An Introduction to Metabolism and Enzymes Substate Active
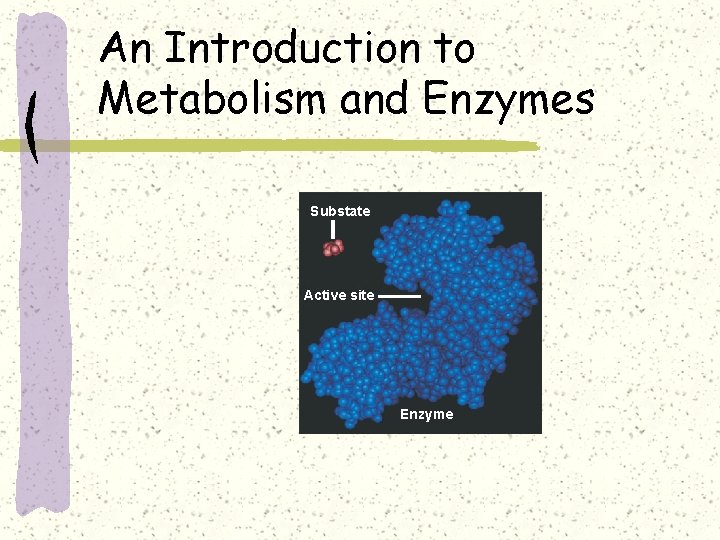
An Introduction to Metabolism and Enzymes Substate Active site Enzyme
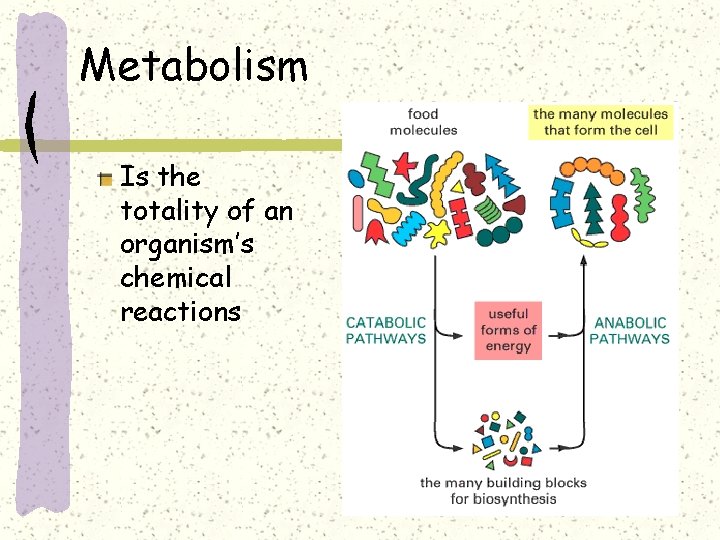
Metabolism Is the totality of an organism’s chemical reactions
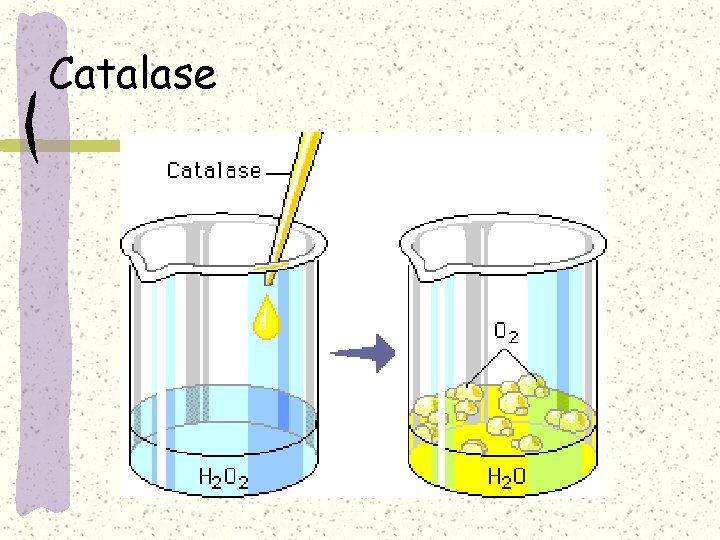
Catalase
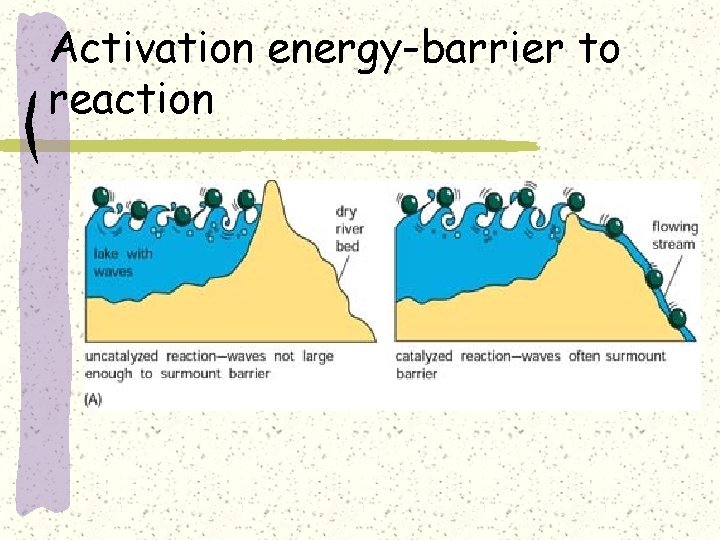
Activation energy-barrier to reaction
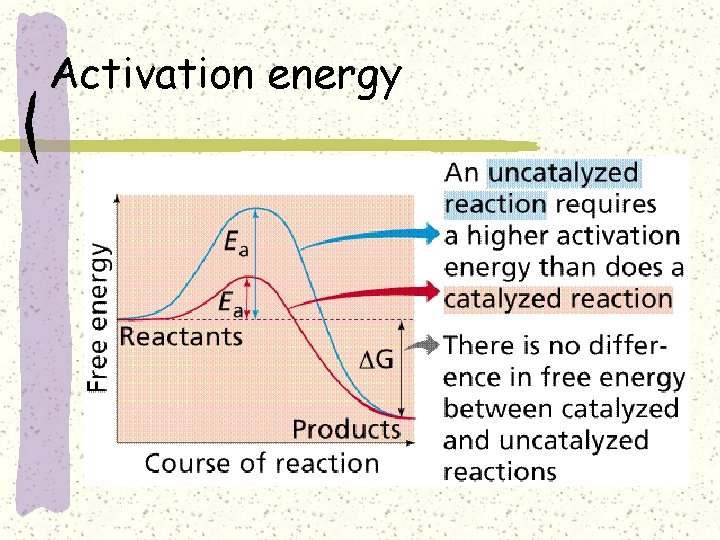
Activation energy
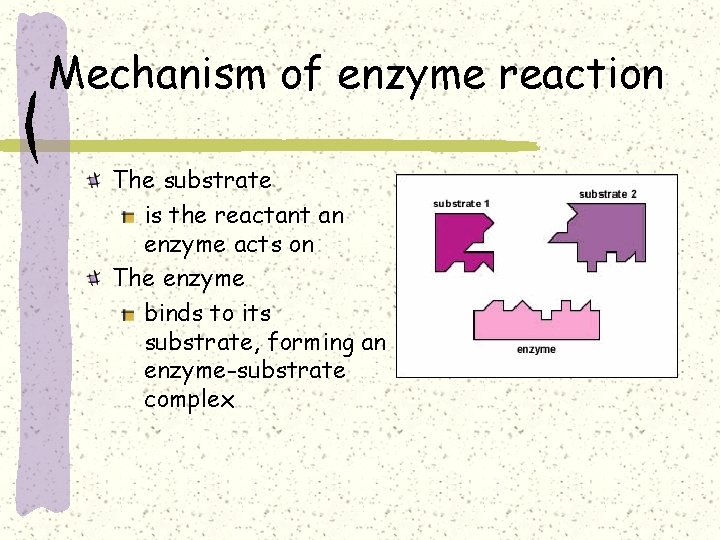
Mechanism of enzyme reaction The substrate is the reactant an enzyme acts on The enzyme binds to its substrate, forming an enzyme-substrate complex
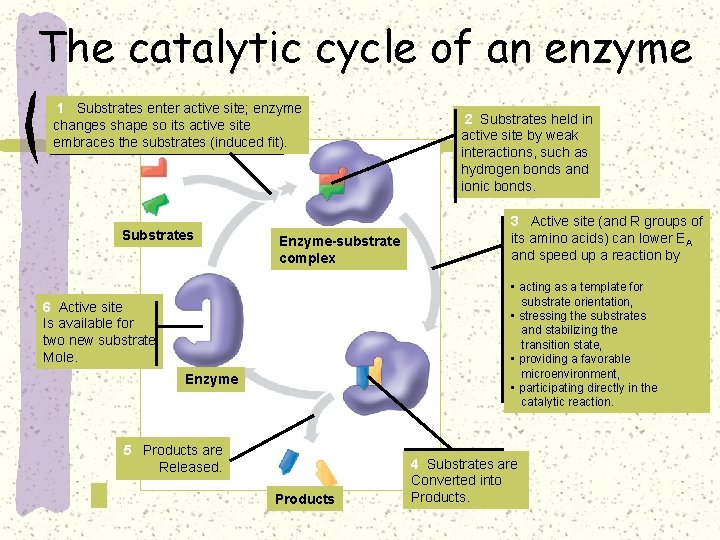
The catalytic cycle of an enzyme 1 Substrates enter active site; enzyme changes shape so its active site embraces the substrates (induced fit). Substrates Enzyme-substrate complex 2 Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. 3 Active site (and R groups of its amino acids) can lower EA and speed up a reaction by • acting as a template for substrate orientation, • stressing the substrates and stabilizing the transition state, • providing a favorable microenvironment, • participating directly in the catalytic reaction. 6 Active site Is available for two new substrate Mole. Enzyme 5 Products are Released. Products 4 Substrates are Converted into Products.
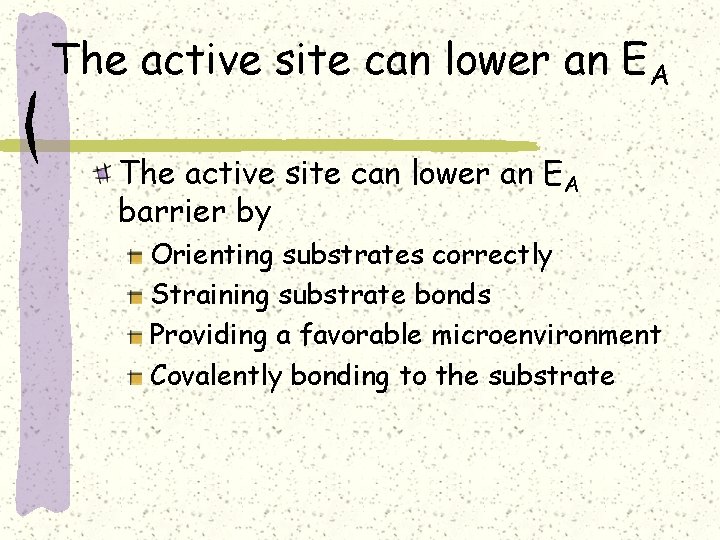
The active site can lower an EA barrier by Orienting substrates correctly Straining substrate bonds Providing a favorable microenvironment Covalently bonding to the substrate
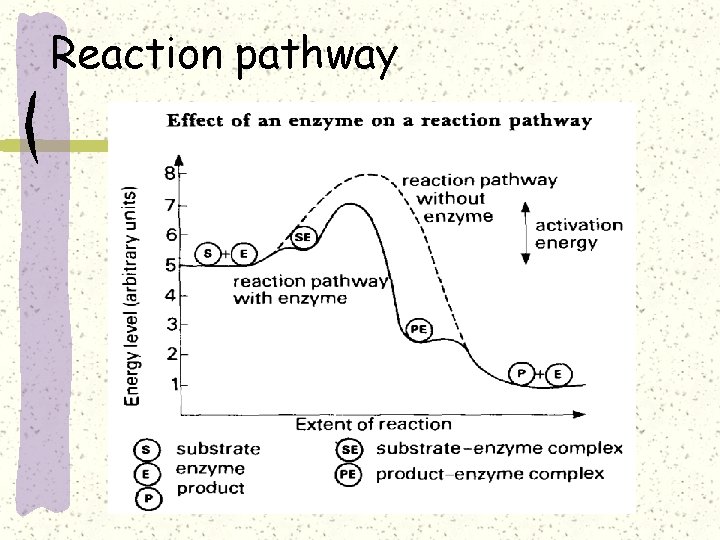
Reaction pathway
3 D / tertiary structure of an enzyme
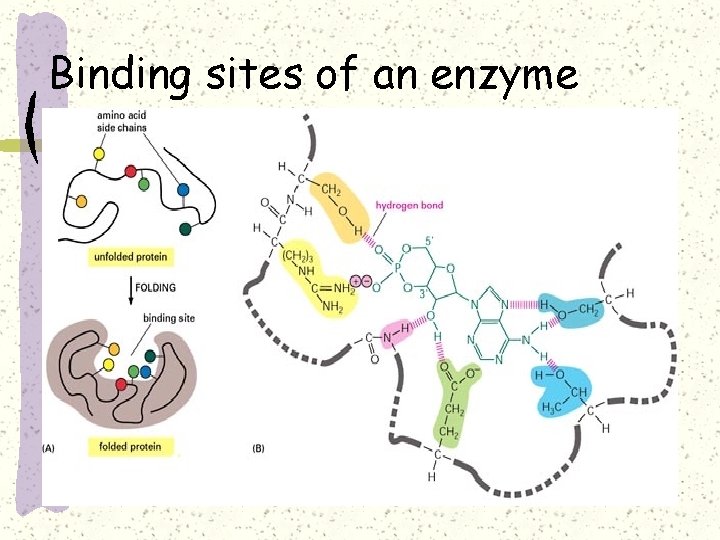
Binding sites of an enzyme
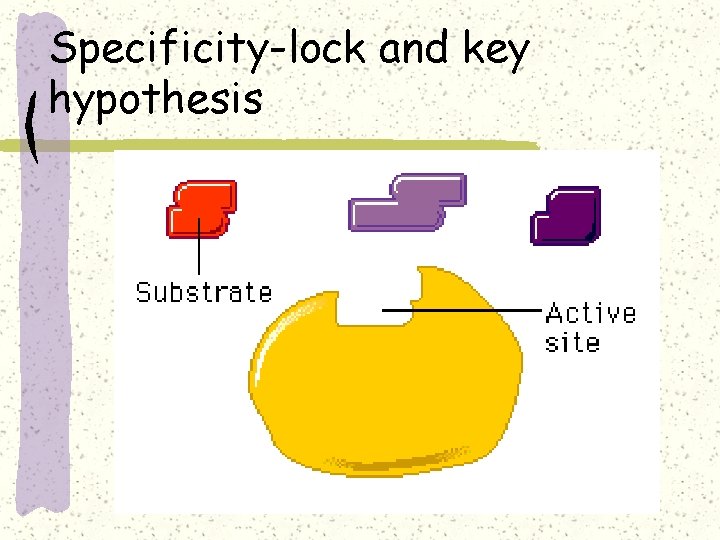
Specificity-lock and key hypothesis
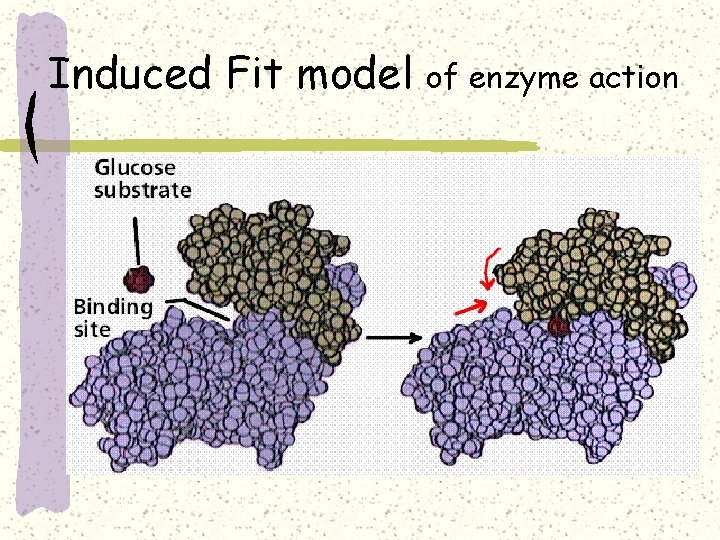
Induced Fit model of enzyme action
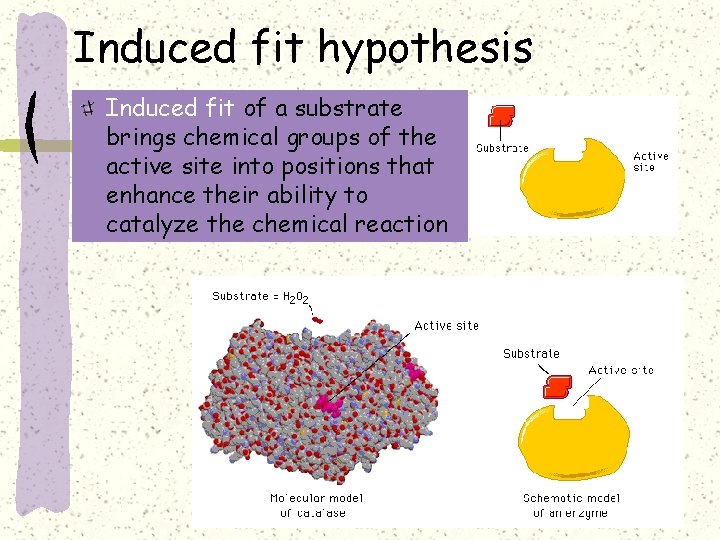
Induced fit hypothesis Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the chemical reaction
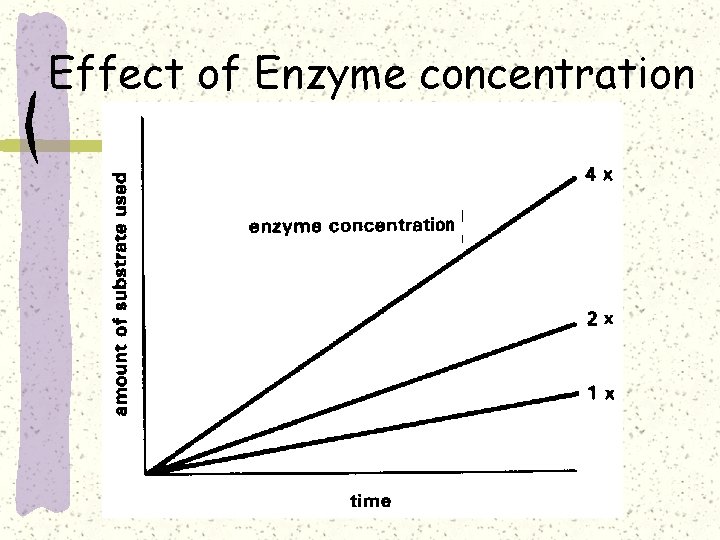
Effect of Enzyme concentration
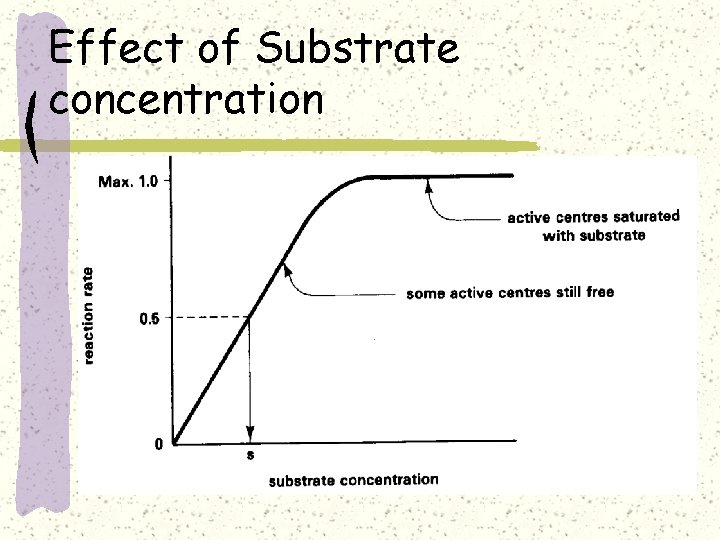
Effect of Substrate concentration
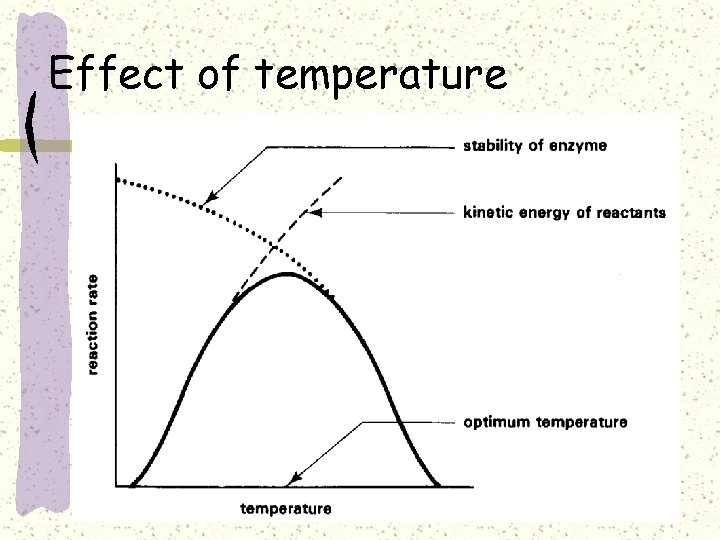
Effect of temperature
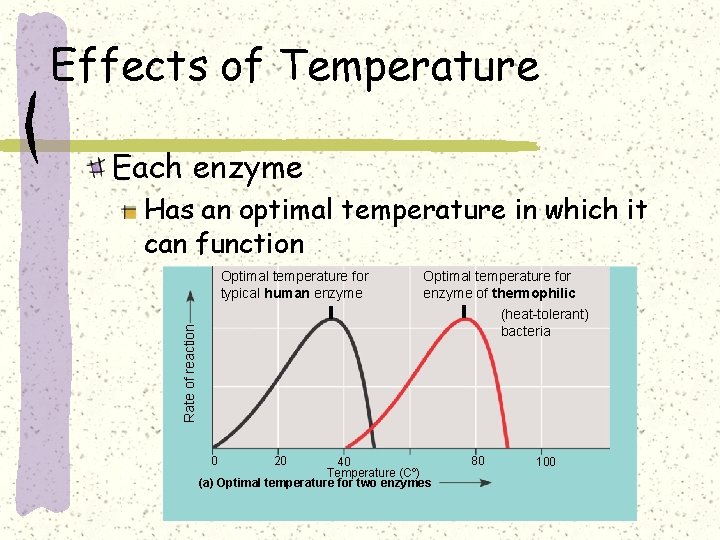
Effects of Temperature Each enzyme Has an optimal temperature in which it can function Optimal temperature for typical human enzyme Optimal temperature for enzyme of thermophilic Rate of reaction (heat-tolerant) bacteria 0 20 40 Temperature (Cº) (a) Optimal temperature for two enzymes 80 100
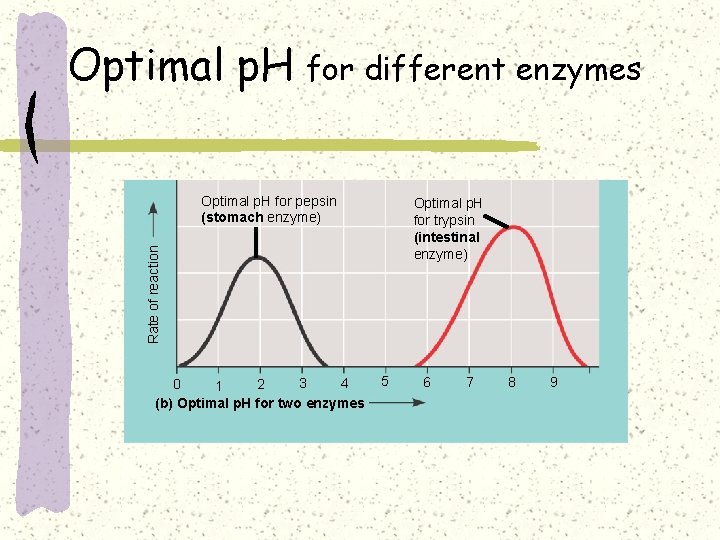
Optimal p. H for different enzymes Optimal p. H for pepsin (stomach enzyme) Rate of reaction Optimal p. H for trypsin (intestinal enzyme) 3 4 0 2 1 (b) Optimal p. H for two enzymes 5 6 7 8 9
Effect of p. H on enzyme

Cofactors -- enzyme helpers Cofactors Are nonprotein enzyme helpers—can be either inorganic or organic Coenzymes Are organic cofactors
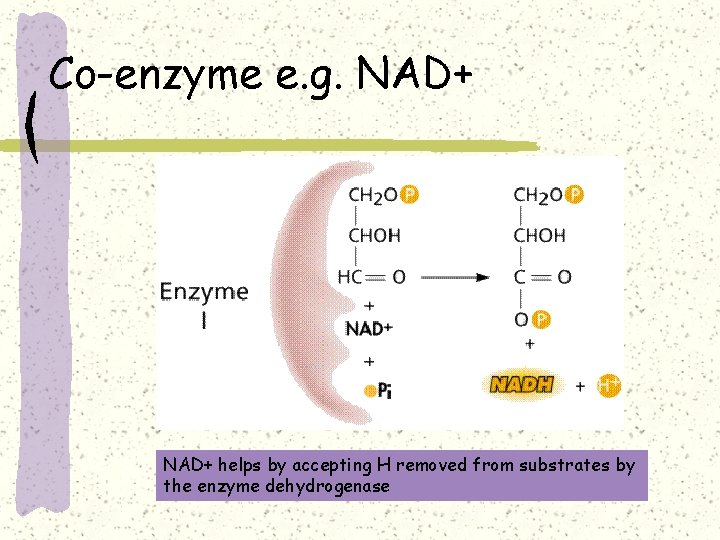
Co-enzyme e. g. NAD+ helps by accepting H removed from substrates by the enzyme dehydrogenase
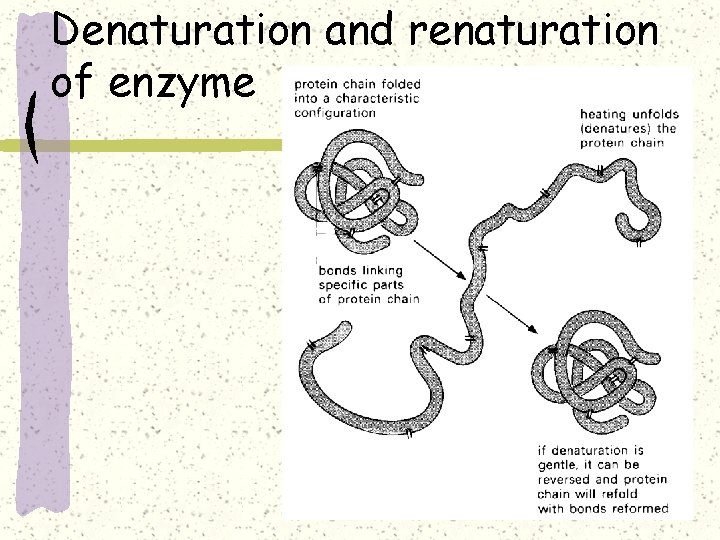
Denaturation and renaturation of enzyme
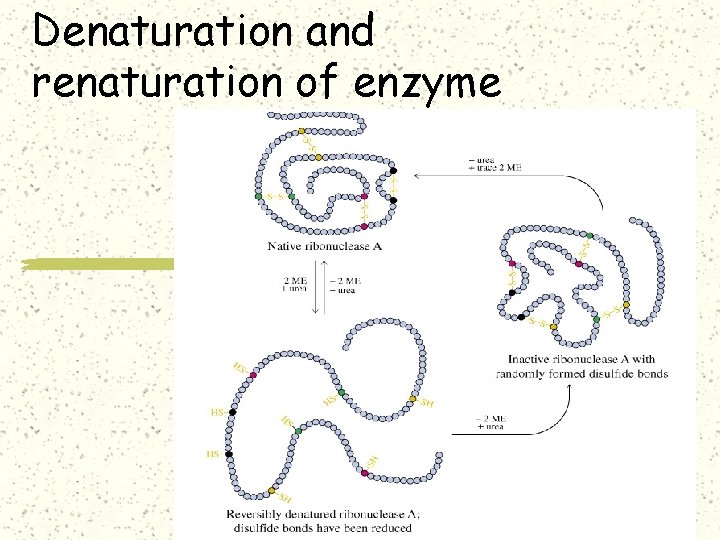
Denaturation and renaturation of enzyme
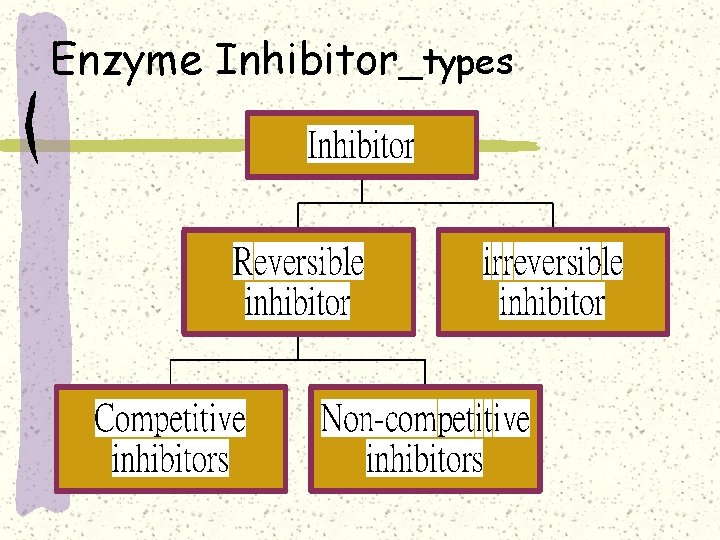
Enzyme Inhibitor_types
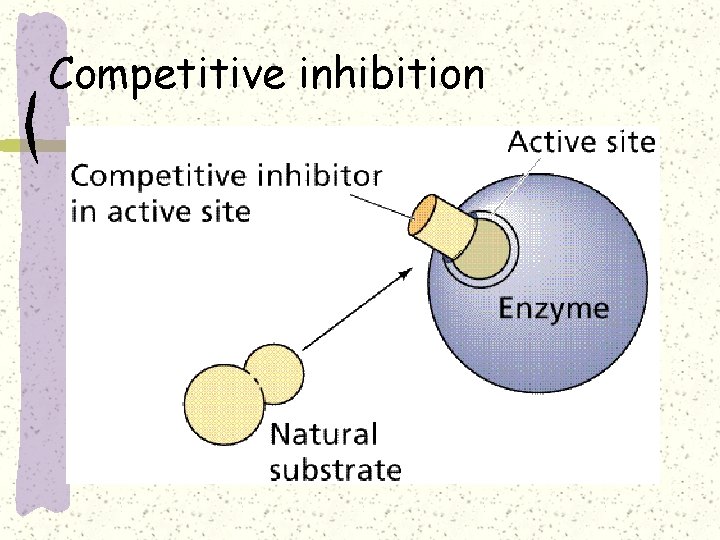
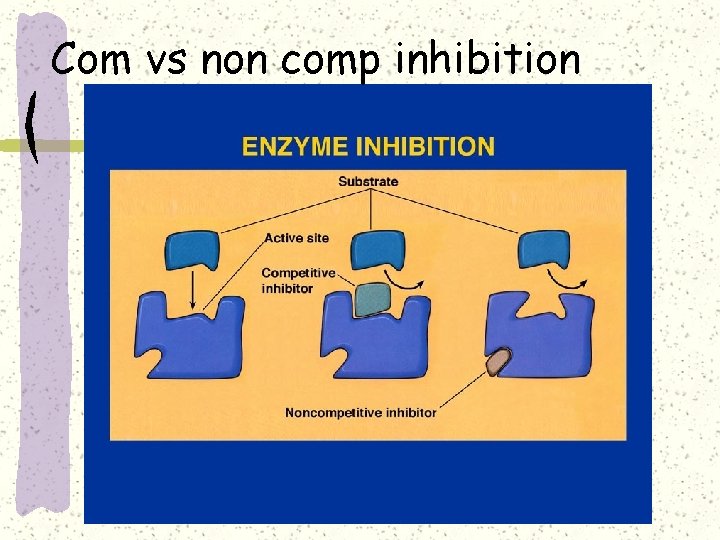
Competitive inhibition
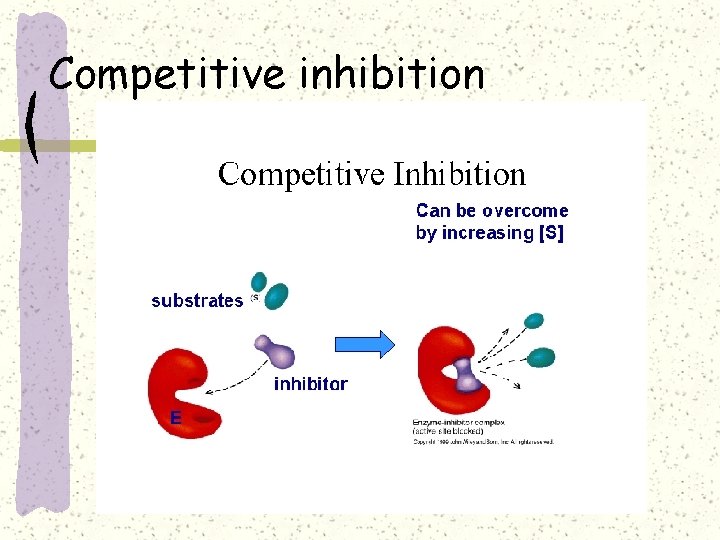
Competitive inhibition
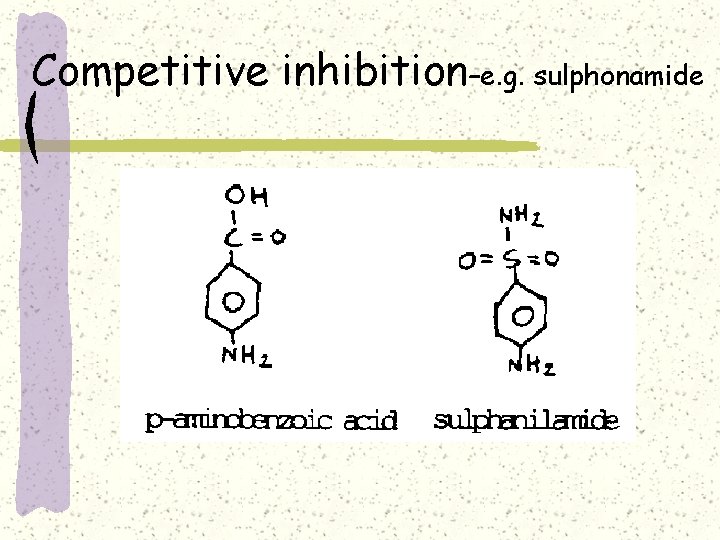
Competitive inhibition–e. g. sulphonamide
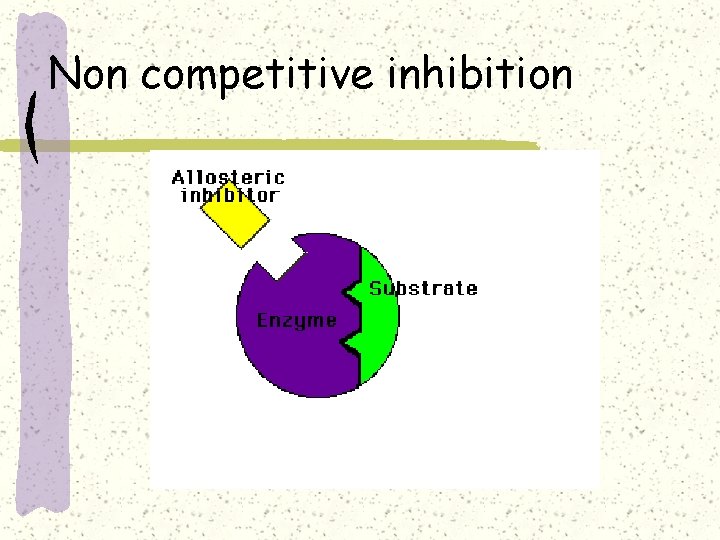
Non competitive inhibition
Com vs non comp inhibition
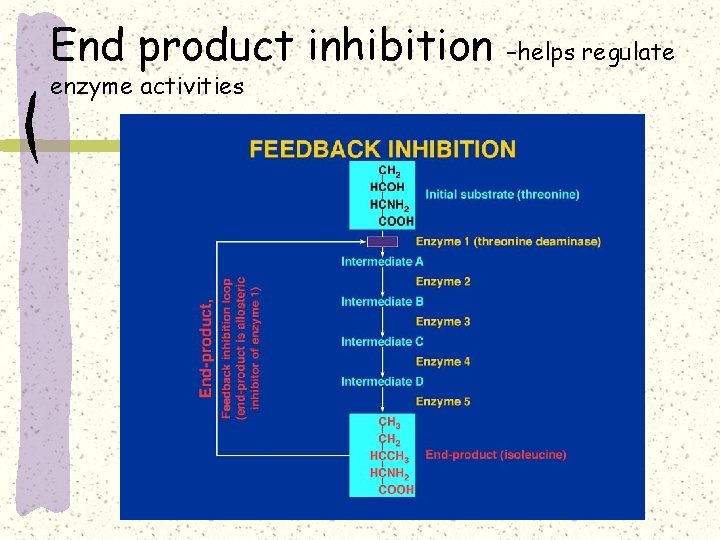
End product inhibition enzyme activities –helps regulate
End product inhibition
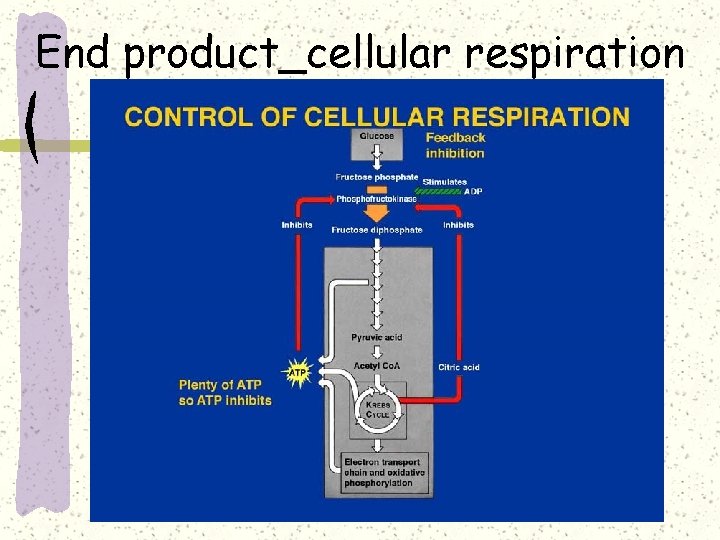
End product_cellular respiration
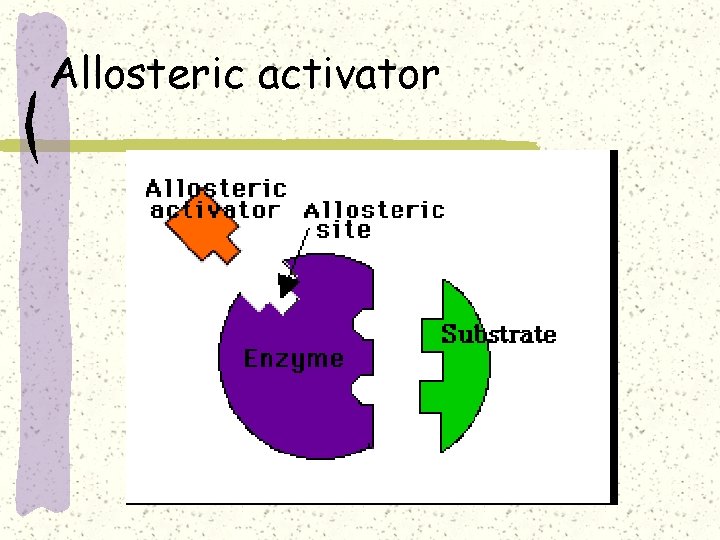
Allosteric activator
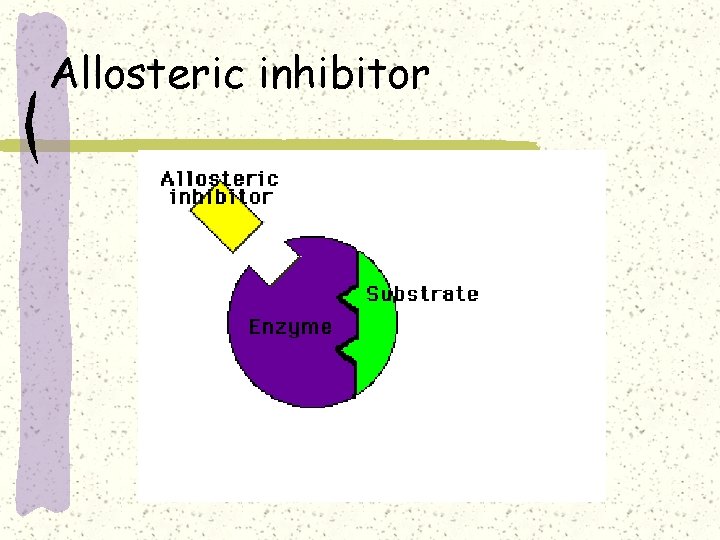
Allosteric inhibitor
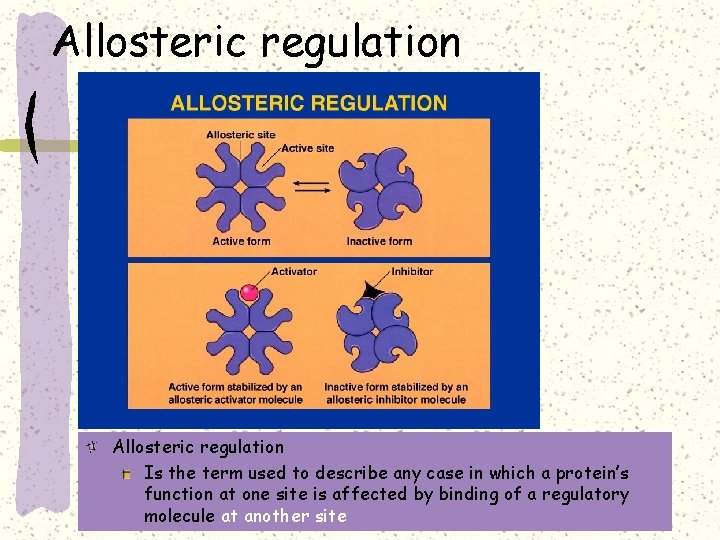
Allosteric regulation Is the term used to describe any case in which a protein’s function at one site is affected by binding of a regulatory molecule at another site
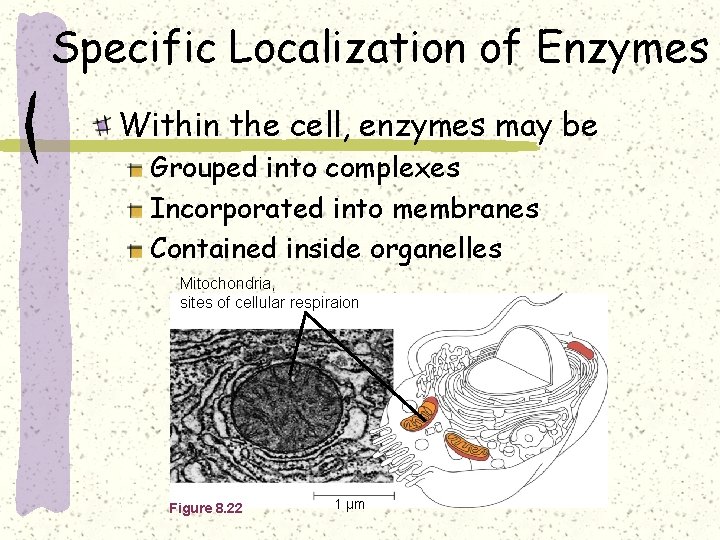
Specific Localization of Enzymes Within the cell, enzymes may be Grouped into complexes Incorporated into membranes Contained inside organelles Mitochondria, sites of cellular respiraion Figure 8. 22 1 µm



Bacteria in Hot springs

Enzyme application_thermophilic
- Slides: 41