An Introduction to Metabolism Energy changes are involved

An Introduction to Metabolism Energy changes are involved in every chemical reaction— even those that occur in our bodies. While many processes involve the release of energy, others are accompanied by the absorption of energy. Sarah Reinertsen is a competitive runner. She relies a great deal on energy produced by the chemical reactions in her cells. Metabolism: The sum of all anabolic and catabolic reactions in a cell or body
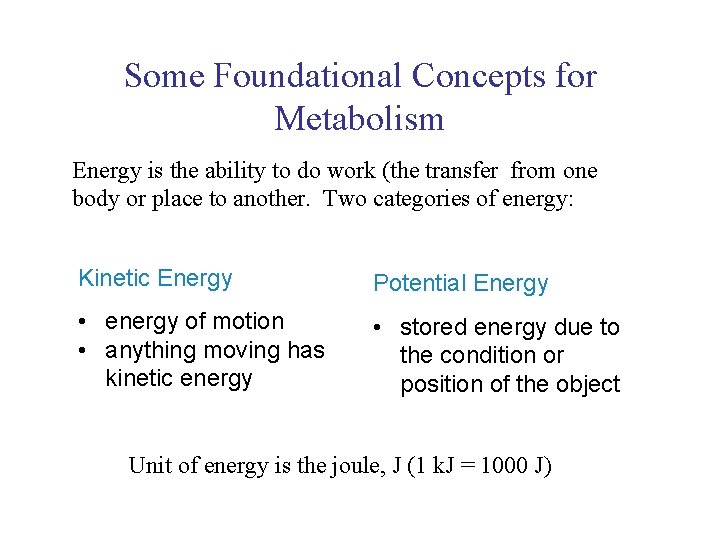
UNIT 3 Chapter 5: Energy Changes Section 5. 1 Some Foundational Concepts for Metabolism Energy is the ability to do work (the transfer from one body or place to another. Two categories of energy: Kinetic Energy Potential Energy • energy of motion • anything moving has kinetic energy • stored energy due to the condition or position of the object Unit of energy is the joule, J (1 k. J = 1000 J)
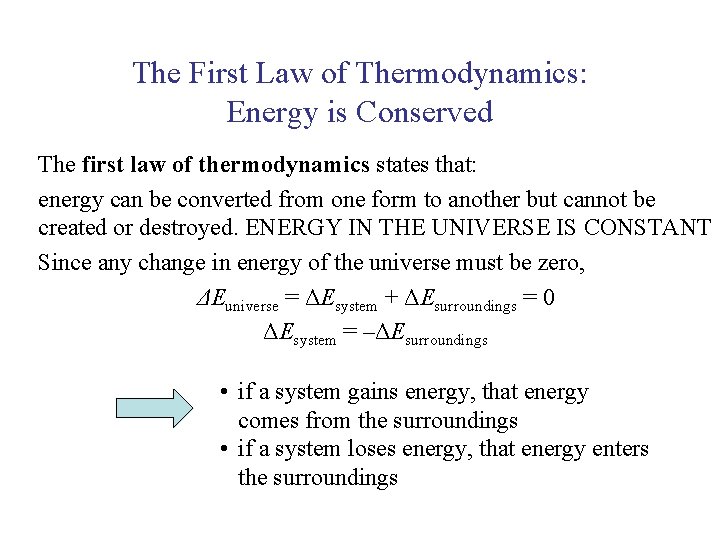
UNIT 3 Chapter 5: Energy Changes Section 5. 1 The First Law of Thermodynamics: Energy is Conserved The first law of thermodynamics states that: energy can be converted from one form to another but cannot be created or destroyed. ENERGY IN THE UNIVERSE IS CONSTANT Since any change in energy of the universe must be zero, ΔEuniverse = ΔEsystem + ΔEsurroundings = 0 ΔEsystem = –ΔEsurroundings • if a system gains energy, that energy comes from the surroundings • if a system loses energy, that energy enters the surroundings

UNIT 3 Chapter 5: Energy Changes Section 5. 1 System and Surroundings universe = system + surroundings • the system is the sample being observed • the surroundings is everything else • interactions between a system and its surroundings involve exchange of energy and matter Three types of systems based on this type of exchange are: can exchange energy and matter with surroundings can exchange energy, not matter, with surroundings cannot exchange matter or energy with surroundings
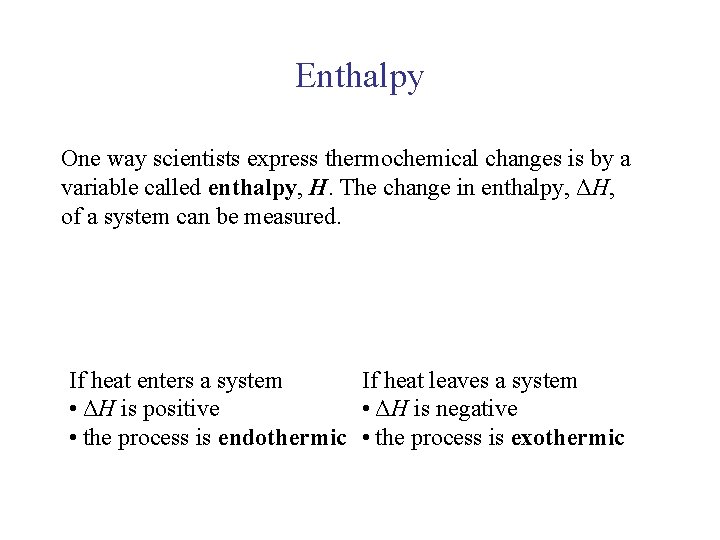
UNIT 3 Chapter 5: Energy Changes Section 5. 1 Enthalpy One way scientists express thermochemical changes is by a variable called enthalpy, H. The change in enthalpy, ΔH, of a system can be measured. If heat enters a system If heat leaves a system • ΔH is positive • ΔH is negative • the process is endothermic • the process is exothermic
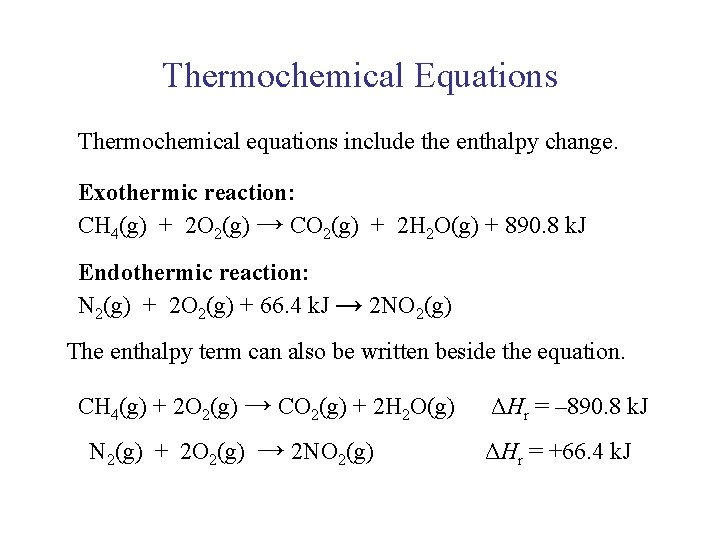
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Thermochemical Equations Thermochemical equations include the enthalpy change. Exothermic reaction: CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) + 890. 8 k. J Endothermic reaction: N 2(g) + 2 O 2(g) + 66. 4 k. J → 2 NO 2(g) The enthalpy term can also be written beside the equation. CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) N 2(g) + 2 O 2(g) → 2 NO 2(g) ΔHr = – 890. 8 k. J ΔHr = +66. 4 k. J
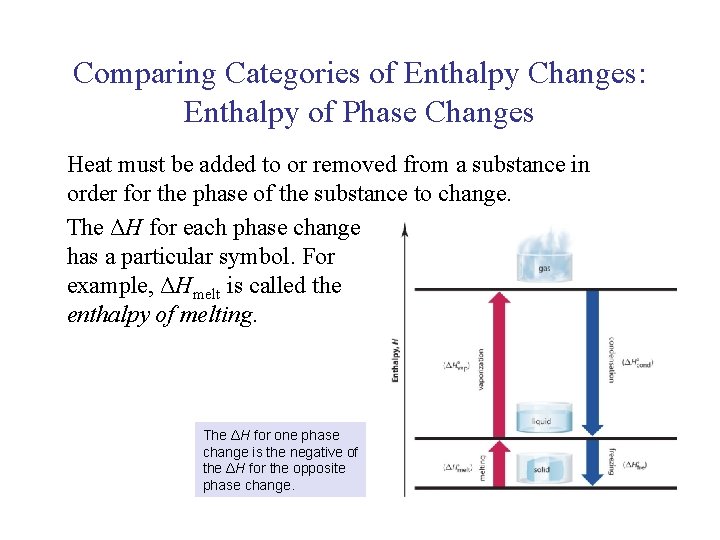
UNIT 3 Chapter 5: Energy Changes Section 5. 1 Comparing Categories of Enthalpy Changes: Enthalpy of Phase Changes Heat must be added to or removed from a substance in order for the phase of the substance to change. The ΔH for each phase change has a particular symbol. For example, ΔHmelt is called the enthalpy of melting. The ΔH for one phase change is the negative of the ΔH for the opposite phase change.
UNIT 3 Chapter 5: Energy Changes Learning Check: Label the following as endothermic or exothermic a) Liquid turning to gas (condensation) b) Combustion c) Liquid turning to a solid (freezing/solidification) Section 5. 2
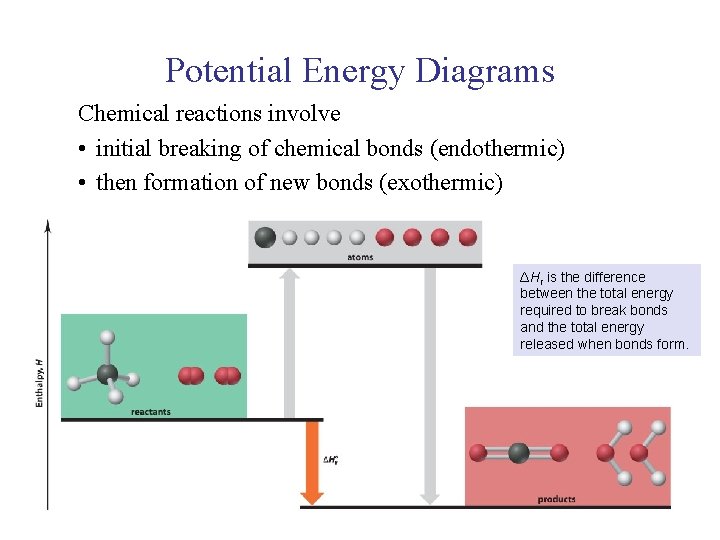
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Potential Energy Diagrams Chemical reactions involve • initial breaking of chemical bonds (endothermic) • then formation of new bonds (exothermic) ΔHr is the difference between the total energy required to break bonds and the total energy released when bonds form.
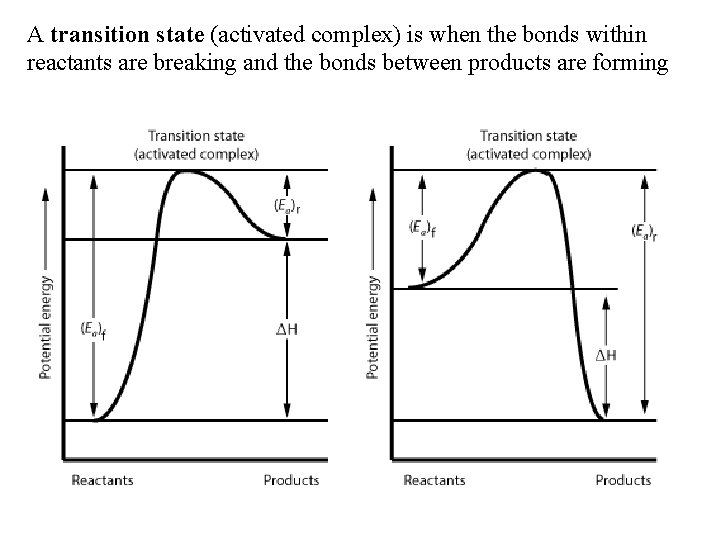
A transition state (activated complex) is when the bonds within reactants are breaking and the bonds between products are forming
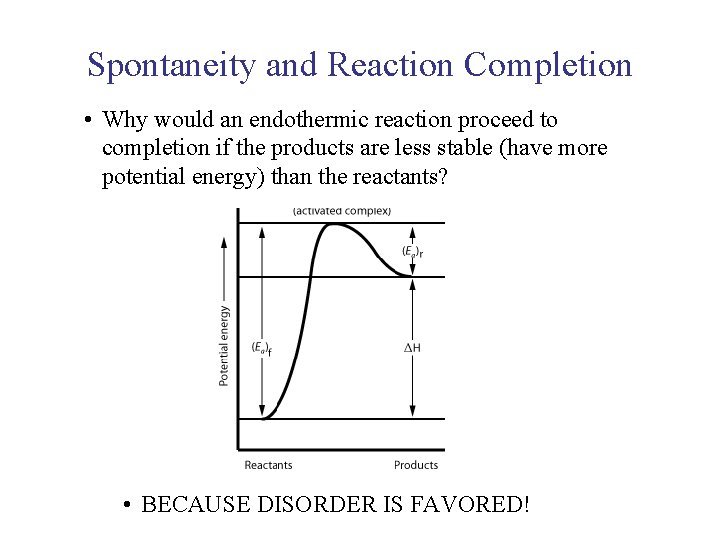
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Spontaneity and Reaction Completion • Why would an endothermic reaction proceed to completion if the products are less stable (have more potential energy) than the reactants? • BECAUSE DISORDER IS FAVORED!

UNIT 3 Chapter 5: Energy Changes Entropy A measure of the disorder in a system The second law of thermodynamics states that the universe tends toward disorder Chaos will reign supreme Section 5. 2
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Learning Check: Which has greater entropy? a) Solid or liquid? b) Polymers or monomers? c) Highly concentrated particles or diffusion of particles? d) Catabolic or anabolic reaction?
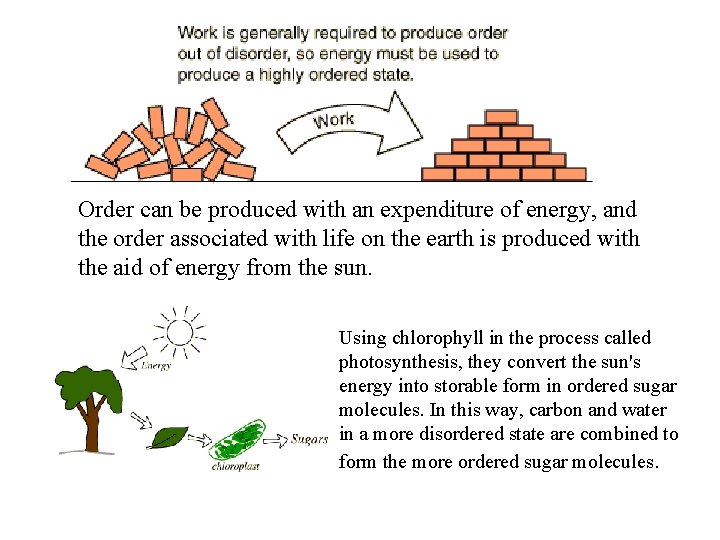
Order can be produced with an expenditure of energy, and the order associated with life on the earth is produced with the aid of energy from the sun. Using chlorophyll in the process called photosynthesis, they convert the sun's energy into storable form in ordered sugar molecules. In this way, carbon and water in a more disordered state are combined to form the more ordered sugar molecules.

UNIT 3 Chapter 5: Energy Changes Section 5. 2 Disorder on Earth (or lack there of) But what about other orderly structures on planet Earth? Our body?
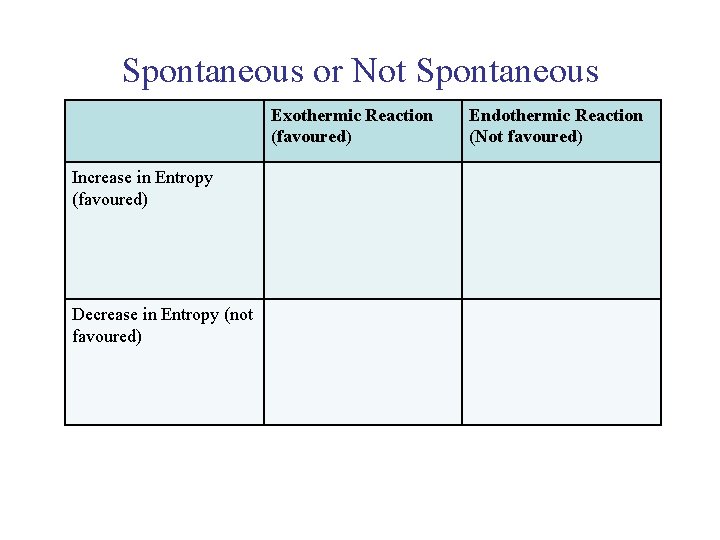
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Spontaneous or Not Spontaneous Exothermic Reaction (favoured) Increase in Entropy (favoured) Decrease in Entropy (not favoured) Endothermic Reaction (Not favoured)

UNIT 3 Chapter 5: Energy Changes Section 5. 2 Gibbs Free Energy that can do useful work If a rxn is not spontaneous: • ΔG is positive • the process is endergonic If a reaction is spontaneous • ΔG is negative • the process is exergonic ΔG tells us how much energy is needed to drive the reaction Learning Check: Will a positive ΔG need energy for the rxn to happen? Will a negative ΔG?
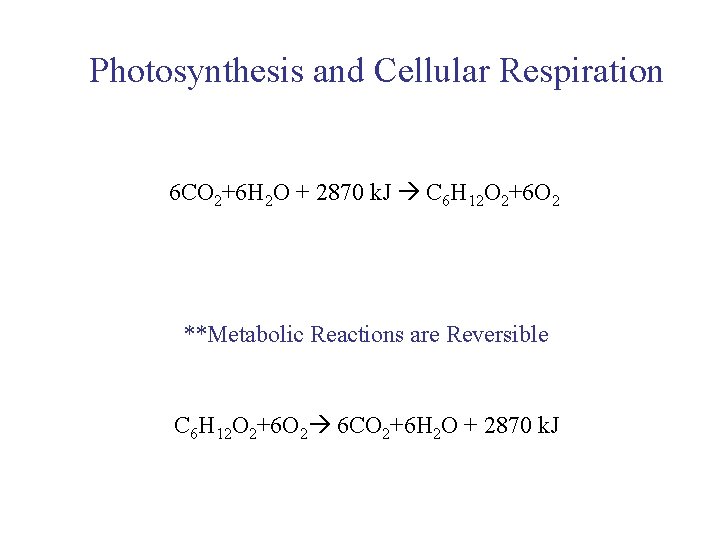
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Photosynthesis and Cellular Respiration 6 CO 2+6 H 2 O + 2870 k. J C 6 H 12 O 2+6 O 2 **Metabolic Reactions are Reversible C 6 H 12 O 2+6 O 2 6 CO 2+6 H 2 O + 2870 k. J

UNIT 3 Chapter 5: Energy Changes Section 5. 2 Hydrolysis of ATP *Catalyzed by ATPase enzyme ATP+H 2 O ADP+Pi What do you think the hydrolysis of ATP would be: 1) 31 k. J written on the reactants or the products side? 2) Would 31 k. J be positive or negative? 3) Is the reaction exergonic or endergonic?
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Draw a Potential Energy Diagram for the Hydrolysis of ATP Label the reactants and products Are the products or the reactants more stable? How do you know?
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Draw a Potential Energy Diagram for the Phosphorylation (adding a P)of ADP to from ATP Label the reactants and products Write the full reaction equation, including the ΔG value
UNIT 3 Chapter 5: Energy Changes Section 5. 2 Think/Pair/Share Do you think the energy produced from the hydrolysis of ATP is released as heat? Why or why not?
- Slides: 23