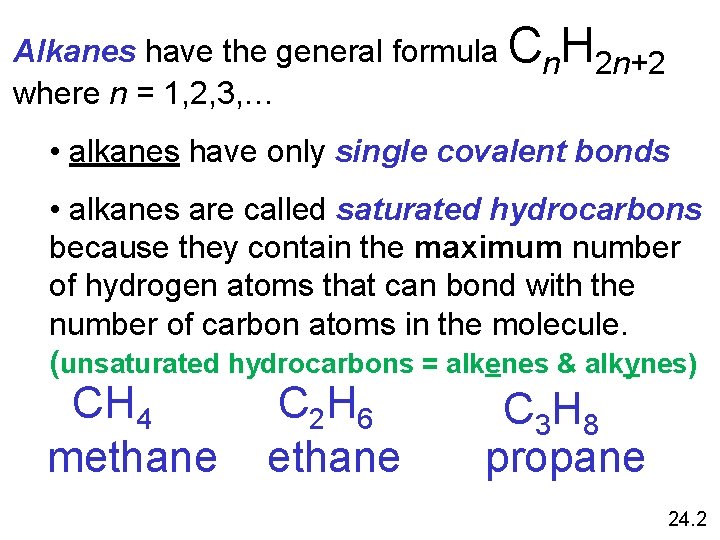
Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • alkanes have only single covalent bonds • alkanes are called saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule. (unsaturated hydrocarbons = alkenes & alkynes) CH 4 methane C 2 H 6 ethane C 3 H 8 propane 24. 2

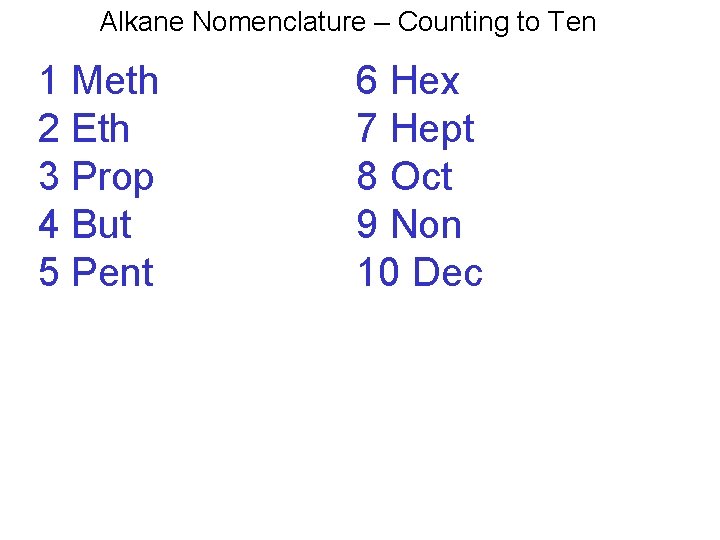
Alkane Nomenclature – Counting to Ten 1 Meth 2 Eth 3 Prop 4 But 5 Pent 6 Hex 7 Hept 8 Oct 9 Non 10 Dec

Practice! Name the following alkanes: CH 4 methane C 5 H 12 pentane C 8 H 18 octane C 6 H 14 hexane Formula of ? ? ethane C 2 H 6 nonane C 9 H 20 propane Quiz on C 3 H 8 this slide butane Monday C 4 H 10 Go 49 ers!

How to draw Lewis structure of alkanes. (Go to promethean board) Methane Ethane Propane Butane Etc.

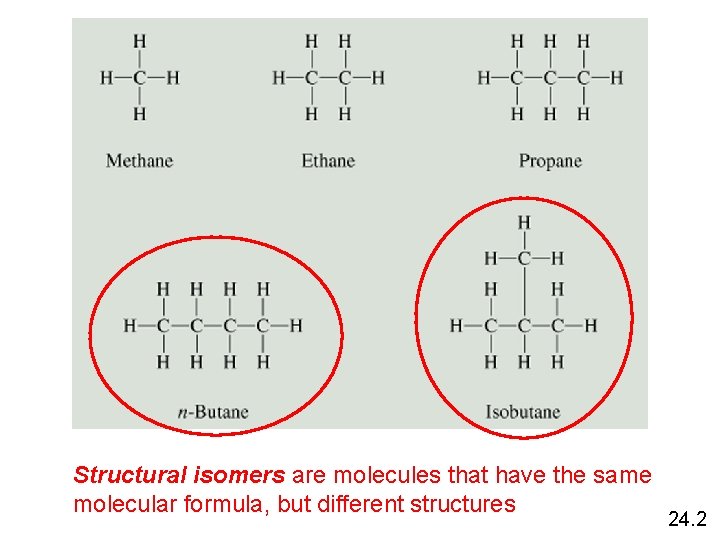
Structural isomers are molecules that have the same molecular formula, but different structures 24. 2

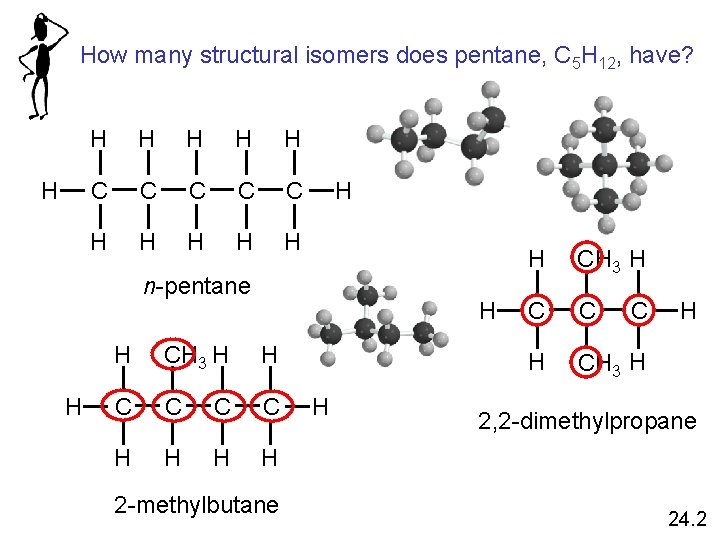
How many structural isomers does pentane, C 5 H 12, have? H H H C C C H H H n-pentane H H H CH 3 H H C C H H 2 -methylbutane H H CH 3 H C C H CH 3 H C H 2, 2 -dimethylpropane 24. 2

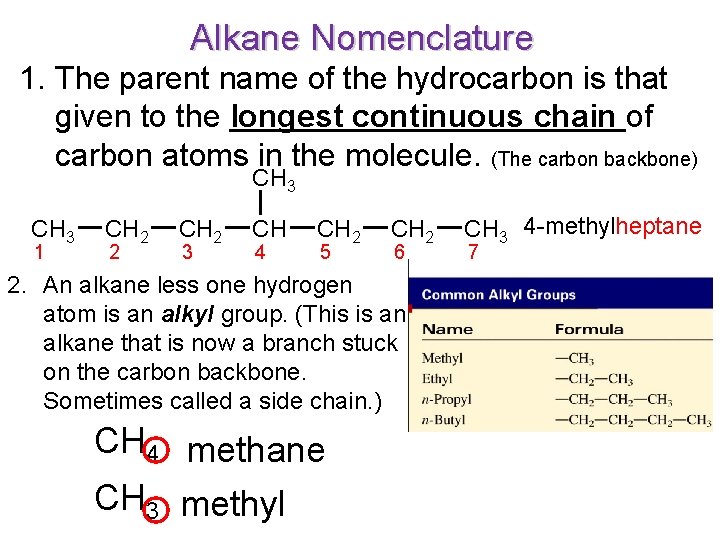
Alkane Nomenclature 1. The parent name of the hydrocarbon is that given to the longest continuous chain of carbon atoms in the molecule. (The carbon backbone) CH 3 1 CH 2 2 CH 2 3 CH 4 CH 2 5 CH 2 6 2. An alkane less one hydrogen atom is an alkyl group. (This is an alkane that is now a branch stuck on the carbon backbone. Sometimes called a side chain. ) CH 4 methane CH 3 methyl CH 3 4 -methylheptane 7

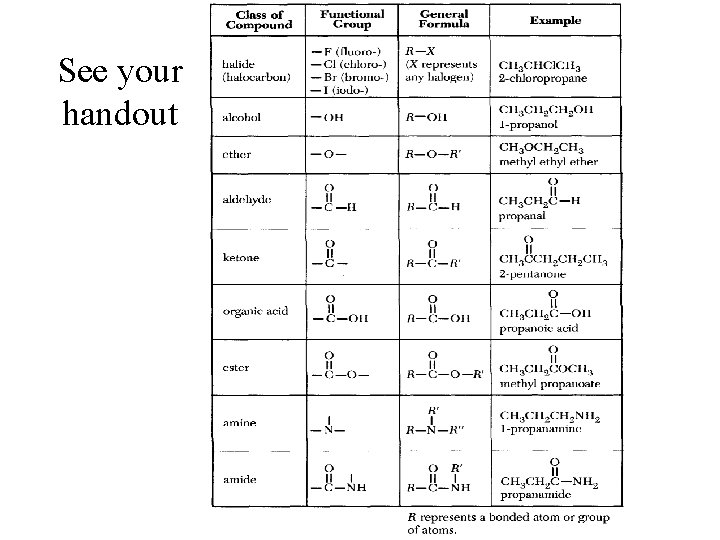
See your handout

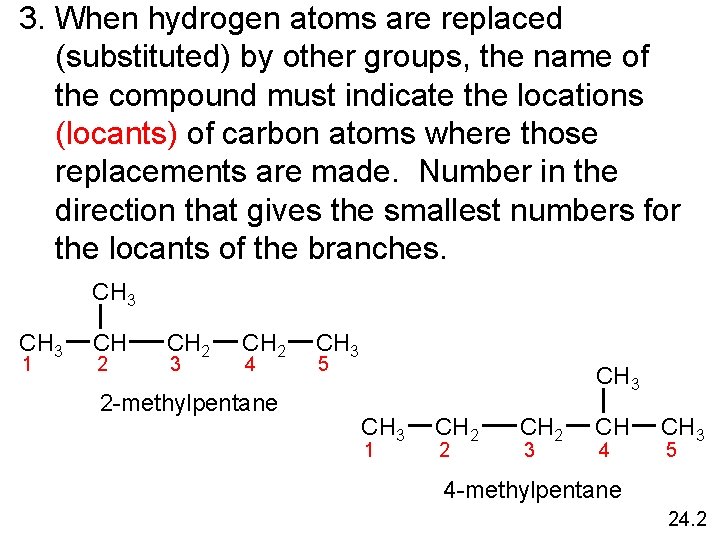
3. When hydrogen atoms are replaced (substituted) by other groups, the name of the compound must indicate the locations (locants) of carbon atoms where those replacements are made. Number in the direction that gives the smallest numbers for the locants of the branches. CH 3 1 CH 2 3 CH 2 4 2 -methylpentane CH 3 5 CH 3 1 CH 2 2 CH 2 3 CH 4 CH 3 5 4 -methylpentane 24. 2

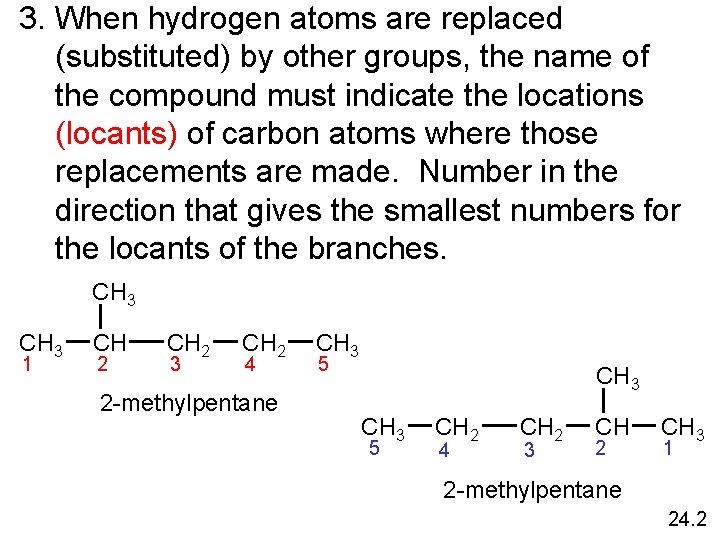
3. When hydrogen atoms are replaced (substituted) by other groups, the name of the compound must indicate the locations (locants) of carbon atoms where those replacements are made. Number in the direction that gives the smallest numbers for the locants of the branches. CH 3 1 CH 2 3 CH 2 4 2 -methylpentane CH 3 5 CH 2 4 CH 2 3 CH 2 CH 3 1 2 -methylpentane 24. 2

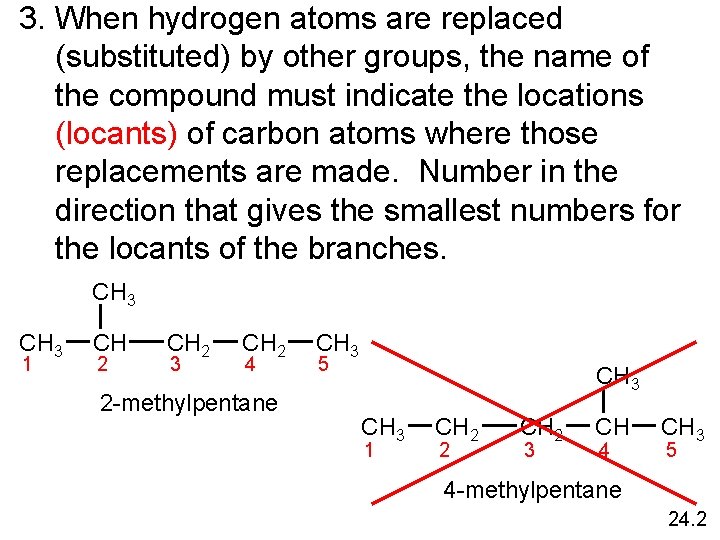
3. When hydrogen atoms are replaced (substituted) by other groups, the name of the compound must indicate the locations (locants) of carbon atoms where those replacements are made. Number in the direction that gives the smallest numbers for the locants of the branches. CH 3 1 CH 2 3 CH 2 4 2 -methylpentane CH 3 5 CH 3 1 CH 2 2 CH 2 3 CH 4 CH 3 5 4 -methylpentane 24. 2

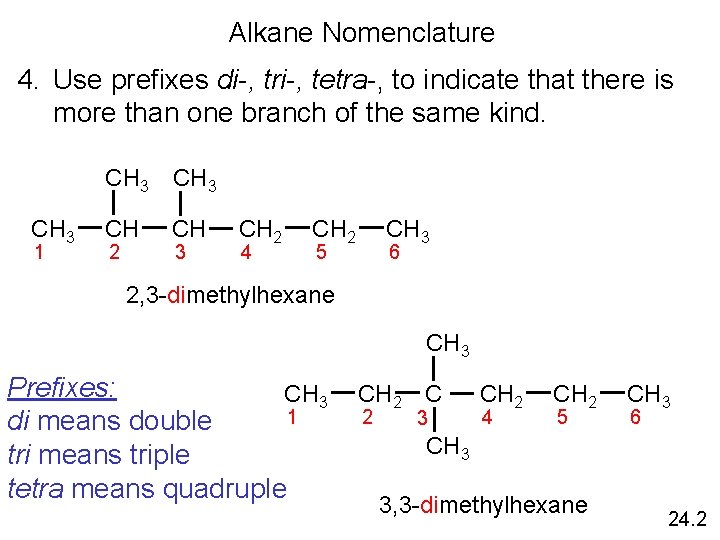
Alkane Nomenclature 4. Use prefixes di-, tri-, tetra-, to indicate that there is more than one branch of the same kind. CH 3 1 CH 3 CH CH 2 3 CH 2 4 CH 2 CH 3 5 6 2, 3 -dimethylhexane CH 3 Prefixes: CH 3 1 di means double tri means triple tetra means quadruple CH 2 C 2 3 CH 2 4 CH 2 5 CH 3 6 CH 3 3, 3 -dimethylhexane 24. 2

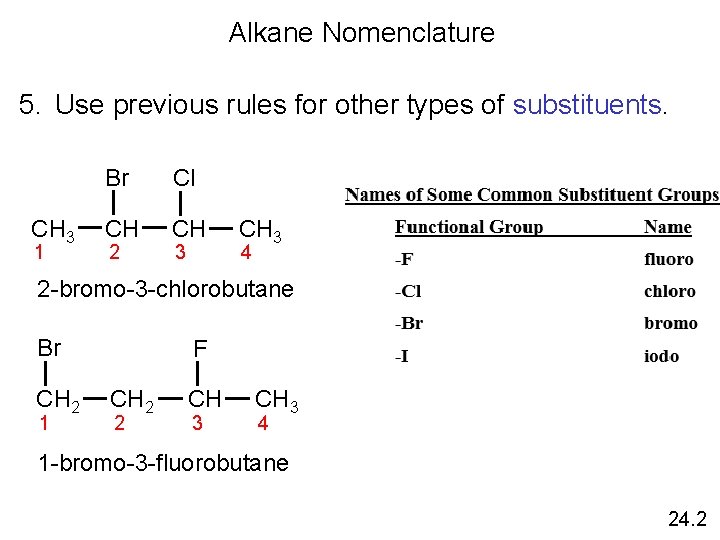
Alkane Nomenclature 5. Use previous rules for other types of substituents. CH 3 1 Br Cl CH CH 2 3 CH 3 4 2 -bromo-3 -chlorobutane Br CH 2 1 F CH 2 2 CH 3 4 1 -bromo-3 -fluorobutane 24. 2

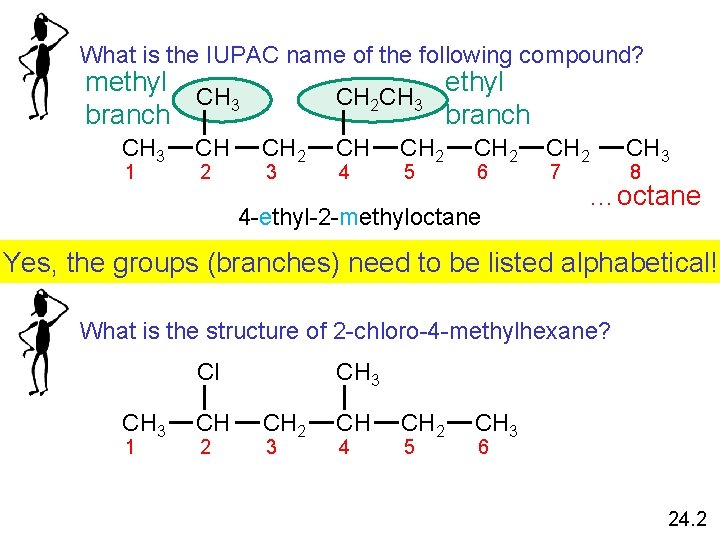
What is the IUPAC name of the following compound? methyl CH 3 branch CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 ethyl branch CH 2 6 4 -ethyl-2 -methyloctane CH 2 7 CH 3 8 …octane Yes, the groups (branches) need to be listed alphabetical! What is the structure of 2 -chloro-4 -methylhexane? Cl CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 CH 3 6 24. 2

Summary of rules for naming a basic alkane: • Assign a parent name based upon the longest continuous carbon chain. (Find the longest C chain) • Assign location numbers (called locants) to all of the groups. Count along the parent chain in both directions: use the version that yields the lowest starting locant for the first branch. • Identify and name the branches coming off the parent chain. • Have same groups in different places? Use prefixes. (di for two, tri for three, or tetra for four) • Shove it all together alphabetically using commas and dashes!

Now build n-heptane

Isomers of heptane • Heptane • 2 -Methylhexane • 3 -Methylhexane • 2, 2 -Dimethylpentane • 2, 3 -Dimethylpentane • 2, 4 -Dimethylpentane • 3, 3 -Dimethylpentane • 3 -Ethylpentane • 2, 2, 3 -Trimethylbutane

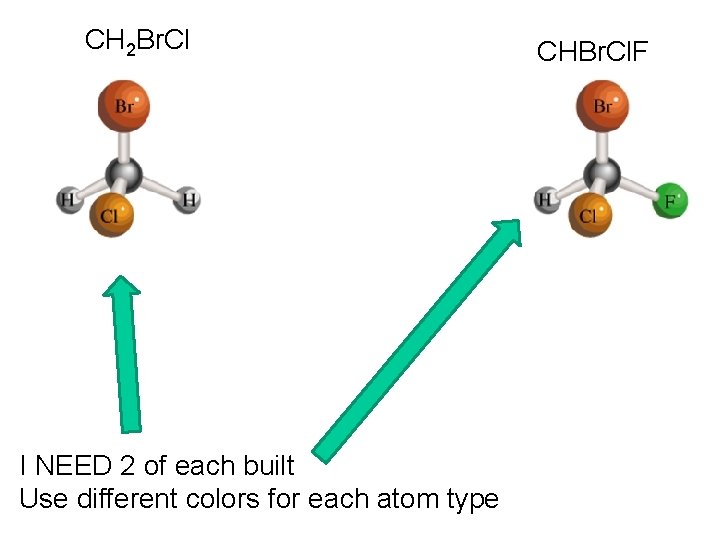
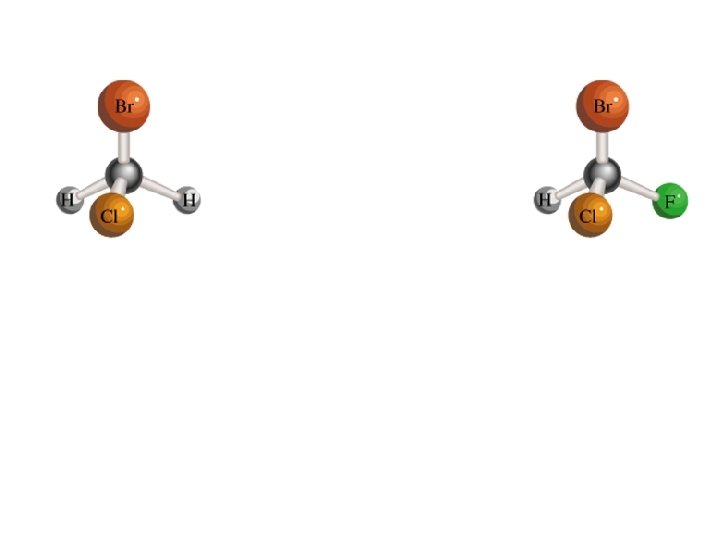
CH 2 Br. Cl I NEED 2 of each built Use different colors for each atom type CHBr. Cl. F


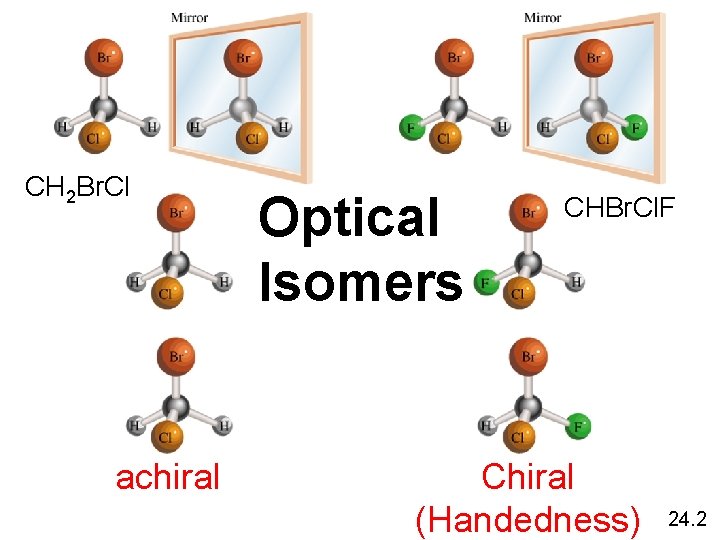
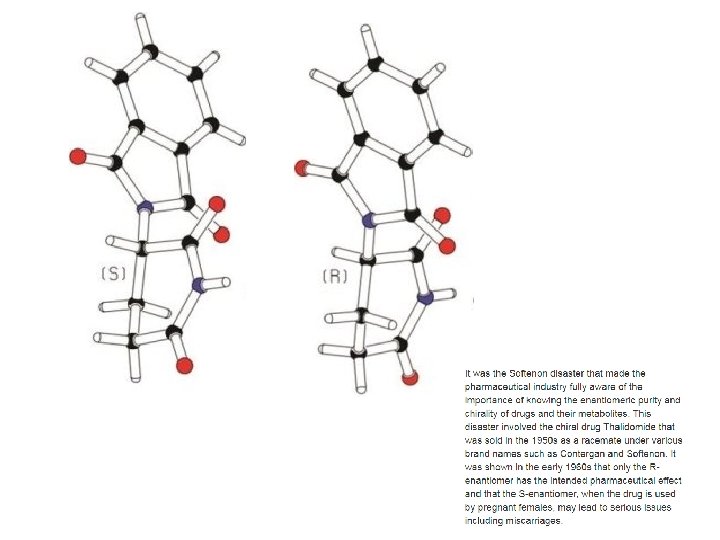
CH 2 Br. Cl achiral Optical Isomers CHBr. Cl. F Chiral (Handedness) 24. 2

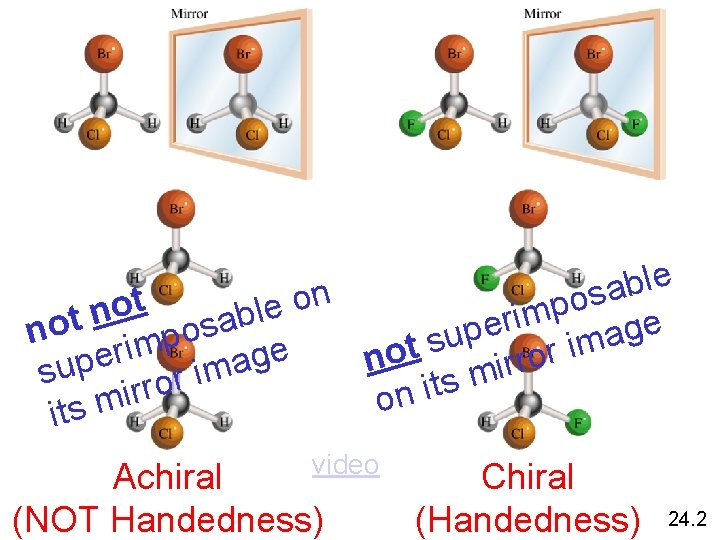
e l b a n s o o t p o e l n m b i t r a e o e s g p n o a u p s m m i t i r e r o e g o n rr i sup ror ima m s t i r i n o its m video Achiral (NOT Handedness) Chiral (Handedness) 24. 2

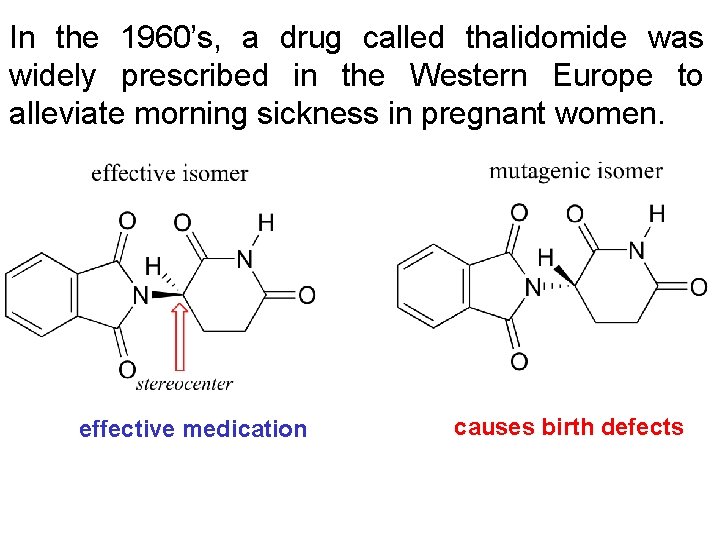
In the 1960’s, a drug called thalidomide was widely prescribed in the Western Europe to alleviate morning sickness in pregnant women. effective medication causes birth defects


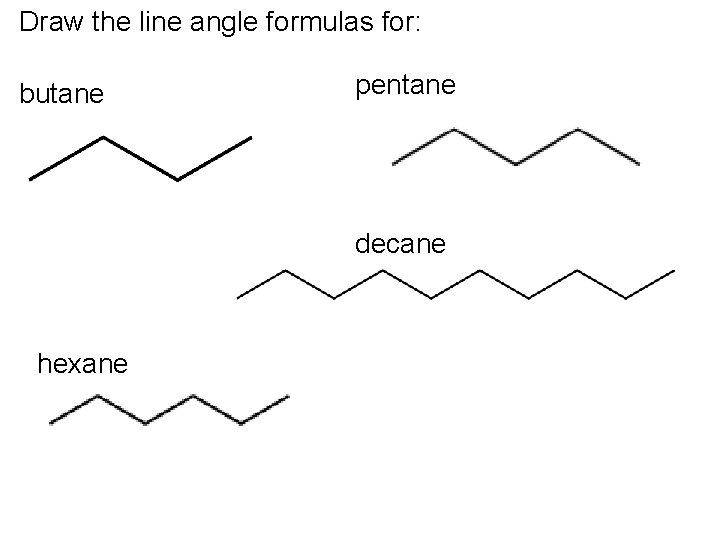
Draw the line angle formulas for: butane pentane decane hexane

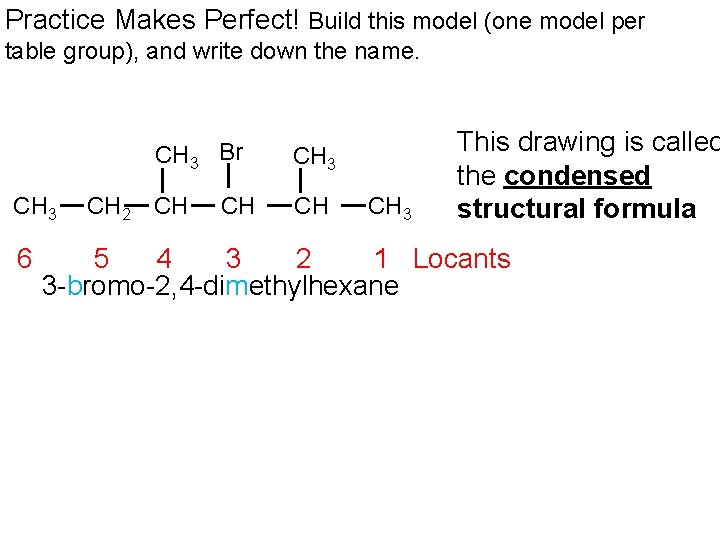
Practice Makes Perfect! Build this model (one model per table group), and write down the name. CH 3 Br CH 3 6 CH 2 CH CH CH 3 This drawing is called the condensed structural formula 5 4 3 2 1 Locants 3 -bromo-2, 4 -dimethylhexane

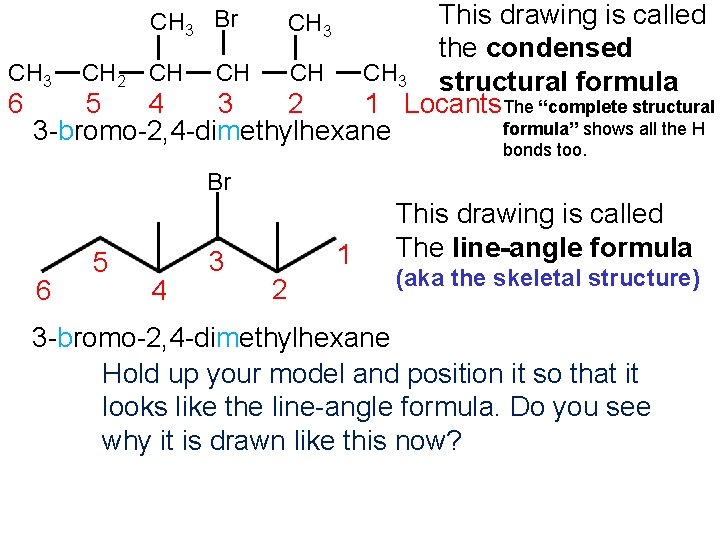
This drawing is called the condensed CH 3 structural formula CH CH 3 CH 2 CH CH 6 5 4 3 2 1 Locants The “complete structural formula” shows all the H 3 -bromo-2, 4 -dimethylhexane CH 3 Br CH 3 bonds too. Br 6 5 4 3 1 2 This drawing is called The line-angle formula (aka the skeletal structure) 3 -bromo-2, 4 -dimethylhexane Hold up your model and position it so that it looks like the line-angle formula. Do you see why it is drawn like this now?

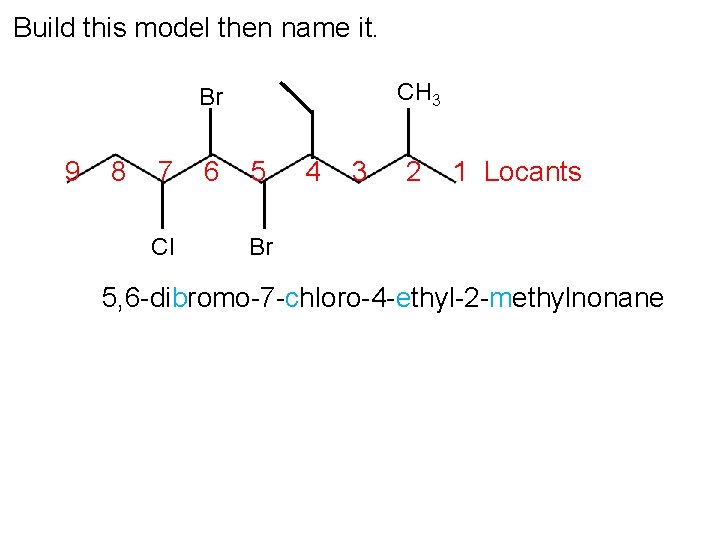
Build this model then name it. CH 3 Br 9 8 7 Cl 6 5 4 3 2 1 Locants Br 5, 6 -dibromo-7 -chloro-4 -ethyl-2 -methylnonane

Draw 2 -bromo-4 -ethyl-3 -methylheptane Draw 1 -chloro-4, 4 -diethyloctane

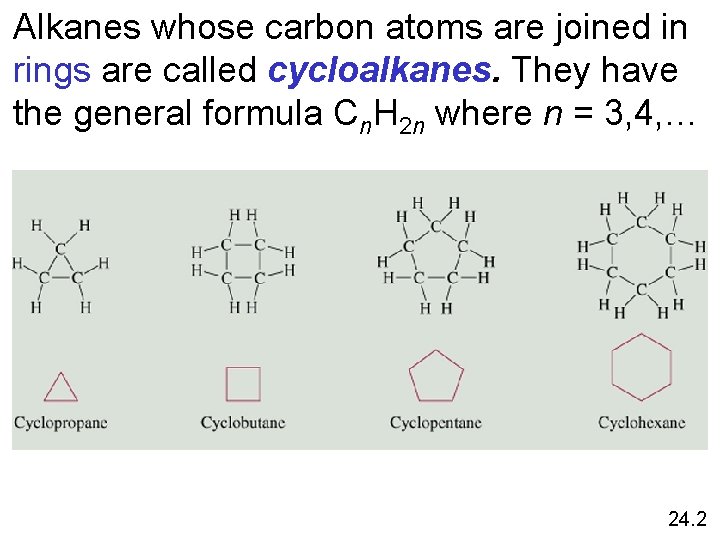
Alkanes whose carbon atoms are joined in rings are called cycloalkanes. They have the general formula Cn. H 2 n where n = 3, 4, … 24. 2

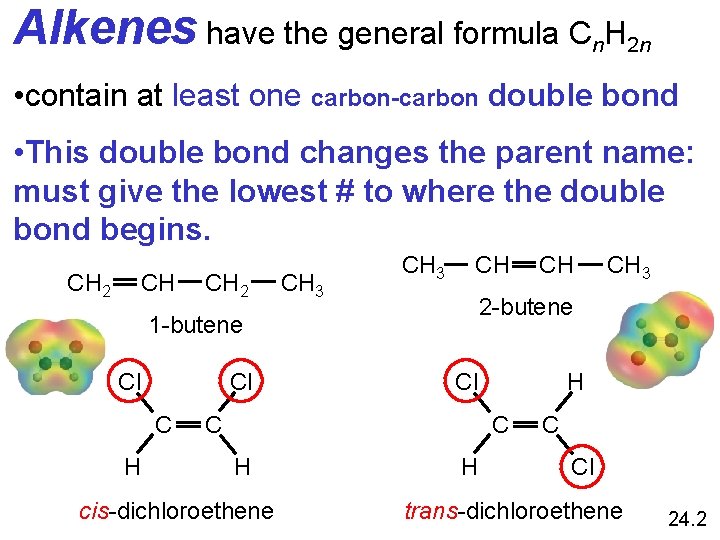
Alkenes have the general formula Cn. H 2 n • contain at least one carbon-carbon double bond • This double bond changes the parent name: must give the lowest # to where the double bond begins. CH CH 2 CH 3 CH Cl C H C H cis-dichloroethene CH 3 2 -butene 1 -butene Cl CH H C Cl trans-dichloroethene 24. 2

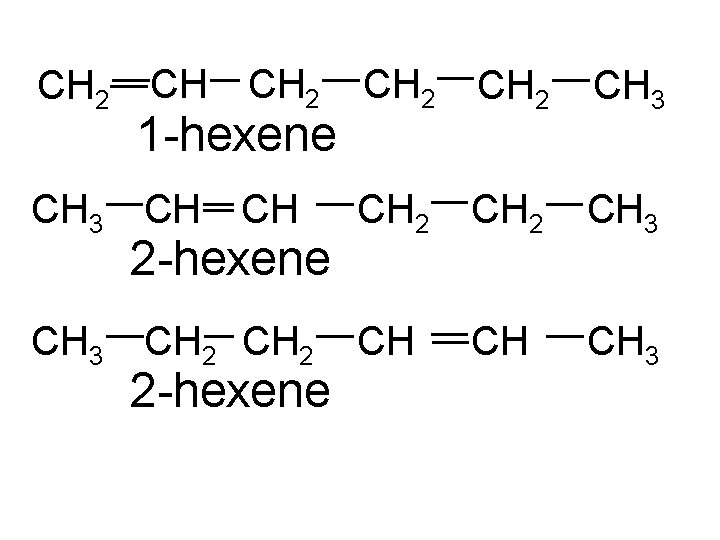
CH 2 CH 3 CH CH 2 CH 3 CH CH CH 3 1 -hexene CH 2 2 -hexene

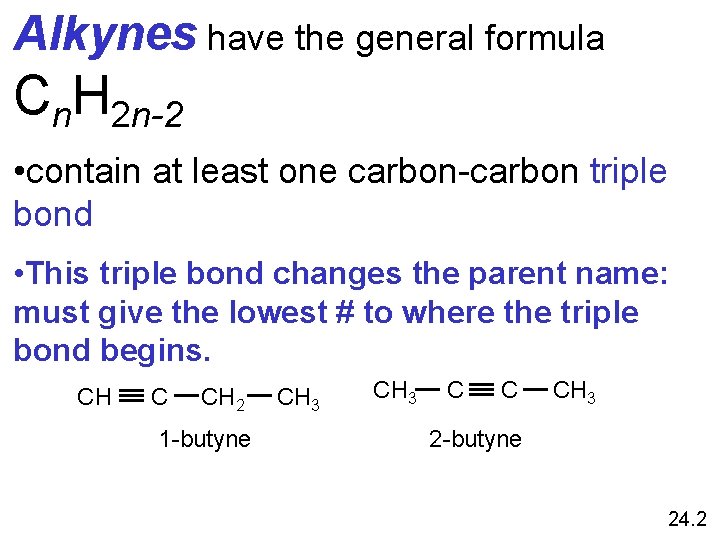
Alkynes have the general formula Cn. H 2 n-2 • contain at least one carbon-carbon triple bond • This triple bond changes the parent name: must give the lowest # to where the triple bond begins. CH C CH 2 1 -butyne CH 3 C C CH 3 2 -butyne 24. 2

Aromatic Hydrocarbons H H C C H H C C C H H H 24. 3

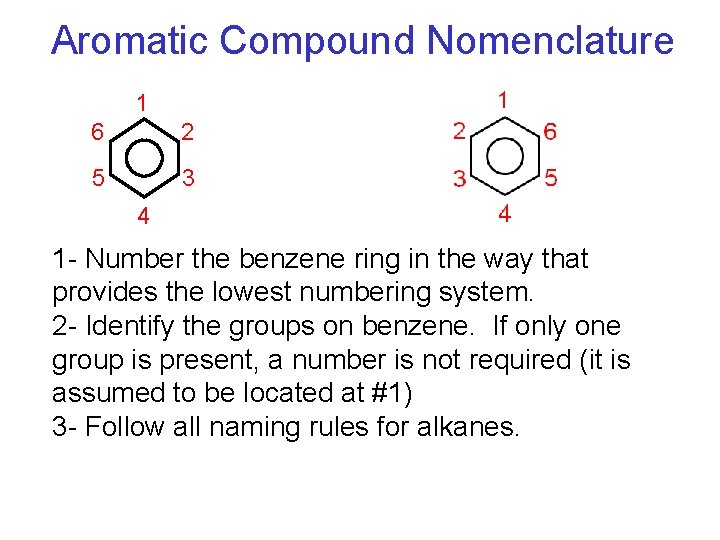
Aromatic Compound Nomenclature 1 6 2 5 3 4 1 - Number the benzene ring in the way that provides the lowest numbering system. 2 - Identify the groups on benzene. If only one group is present, a number is not required (it is assumed to be located at #1) 3 - Follow all naming rules for alkanes.

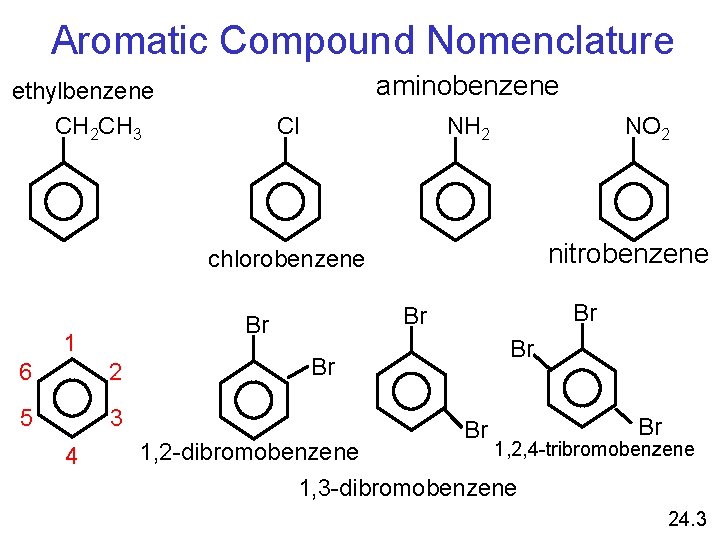
Aromatic Compound Nomenclature aminobenzene ethylbenzene CH 2 CH 3 NH 2 Cl NO 2 nitrobenzene chlorobenzene 1 6 2 5 3 4 Br Br 1, 2, 4 -tribromobenzene 1, 2 -dibromobenzene 1, 3 -dibromobenzene 24. 3

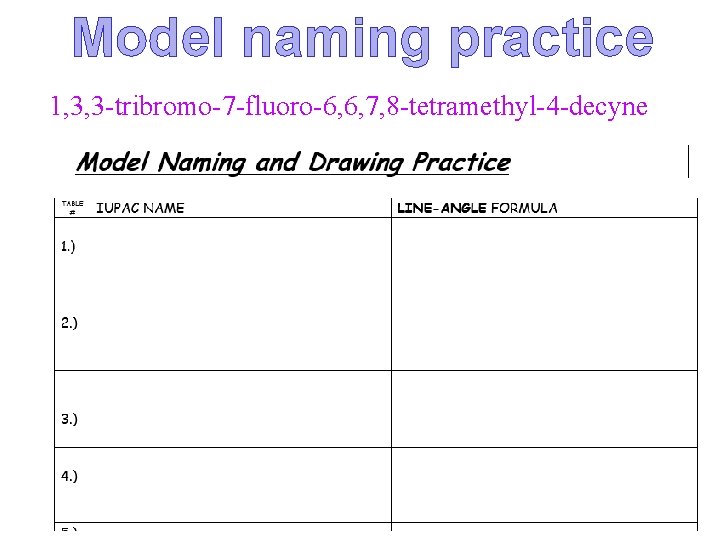
Model naming practice 1, 3, 3 -tribromo-7 -fluoro-6, 6, 7, 8 -tetramethyl-4 -decyne

Organic Chemistry: Other Functional Groups

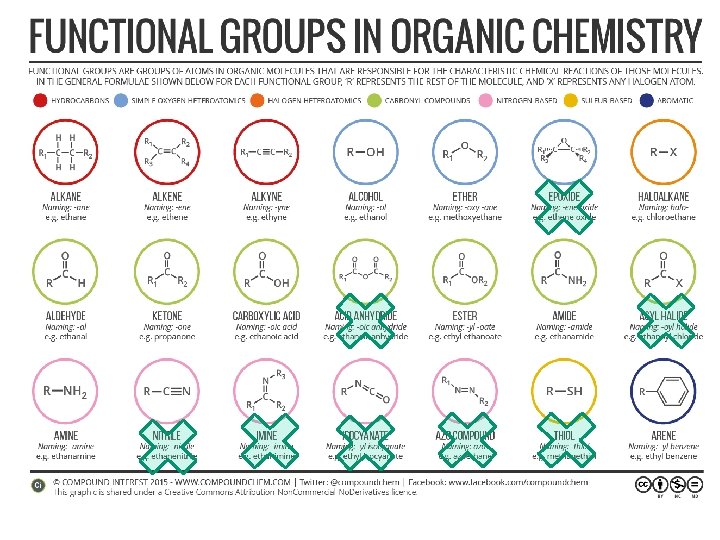
Functional groups • Functional groups are parts of molecules that result in characteristic features • About 100 functional groups exist, we will focus on about 10


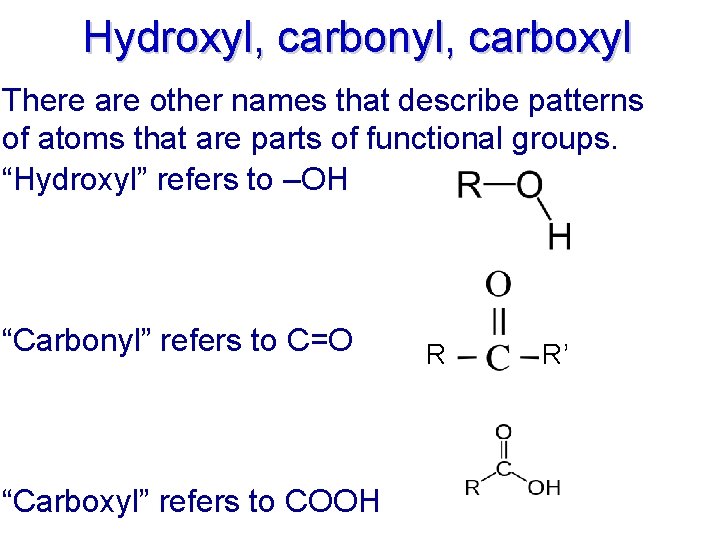
Hydroxyl, carbonyl, carboxyl There are other names that describe patterns of atoms that are parts of functional groups. “Hydroxyl” refers to –OH “Carbonyl” refers to C=O “Carboxyl” refers to COOH R R’

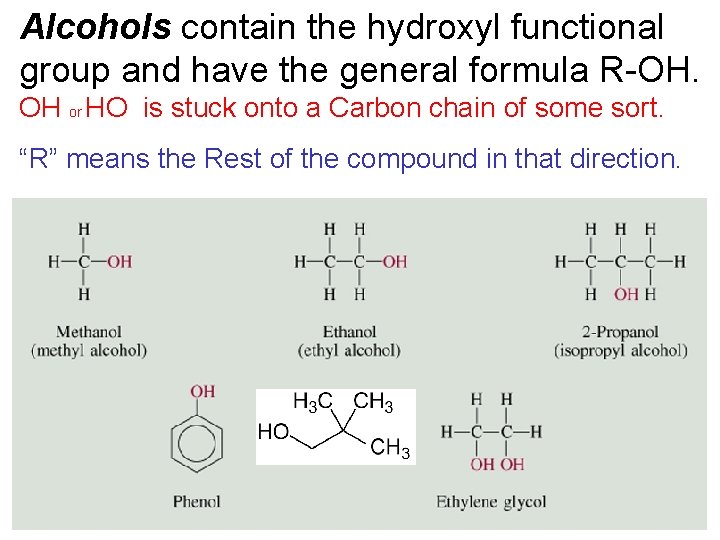
Alcohols contain the hydroxyl functional group and have the general formula R-OH. OH or HO is stuck onto a Carbon chain of some sort. “R” means the Rest of the compound in that direction. 24. 4

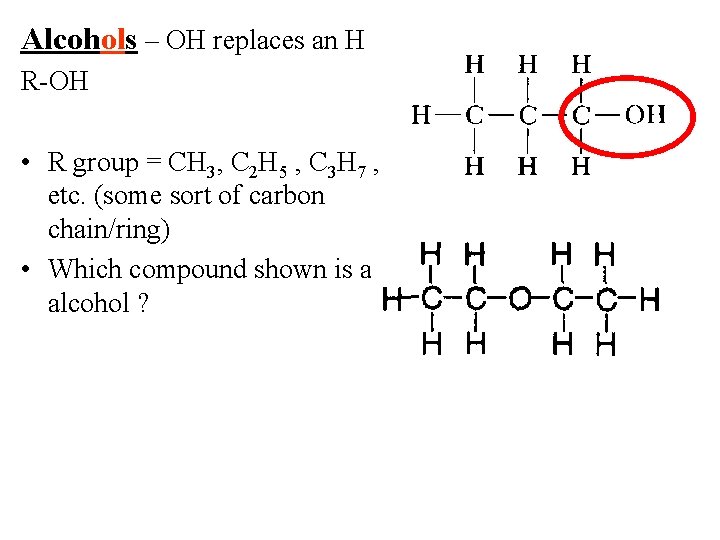
Alcohols – OH replaces an H R-OH • R group = CH 3, C 2 H 5 , C 3 H 7 , etc. (some sort of carbon chain/ring) • Which compound shown is an alcohol ?

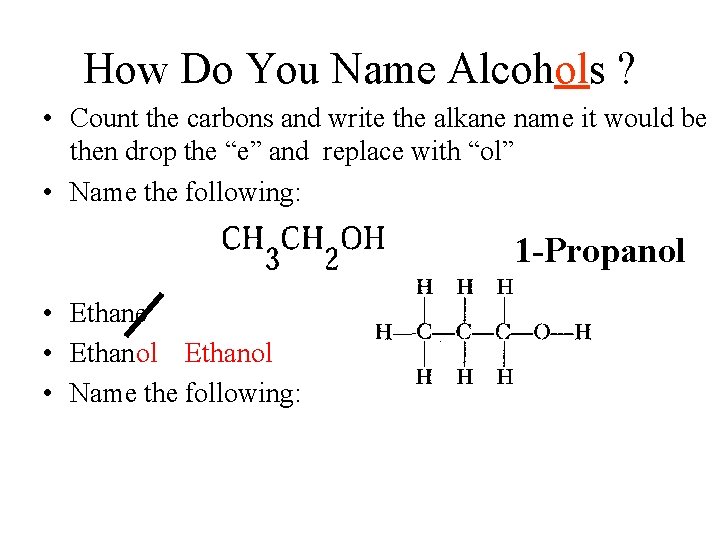
How Do You Name Alcohols ? • Count the carbons and write the alkane name it would be then drop the “e” and replace with “ol” • Name the following: 1 -Propanol • Ethane • Ethanol • Name the following:

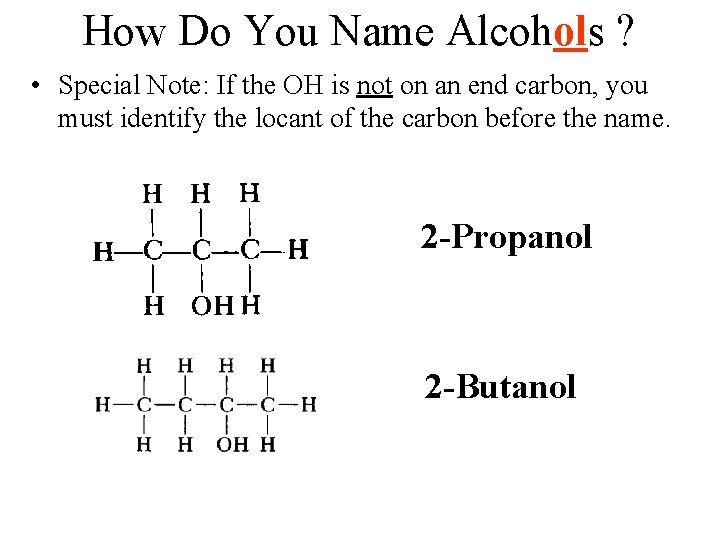
How Do You Name Alcohols ? • Special Note: If the OH is not on an end carbon, you must identify the locant of the carbon before the name. 2 -Propanol 2 -Butanol

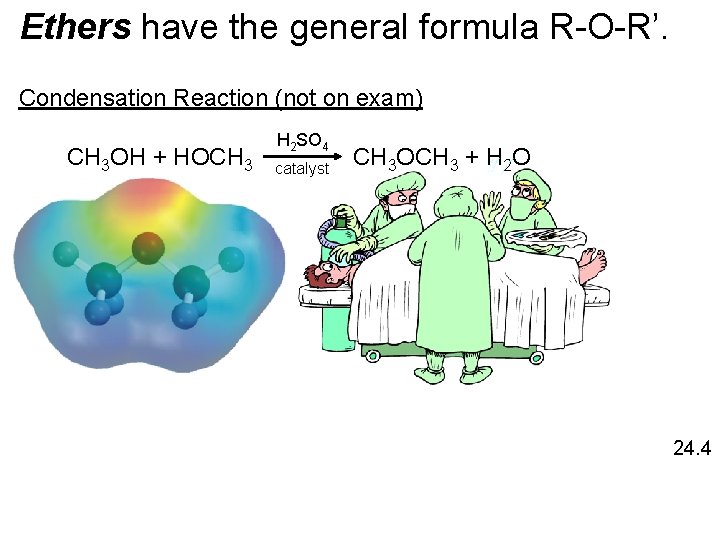
Ethers have the general formula R-O-R’. Condensation Reaction (not on exam) CH 3 OH + HOCH 3 H 2 SO 4 catalyst CH 3 OCH 3 + H 2 O 24. 4



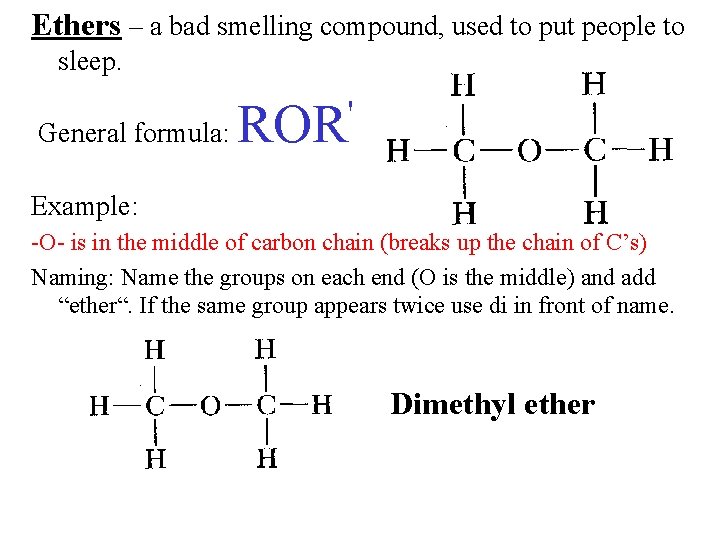
Ethers – a bad smelling compound, used to put people to sleep. ' General formula: ROR Example: -O- is in the middle of carbon chain (breaks up the chain of C’s) Naming: Name the groups on each end (O is the middle) and add “ether“. If the same group appears twice use di in front of name. Dimethyl ether

Using ethers on someone is a CRIME! You better pray for ROR.

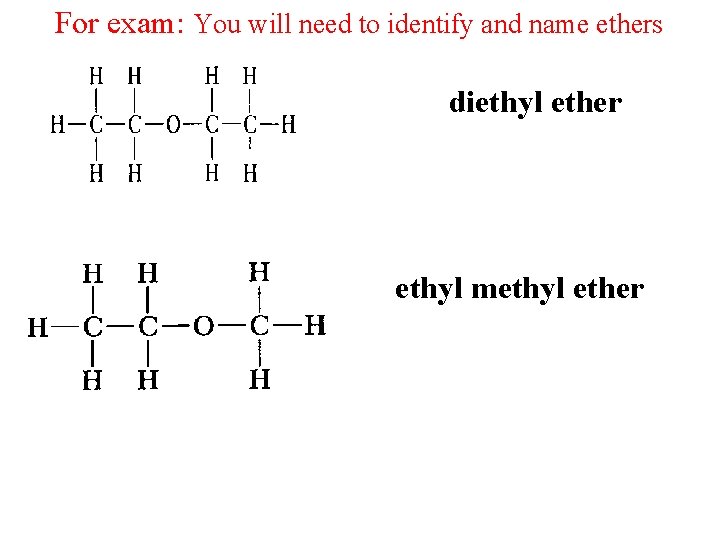
For exam: You will need to identify and name ethers diethyl ether ethyl methyl ether

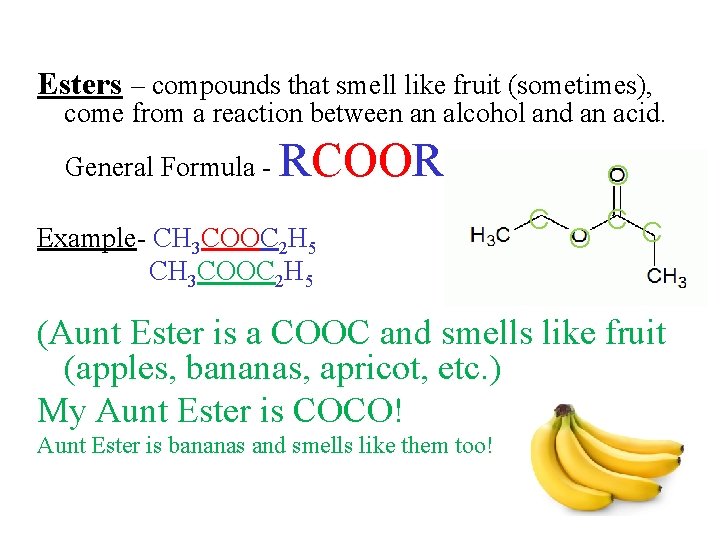
Esters – compounds that smell like fruit (sometimes), come from a reaction between an alcohol and an acid. General Formula - RCOOR Example- CH 3 COOC 2 H 5 O C C C O (Aunt Ester is a COOC and smells like fruit (apples, bananas, apricot, etc. ) My Aunt Ester is COCO! Aunt Ester is bananas and smells like them too!

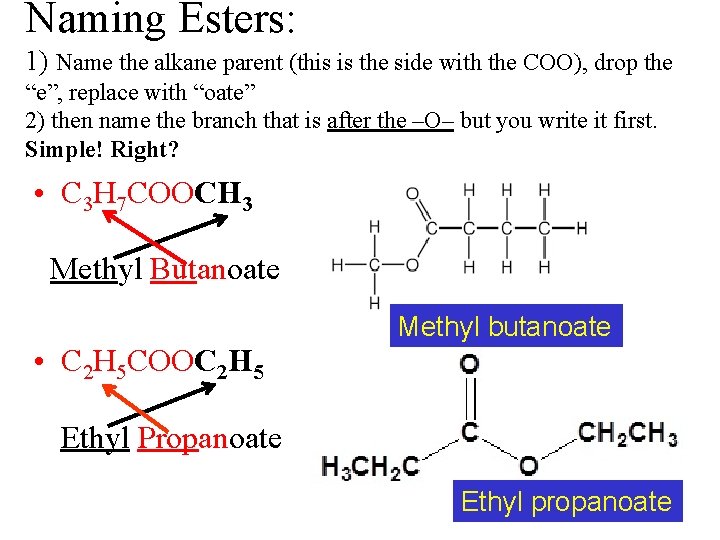
Naming Esters: 1) Name the alkane parent (this is the side with the COO), drop the “e”, replace with “oate” 2) then name the branch that is after the –O– but you write it first. Simple! Right? • C 3 H 7 COOCH 3 Methyl Butanoate Methyl butanoate • C 2 H 5 COOC 2 H 5 Ethyl Propanoate Ethyl propanoate

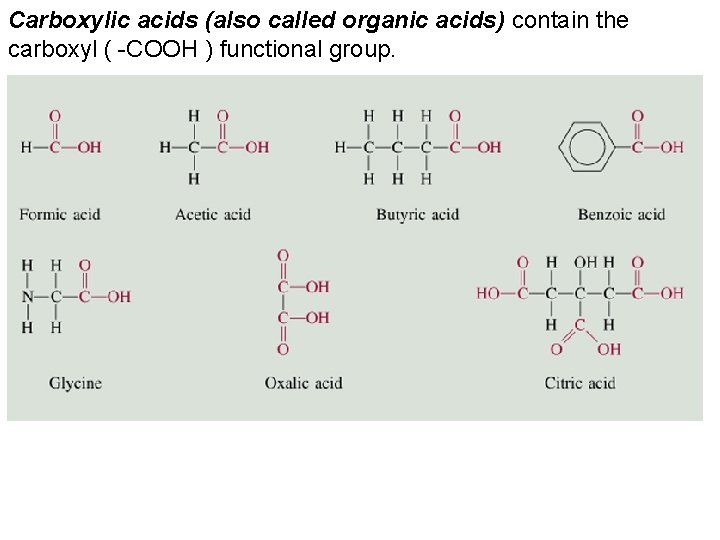
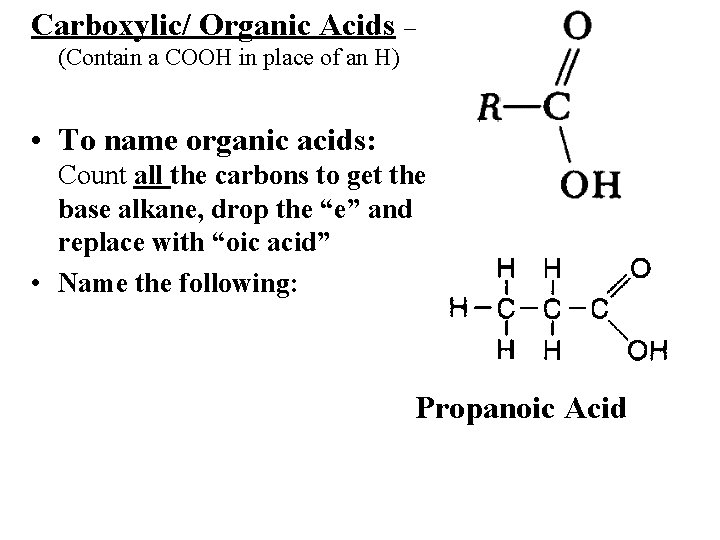
Carboxylic acids (also called organic acids) contain the carboxyl ( -COOH ) functional group.

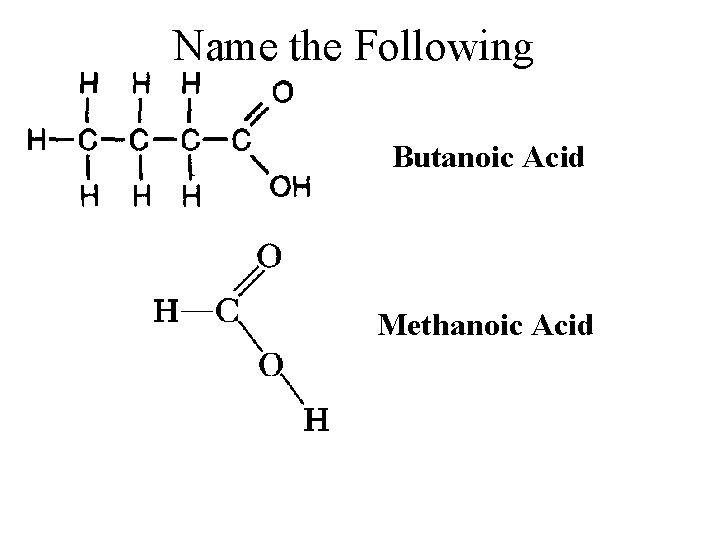
Carboxylic/ Organic Acids – (Contain a COOH in place of an H) • To name organic acids: Count all the carbons to get the base alkane, drop the “e” and replace with “oic acid” • Name the following: Propanoic Acid

Name the Following Butanoic Acid Methanoic Acid

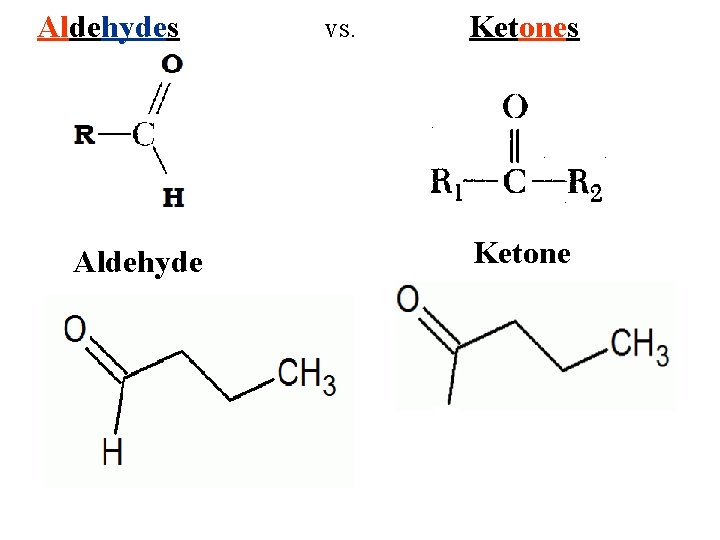
Aldehydes Aldehyde vs. Ketones Ketone

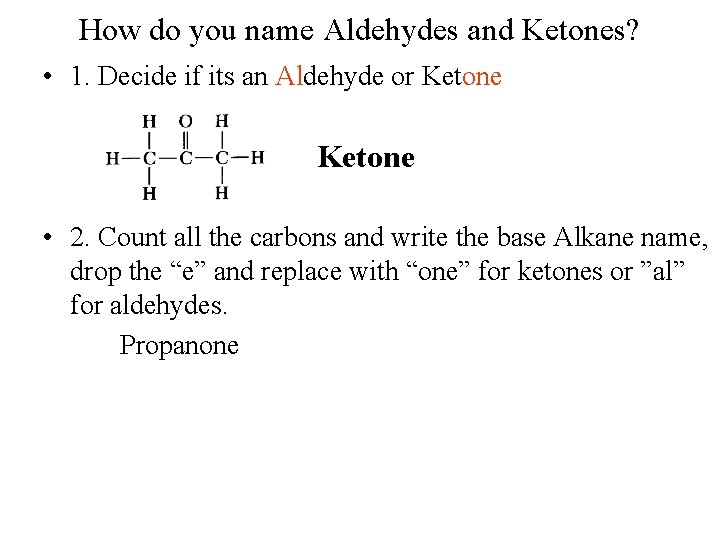
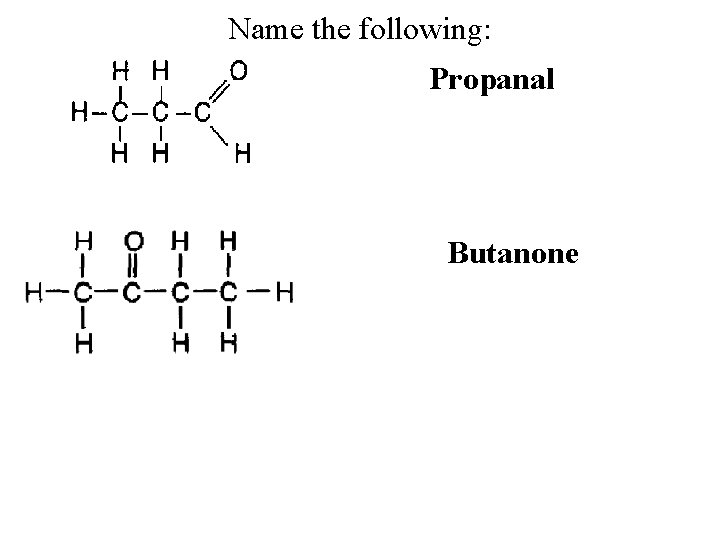
How do you name Aldehydes and Ketones? • 1. Decide if its an Aldehyde or Ketone • 2. Count all the carbons and write the base Alkane name, drop the “e” and replace with “one” for ketones or ”al” for aldehydes. Propanone

Name the following: Propanal Butanone

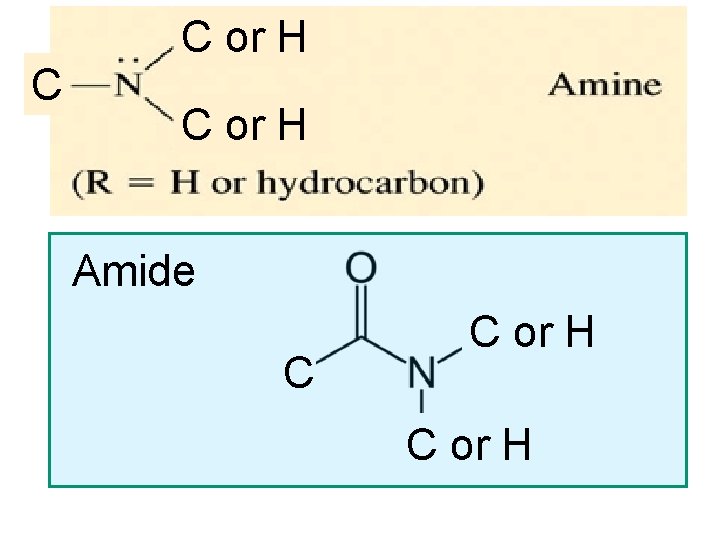
C C or H Amide C C or. Amide H C or H

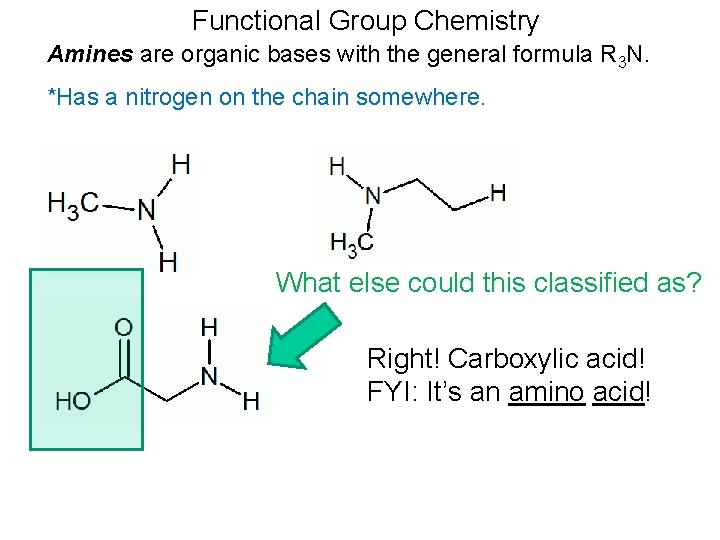
Functional Group Chemistry Amines are organic bases with the general formula R 3 N. *Has a nitrogen on the chain somewhere. What else could this classified as? Right! Carboxylic acid! FYI: It’s an amino acid!

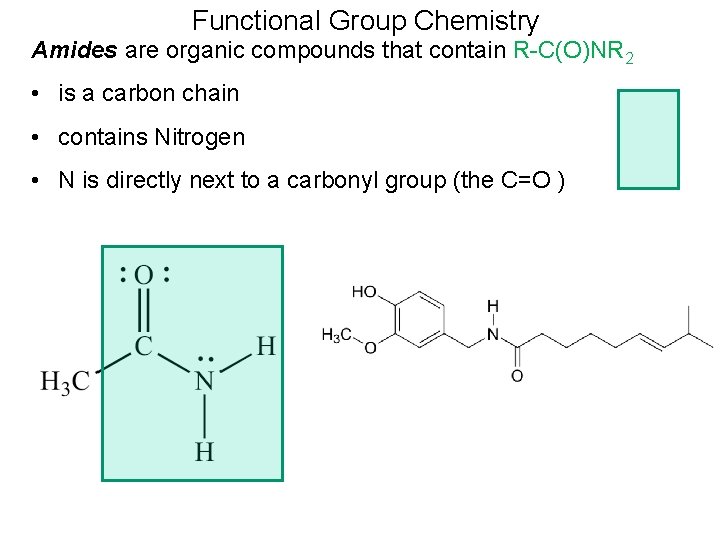
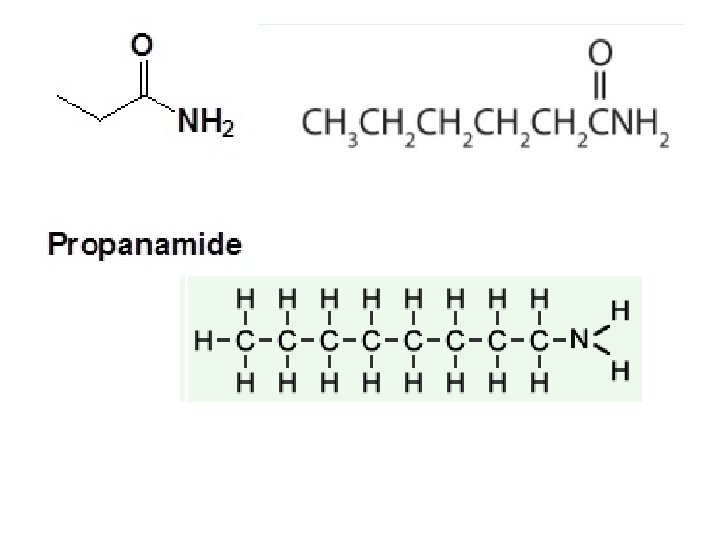
Functional Group Chemistry Amides are organic compounds that contain R-C(O)NR 2 • is a carbon chain • contains Nitrogen • N is directly next to a carbonyl group (the C=O )

How to name amines/amides • • • Amines Count parent carbon chain Assign locant to amine (lowest #) Name of a normal hydrocarbon Drop “e” Add “amine” Put locant in front • • Amides Count parent carbon chain Name of a normal hydrocarbon Drop “e” Add “amide”

See your handout

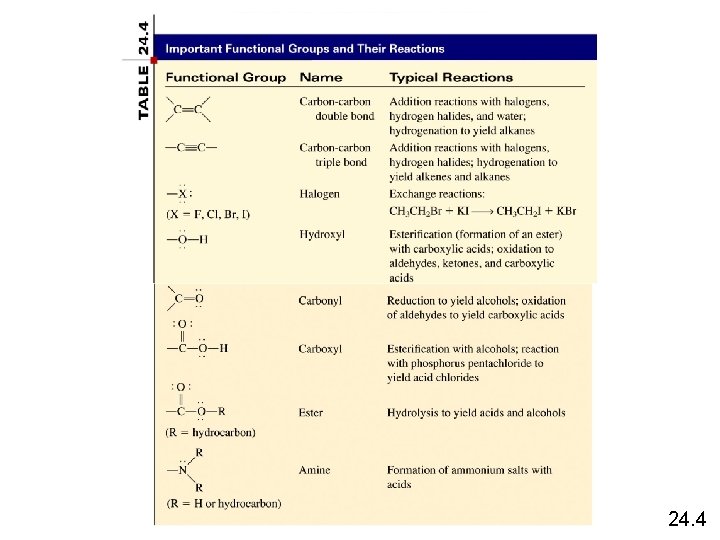
24. 4

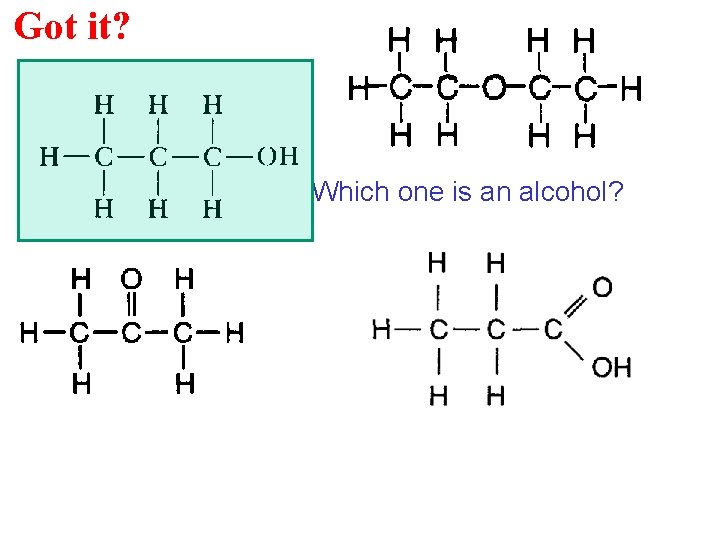
Got it? Which one is an alcohol?

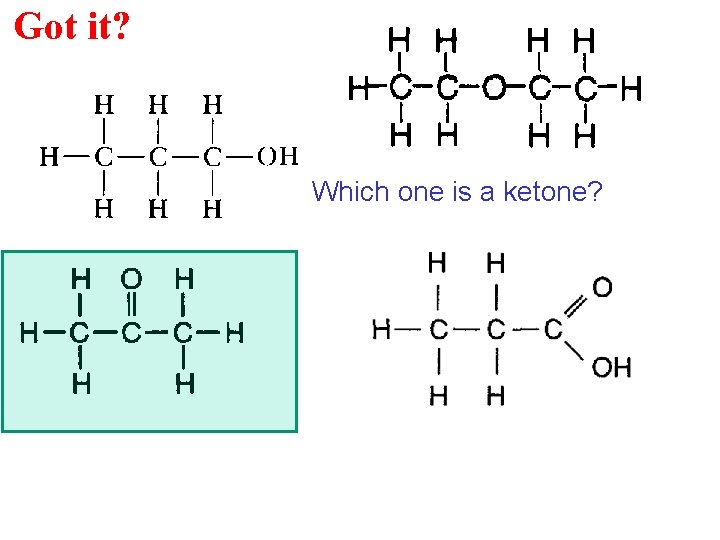
Got it? Which one is a ketone?

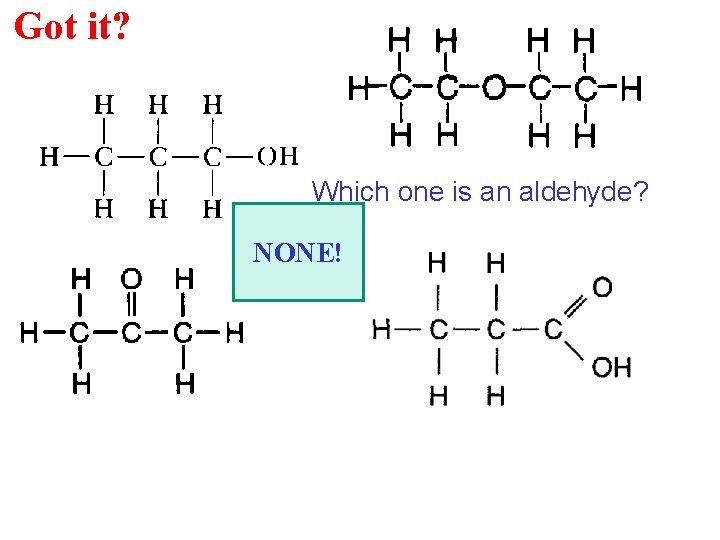
Got it? Which one is an aldehyde? NONE!

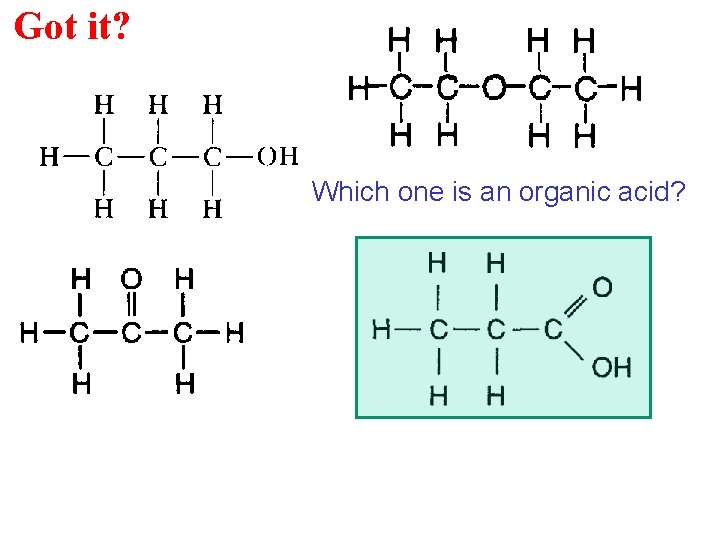
Got it? Which one is an organic acid?

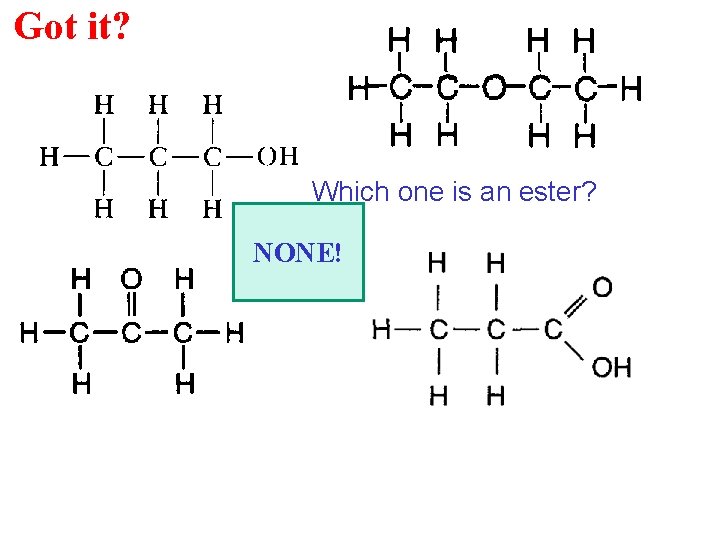
Got it? Which one is an ester? NONE!

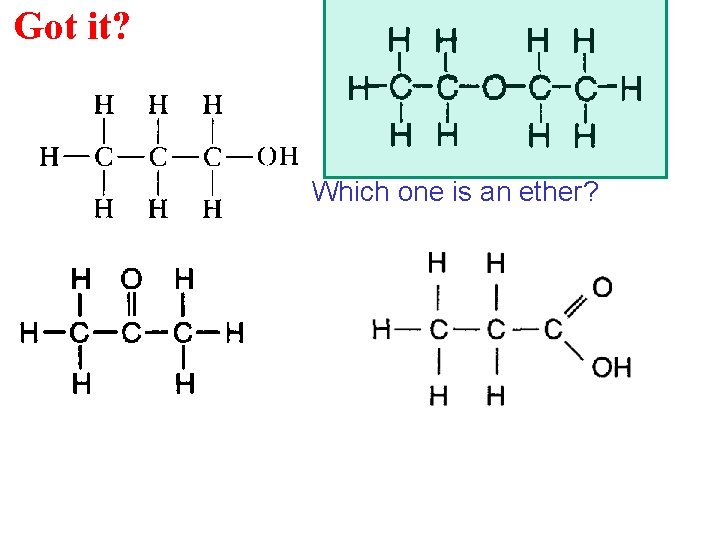
Got it? Which one is an ether?

Practice Questions • • • Type of compound? CH 3 OCH 3 Named: Dimethyl Ether CH 3 CH 2 COOH Named Butanoic Acid (Carboxylic/Organic acid COOH) • Name each compound in the following reaction. CH 3 OH+ C 2 H 5 COOH HOH + C 2 H 5 COOCH 3 Methanol Propanoic Acid Water Methyl Propanoate (is an ester)

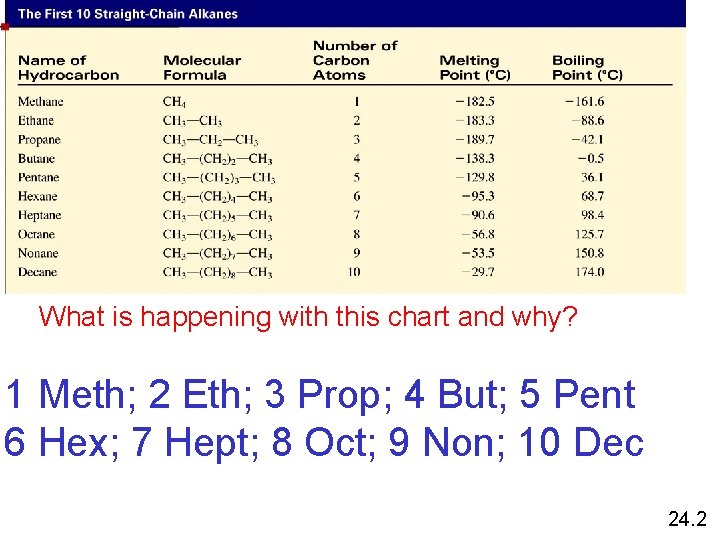
Alkane Nomenclature What is happening with this chart and why? 1 Meth; 2 Eth; 3 Prop; 4 But; 5 Pent 6 Hex; 7 Hept; 8 Oct; 9 Non; 10 Dec 24. 2

Hydroxyl, carbonyl, carboxyl Q: which functional groups contain a hydroxyl group? A carbonyl group? A carboxyl group? Hydroxyl: alcohols, carboxylic acids. Carbonyl: aldehydes, ketones, carboxylic acids, amides, esters. Carboxyl: carboxylic acids Note that properties such as boiling and melting point change due to functional groups

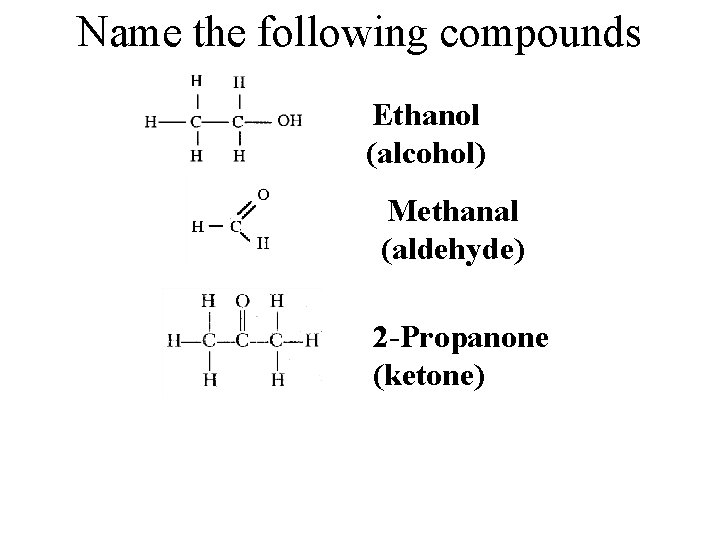
Name the following compounds Ethanol (alcohol) Methanal (aldehyde) 2 -Propanone (ketone)

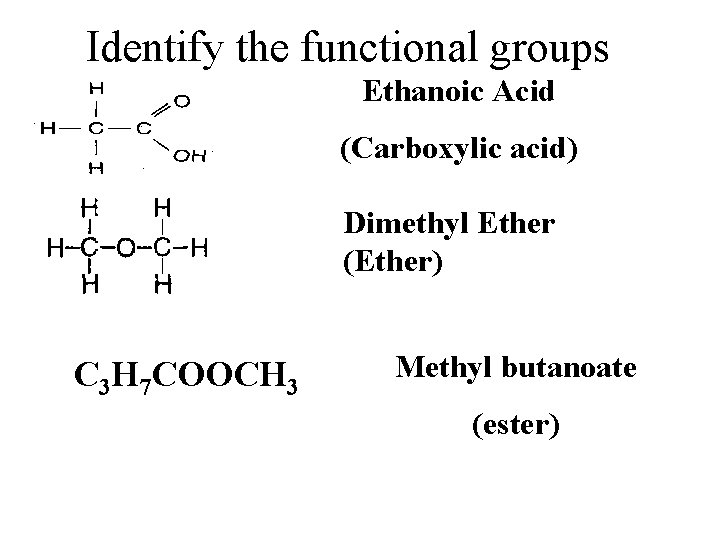
Identify the functional groups Ethanoic Acid (Carboxylic acid) Dimethyl Ether (Ether) C 3 H 7 COOCH 3 Methyl butanoate (ester)



Do now: Summarize your Organic notes onto one page. • You will be making a poster next week for the entire unit. • Alkanes, alkenes, alkynes, cyclo and benzene containing compounds, & all functional groups. • Drawing, identifying, and naming. • When done, work on practice worksheets. • Stamp 1: ANE ENE YNE PRACTICE • Stamp 2: ORGANIC PRACTICE 3

1 meth 2 eth 3 prop 4 but 5 pent 6 hex 7 hept 8 oct 9 non 10 dec Alkane – single bonds Alkene – double bonds Alkyne – triple bonds Cyclo – chained ring (The above are all aliphatic) Benzene – special ring (has 6 carbons in an alternating double/single bond set up. The only aromatic type. )

ORGANIC PARTNER POSTER Intermolecular Forces (IMFs) To be discussed in later unit