An Introduction to molecular diagnostics Dr Catherine Cargo














- Slides: 57

An Introduction to molecular diagnostics Dr Catherine Cargo HMDS

Overview �Basic molecular biology �Mutations �DNA sequencing �Sanger sequencing �Next generation/high throughput sequencing �Impact on haematology


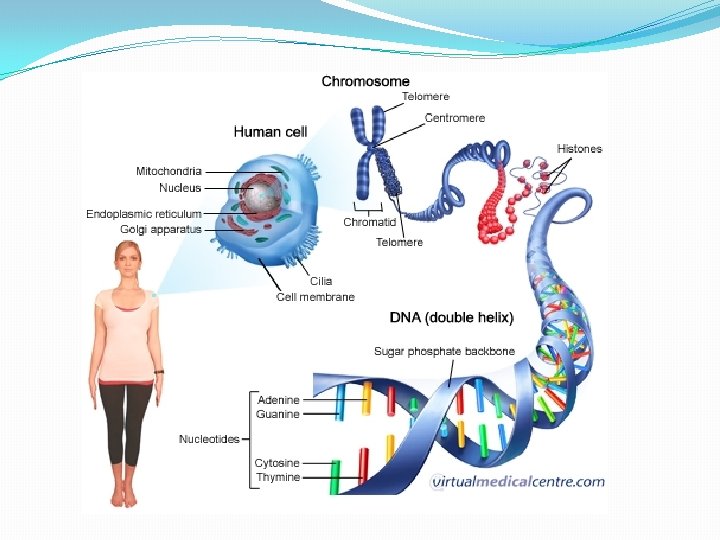
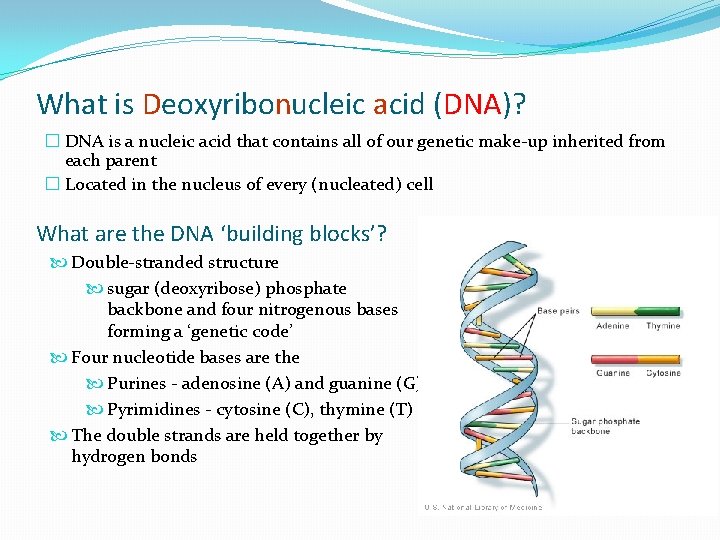
What is Deoxyribonucleic acid (DNA)? � DNA is a nucleic acid that contains all of our genetic make-up inherited from each parent � Located in the nucleus of every (nucleated) cell What are the DNA ‘building blocks’? Double-stranded structure sugar (deoxyribose) phosphate backbone and four nitrogenous bases forming a ‘genetic code’ Four nucleotide bases are the Purines - adenosine (A) and guanine (G) Pyrimidines - cytosine (C), thymine (T) The double strands are held together by hydrogen bonds

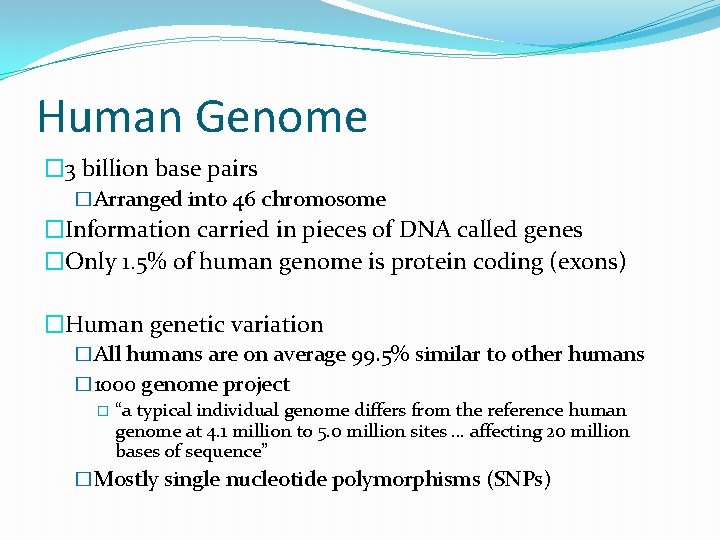
Human Genome � 3 billion base pairs �Arranged into 46 chromosome �Information carried in pieces of DNA called genes �Only 1. 5% of human genome is protein coding (exons) �Human genetic variation �All humans are on average 99. 5% similar to other humans � 1000 genome project � “a typical individual genome differs from the reference human genome at 4. 1 million to 5. 0 million sites … affecting 20 million bases of sequence” �Mostly single nucleotide polymorphisms (SNPs)

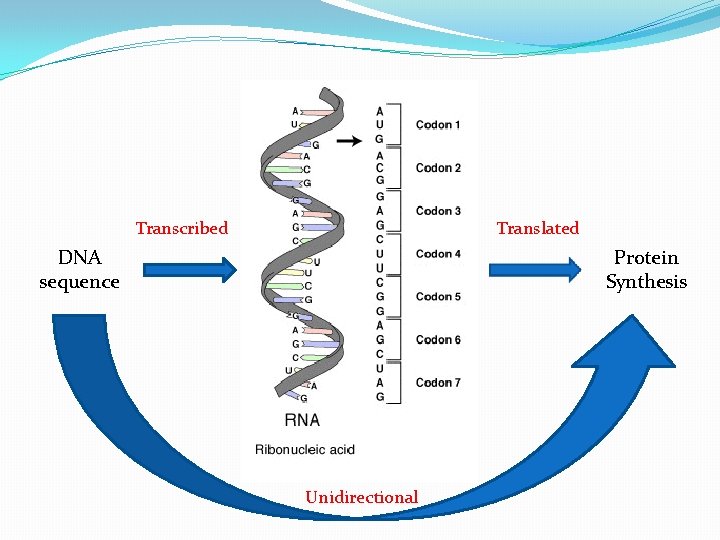
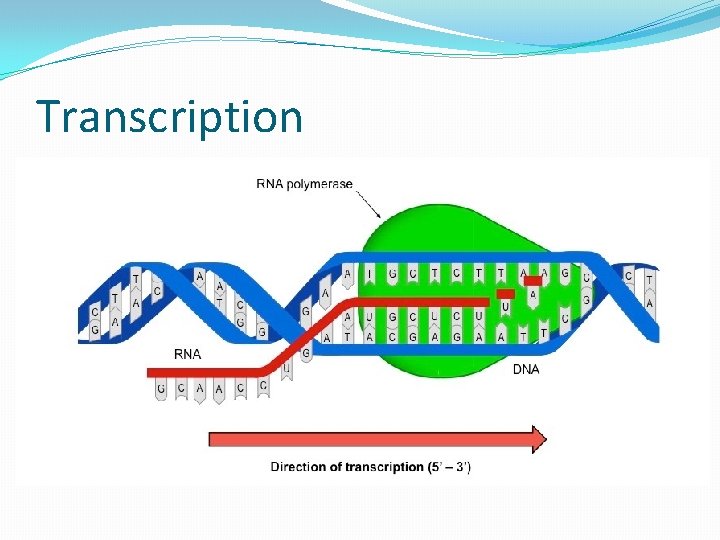
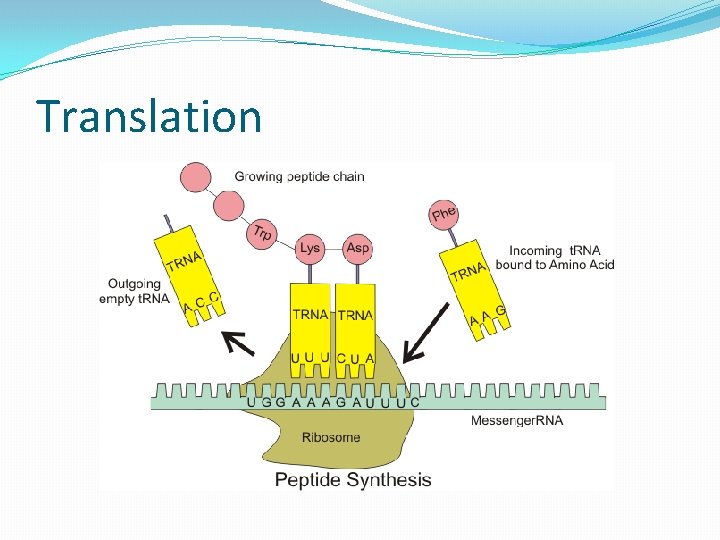
Transcribed Translated DNA sequence Protein Synthesis Unidirectional

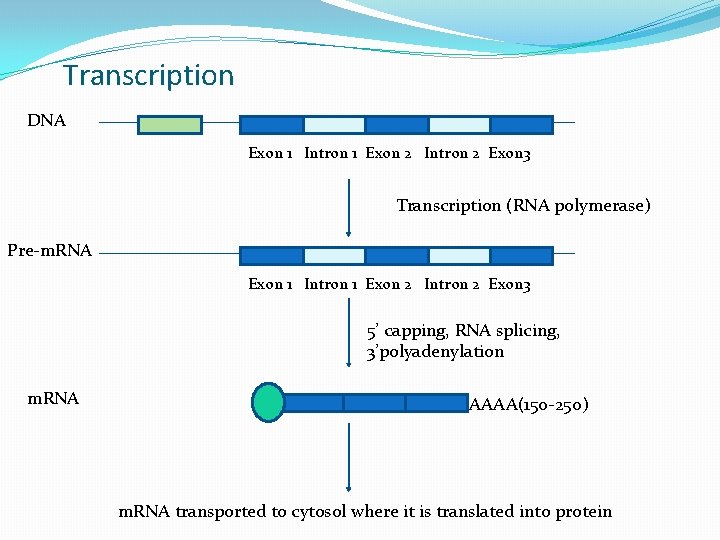
Transcription

Transcription DNA Exon 1 Intron 1 Exon 2 Intron 2 Exon 3 Transcription (RNA polymerase) Pre-m. RNA Exon 1 Intron 1 Exon 2 Intron 2 Exon 3 5’ capping, RNA splicing, 3’polyadenylation m. RNA AAAA(150 -250) m. RNA transported to cytosol where it is translated into protein

Translation

DNA damage � DNA is prone to damage from environmental insults and also during DNA replication � Protective mechanisms in place to eliminate detrimental abnormalities � � DNA repair through various pathways Proof-reading by DNA polymerase. � If these pathways fail � cell’s final ‘line of defence’ is apoptosis (i. e. cell death). � Survival advantage � Genes that regulate cell growth and differentiation are altered � e. g a gene that is part of the protective process (-a ‘tumour supressor’) � Cells may be open to more abnormalities as a result of its ability to evade protection becoming increasing unstable – ‘genetic instability’.

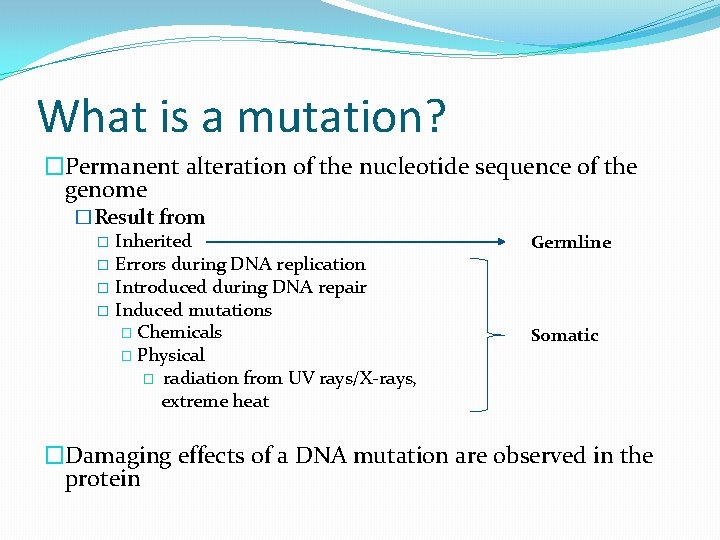
What is a mutation? �Permanent alteration of the nucleotide sequence of the genome �Result from � Inherited � Errors during DNA replication � Introduced during DNA repair � Induced mutations � Chemicals � Physical � radiation from UV rays/X-rays, extreme heat Germline Somatic �Damaging effects of a DNA mutation are observed in the protein

What is a mutation?

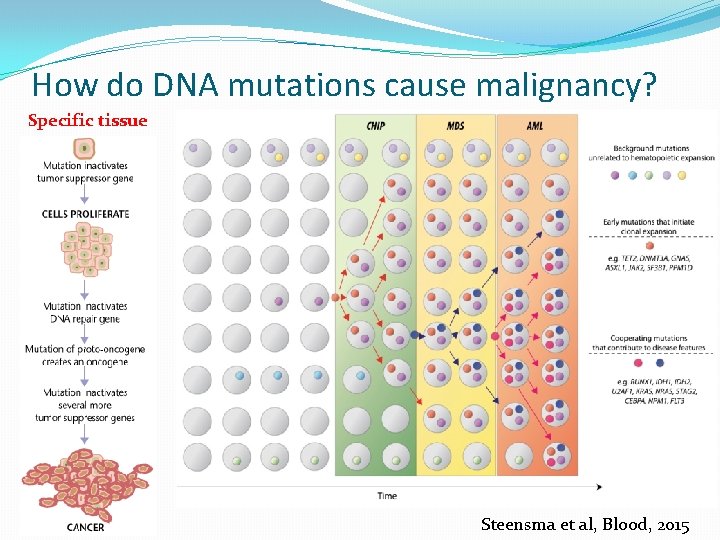
How do DNA mutations cause malignancy? Specific tissue Steensma et al, Blood, 2015

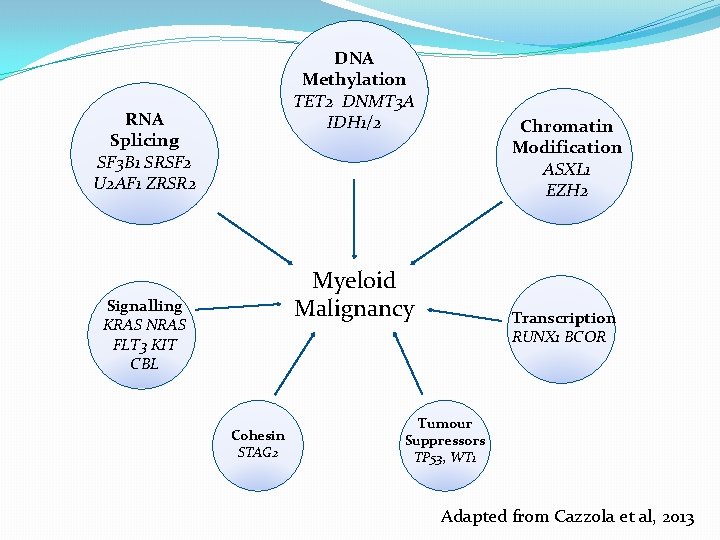
DNA Methylation TET 2 DNMT 3 A IDH 1/2 RNA Splicing SF 3 B 1 SRSF 2 U 2 AF 1 ZRSR 2 Chromatin Modification ASXL 1 EZH 2 Myeloid Malignancy Receptors Signalling / Kinases KRAS NRAS JAK 2 FLT 3 KIT CBL Cohesin STAG 2 Transcription RUNX 1 BCOR Tumour Suppressors TP 53, WT 1 Adapted from Cazzola et al, 2013

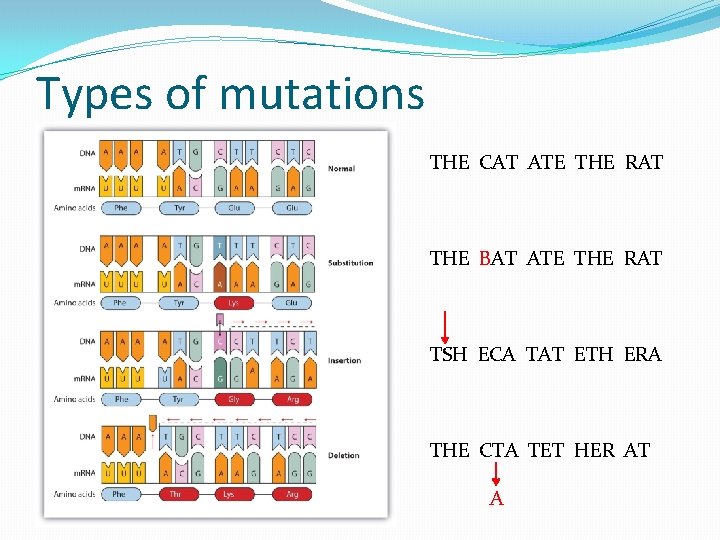
Types of mutations THE CAT ATE THE RAT THE BAT ATE THE RAT TSH ECA TAT ETH ERA THE CTA TET HER AT A

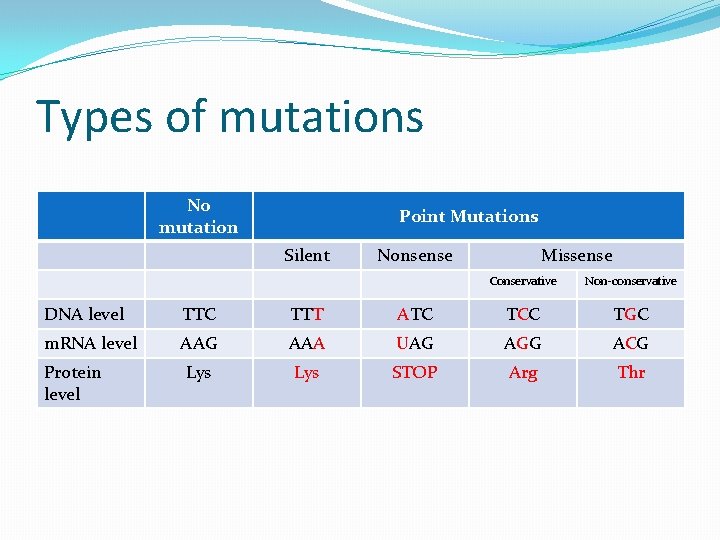
Types of mutations No mutation Point Mutations Silent Nonsense Missense Conservative Non-conservative DNA level TTC TTT ATC TCC TGC m. RNA level AAG AAA UAG AGG ACG Lys STOP Arg Thr Protein level

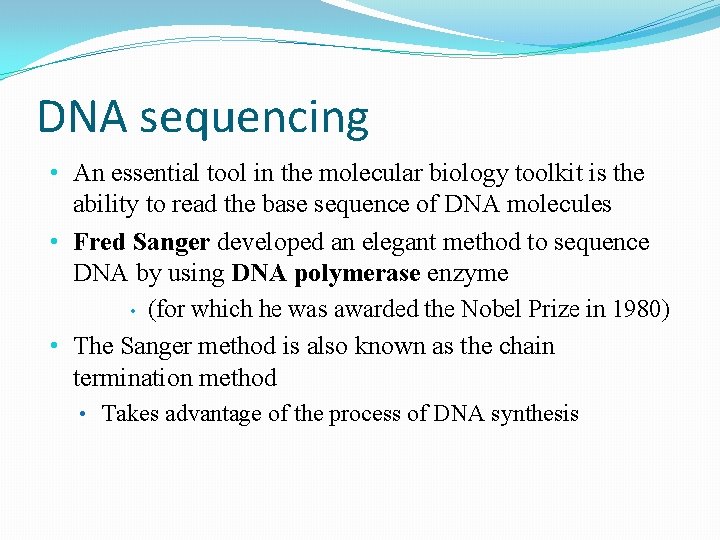
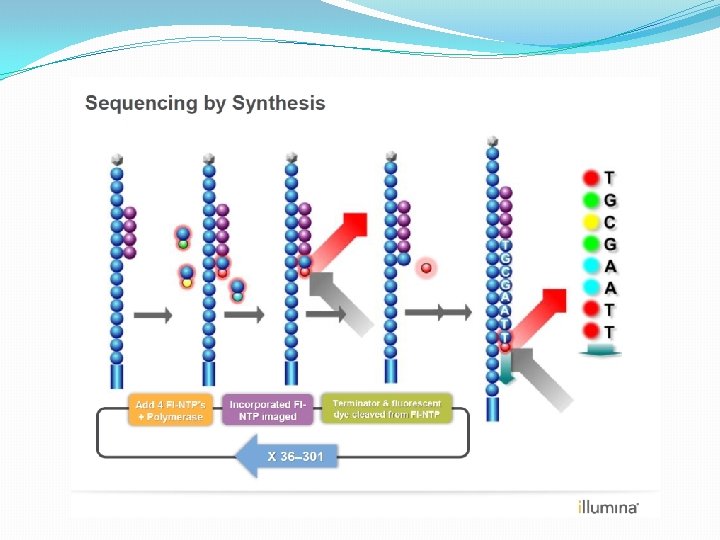
DNA sequencing • An essential tool in the molecular biology toolkit is the ability to read the base sequence of DNA molecules • Fred Sanger developed an elegant method to sequence DNA by using DNA polymerase enzyme • (for which he was awarded the Nobel Prize in 1980) • The Sanger method is also known as the chain termination method • Takes advantage of the process of DNA synthesis


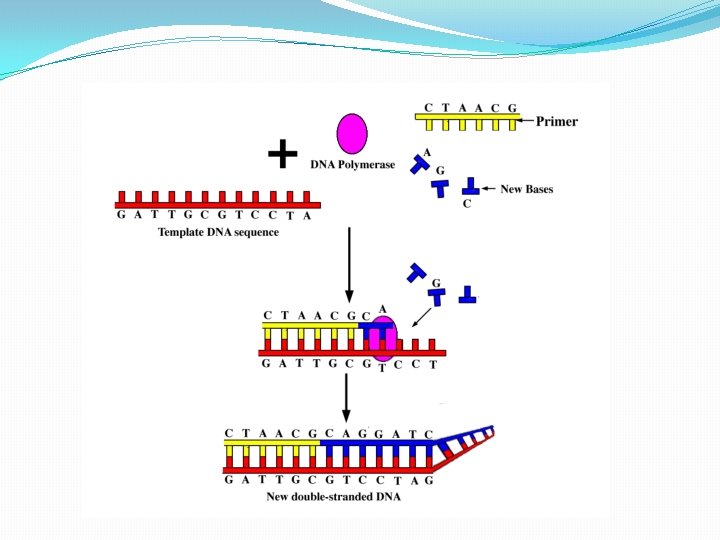
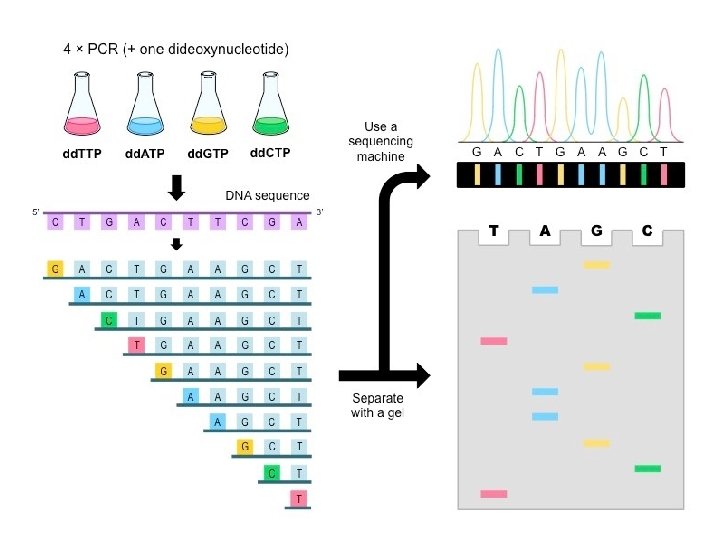
Sanger Method �Chain-terminator method �Key principle � Use of dideoxynucleotide triphosphates (dd. NTPs) as chain terminators �DNA divided into 4 separate sequencing reactions containing – d. ATP, d. GTP, d. CTP, d. TTP and DNA polymerase �To each reaction is added 1 of 4 dideoxynucleotides �dd. ATP, dd. GTP, dd. CTP, dd. TTP �Results in DNA fragments of varying length �Heat denatured and separated by gel electophoresis �Autoradiography


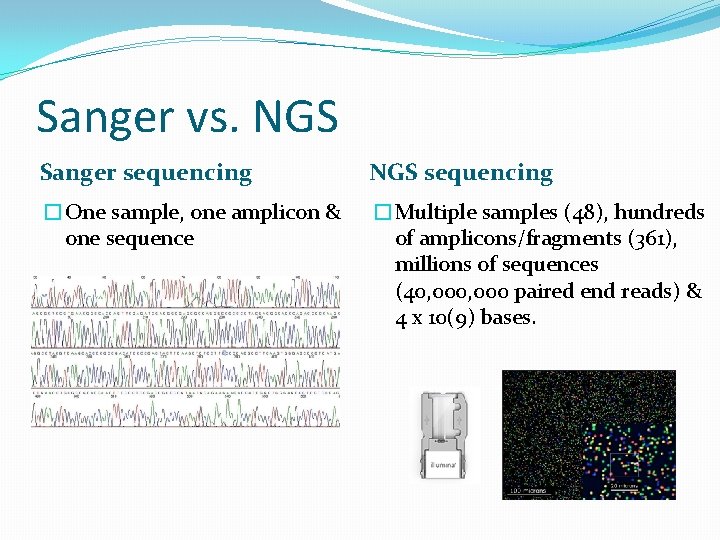
Sanger vs. NGS Sanger sequencing NGS sequencing �One sample, one amplicon & one sequence �Multiple samples (48), hundreds of amplicons/fragments (361), millions of sequences (40, 000 paired end reads) & 4 x 10(9) bases.

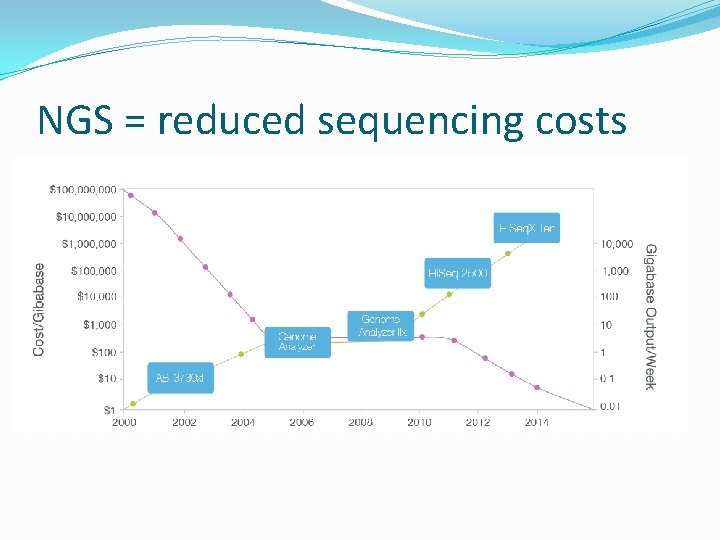
NGS = reduced sequencing costs

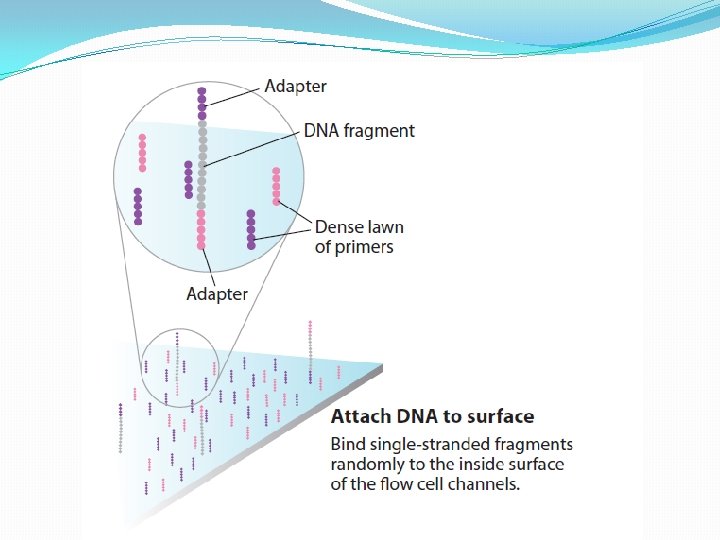
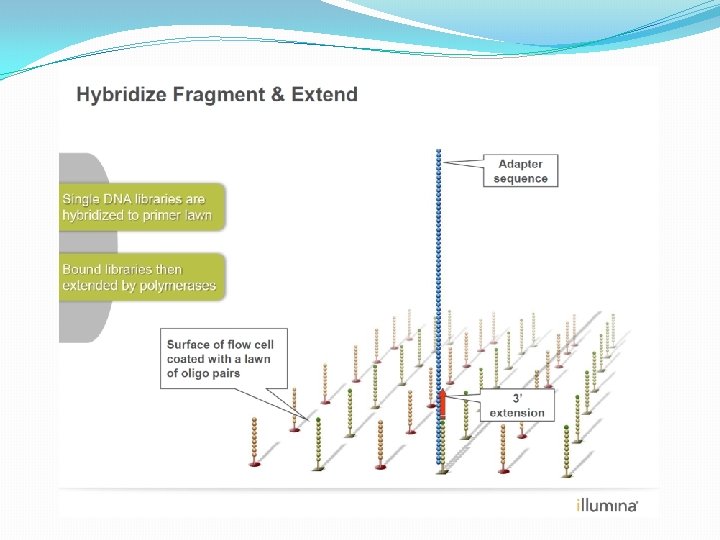
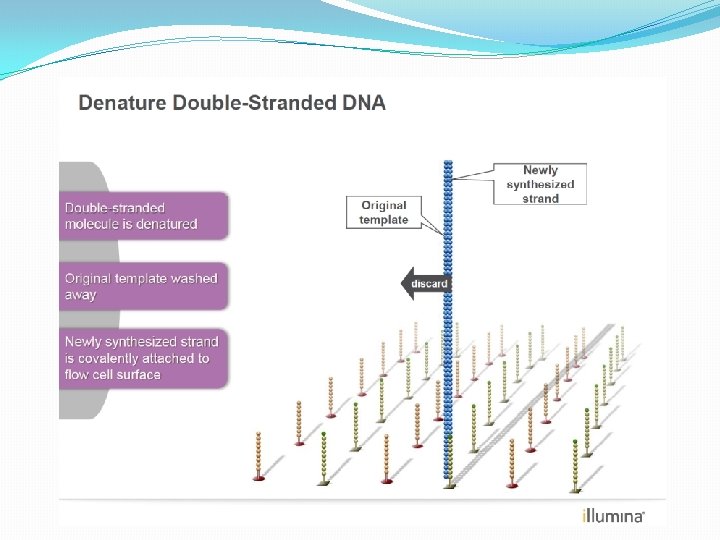
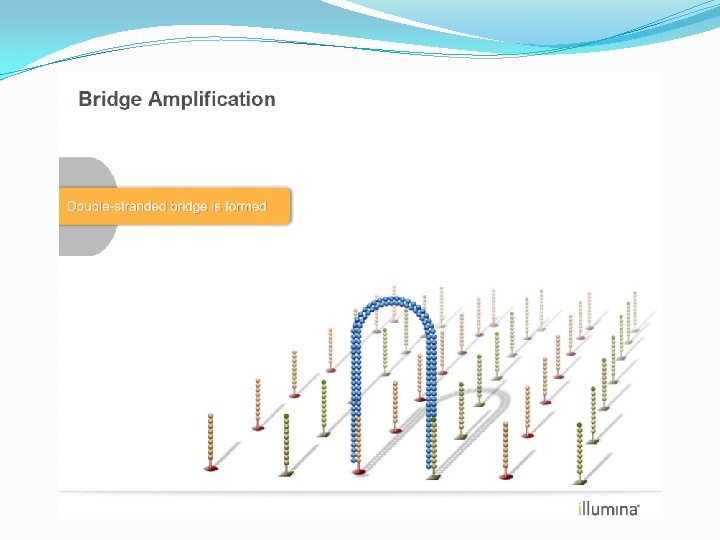
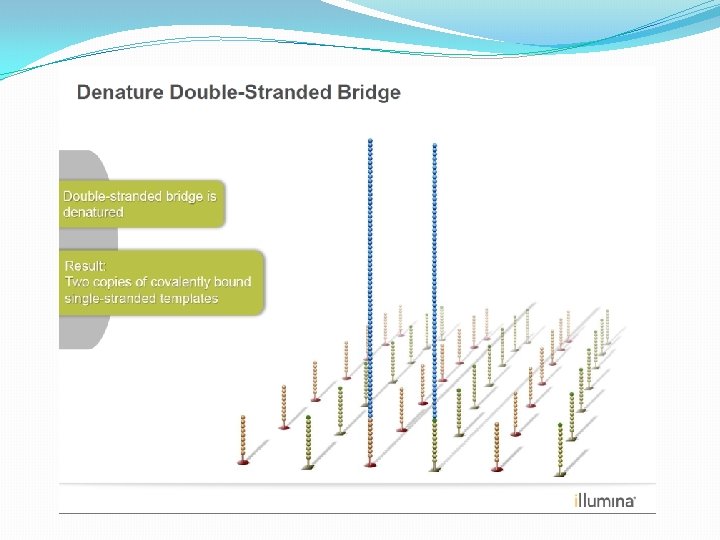
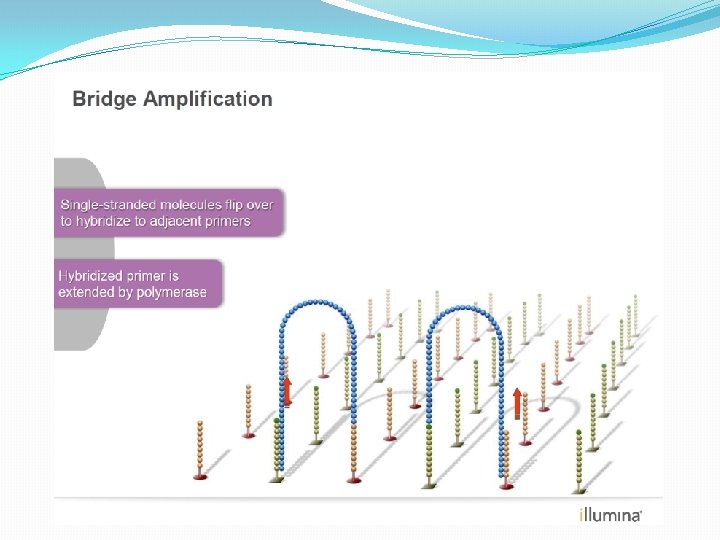
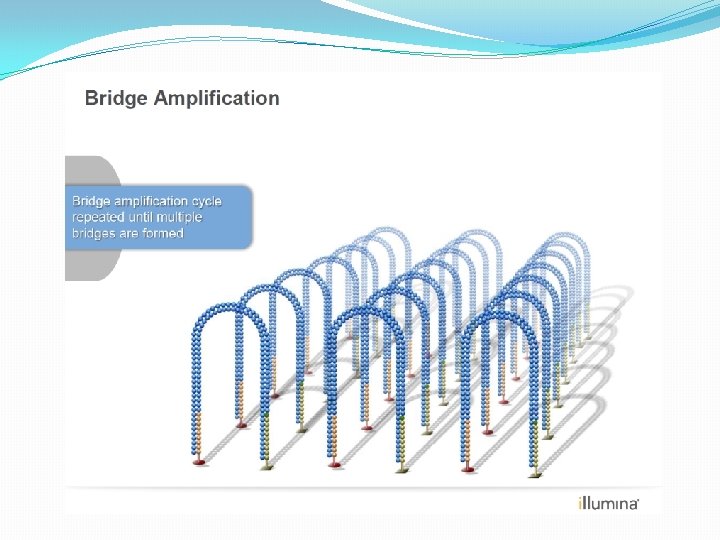
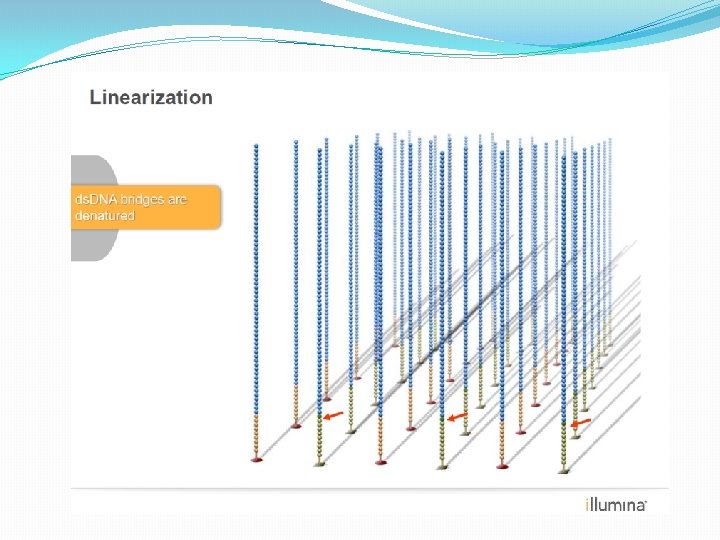
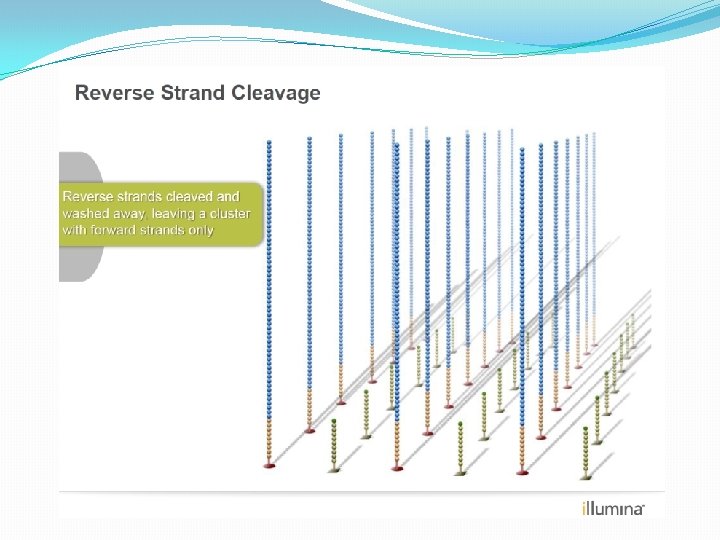
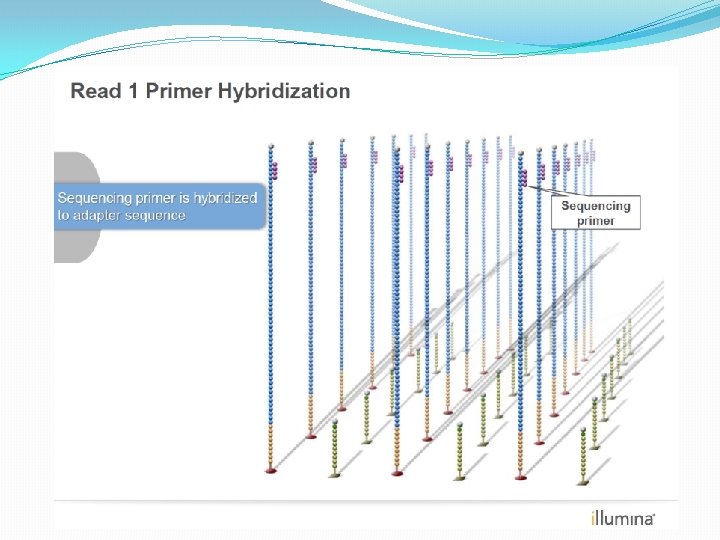
NGS: The basics �Library Preparation �Cluster generation / bead capture �Sequencing �Data analysis (Bioinformatics)

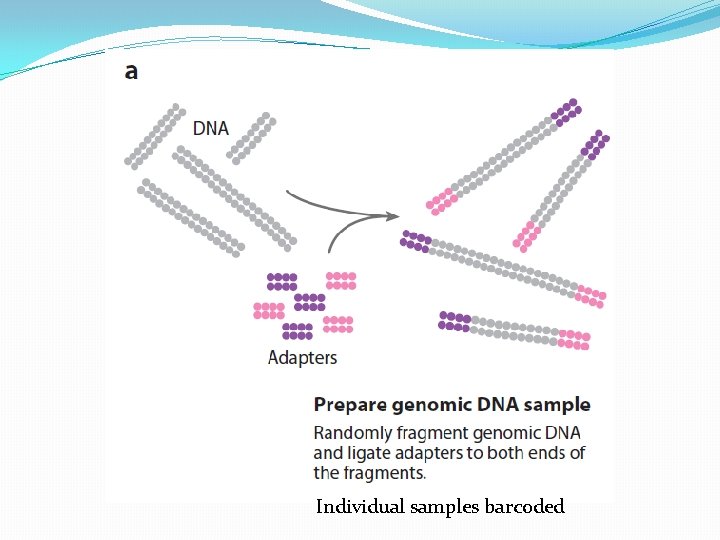
Library Preparation The Library is a pooled tube of ALL the barcoded DNA fragments from ALL the patients.

Individual samples barcoded

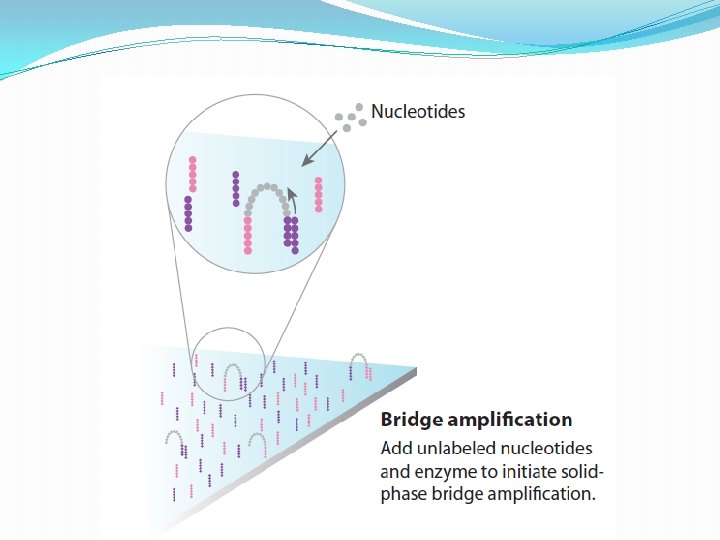
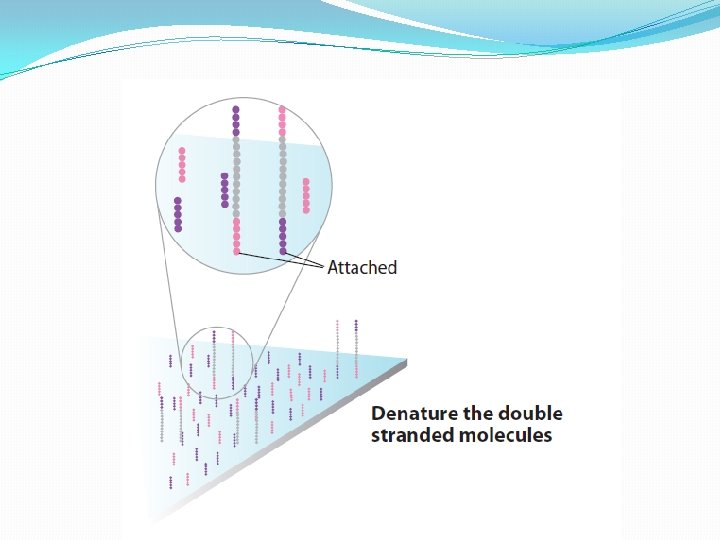
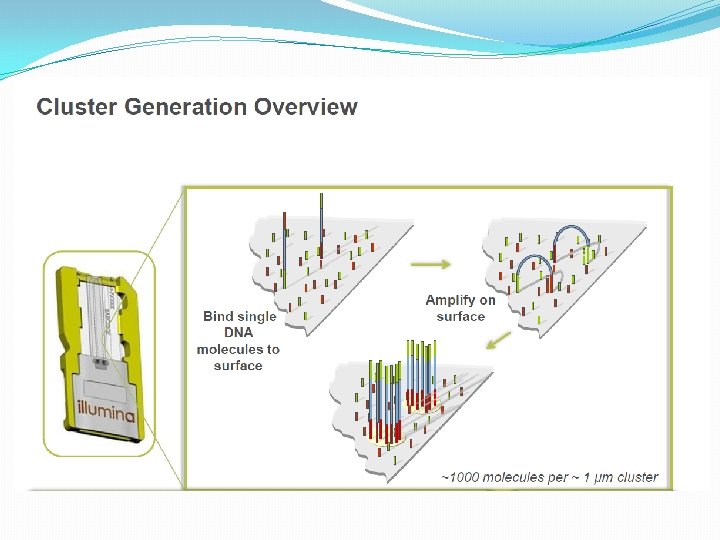
NGS: The basics �Library Preparation �Cluster generation / bead capture �Sequencing �Data analysis (Bioinformatics)

The black box…. .







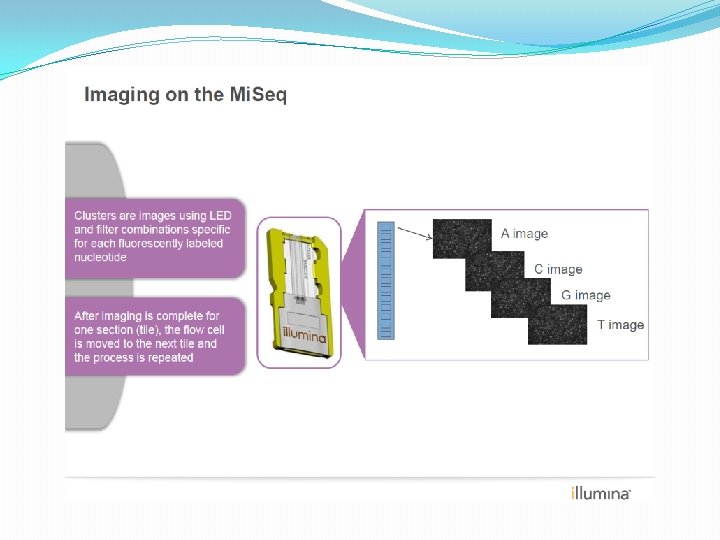
NGS: The basics �Library Preparation �Cluster generation / bead capture �Sequencing �Data analysis (Bioinformatics)

Low throughput Sample preparation assay Interpretation of results High throughput

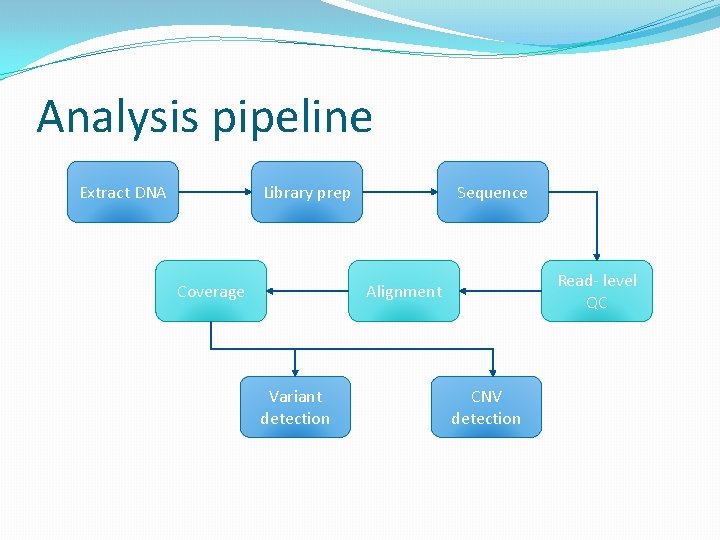
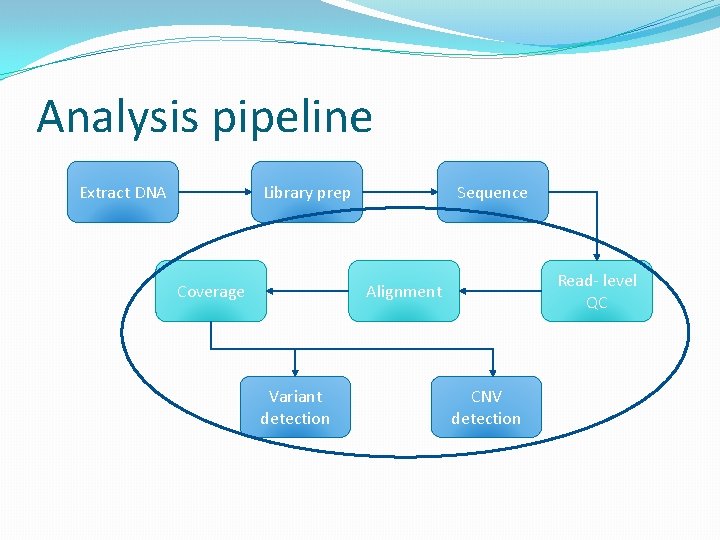
Analysis pipeline Extract DNA Library prep Coverage Sequence Read- level QC Alignment Variant detection CNV detection

Analysis pipeline Extract DNA Library prep Coverage Sequence Read- level QC Alignment Variant detection CNV detection

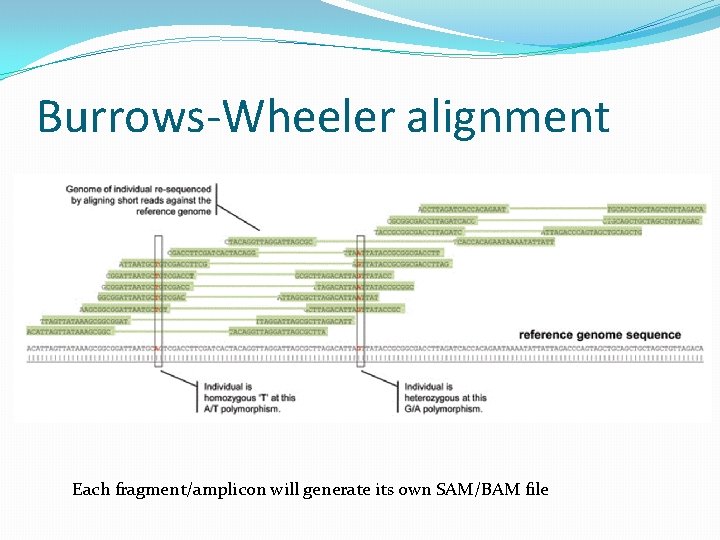
Burrows-Wheeler alignment Each fragment/amplicon will generate its own SAM/BAM file

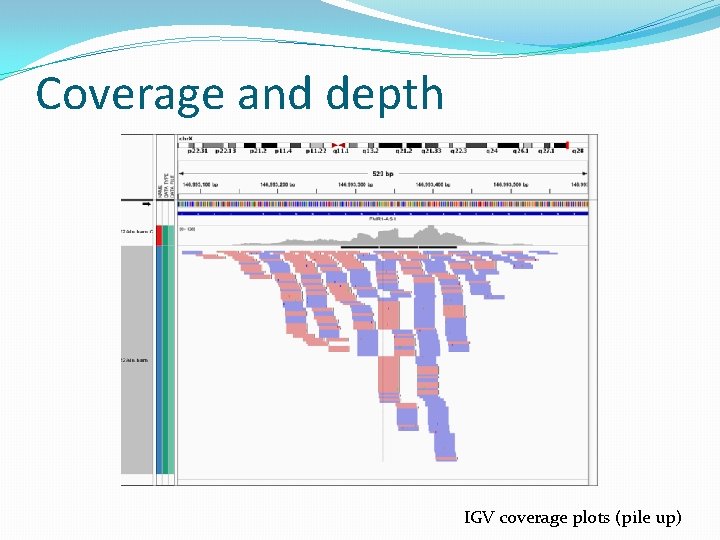
Coverage and depth IGV coverage plots (pile up)

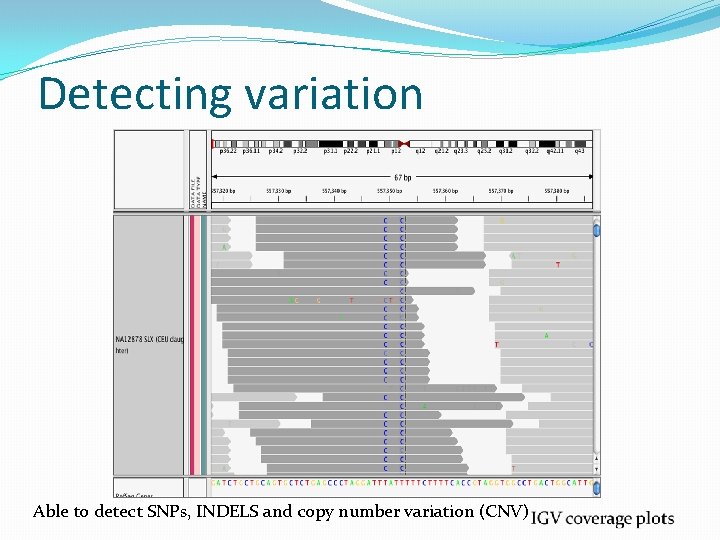
Detecting variation Able to detect SNPs, INDELS and copy number variation (CNV)

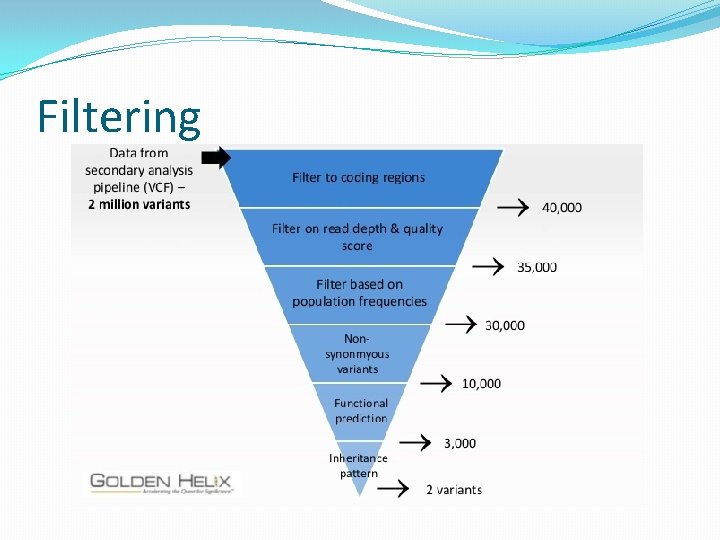
Filtering

Potential applications of NGS in haematology �Diagnostic tool �Objective evidence of disease �Subclassification �Prognostic tool �Disease monitoring �Identify targets for therapy (in future!)

How will this impact on you? �Potential to greatly impact on patient care �Early diagnosis �Information on prognosis �Access to therapies �Early diagnosis = more patients in clinic �Patients will become increasingly aware of this �Ask questions on the significance of mutations �Personalised medicine �Quality results require quality samples!

Conclusions �NGS is set to become a key tool in the diagnostic armoury of the laboratory �NGS is cost effective, rapid, reliable and uses minimal amounts of clinical material. �Targeted sequence analysis of key myeloid and lymphoid driver mutations will form the basis of this testing �Guiding treatment based on the identification of some of these driver mutations is already happening in the clinic


Fluidigm Access Array system -uses micro-fluidics

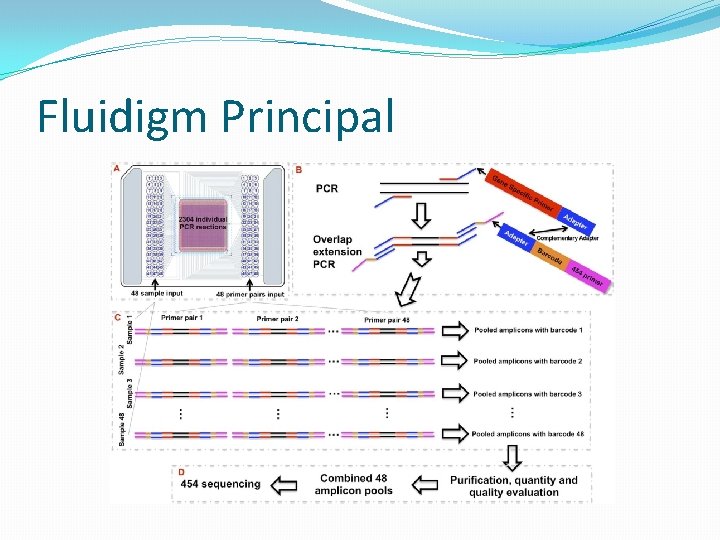
Fluidigm Principal

Library preparation � 27 genes, 370 amplicons � 1 run will perform nearly 18 thousand reactions �this would take weeks to do by old technology…








