An introduction to EMAs support for medicines development









- Slides: 13

An introduction to EMA’s support for medicines development Bio. Capital Europe 2019, Amsterdam 14 March 2019 Presented by Michael Berntgen Head of Product Development Scientific Support Department An agency of the European Union

These Power. Point slides are copyright of the European Medicines Agency. Reproduction is permitted provided the source is acknowledged. 1 An introduction to EMA’s support for medicines development 14 March 2019

EMA’s early development advice services Innovation task force (ITF) as discussion platform for early dialogue with sponsors Scientific advice on the appropriate tests and studies in the development of a medicine, including engagement with other decision makers PRIME scheme for enhanced support of medicines targeting an unmet medical need Qualification of novel methodologies in the context of research and development Specific frameworks for paediatric development and orphan medicines SME support including briefing meetings to discuss regulatory strategies as well as certification of quality and non-clinical data for ATMPs PRIME = PRIority MEdicines; SME = micro, small and medium-sized enterprises; ATMP = Advanced therapy medicinal product 2 An introduction to EMA’s support for medicines development 14 March 2019

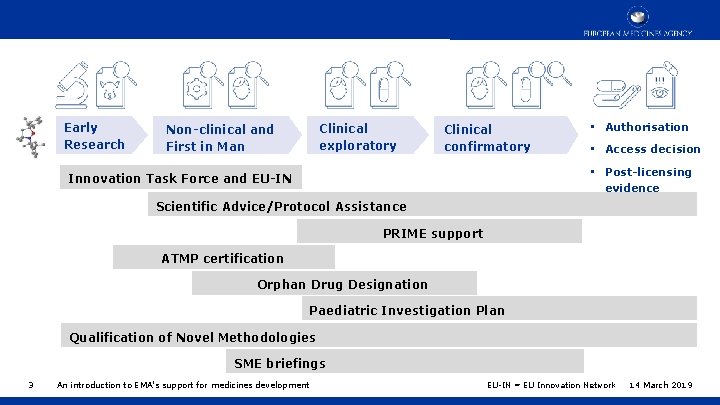
Early Research Clinical exploratory Non-clinical and First in Man Clinical confirmatory • Authorisation • Access decision • Post-licensing evidence Innovation Task Force and EU-IN Scientific Advice/Protocol Assistance PRIME support ATMP certification Orphan Drug Designation Paediatric Investigation Plan Qualification of Novel Methodologies SME briefings 3 An introduction to EMA’s support for medicines development EU-IN = EU Innovation Network 14 March 2019

Innovation Task Force and EU Innovation Network Product/technology/methodology-specific • Support drug development via early informal dialogue in so-called “ITF briefing meetings” on scientific, legal and regulatory issues • Preparing formal procedures Cross-development • Address impact of emerging therapies and technologies on the regulatory system • Identifying emerging trends that may require regulatory guidance and support • Assist knowledge exchange on innovative strategies involving regulatory network • Collaboration with the national innovation offices through the EU Innovation Network, addressing gaps in early regulatory support to innovation 4 An introduction to EMA’s support for medicines development 14 March 2019

Scientific Advice and Protocol Assistance • Advising Applicants on the various tests and trials necessary to demonstrate the quality, safety and efficacy of medicinal products". • Clarifying the scientific requirements for marketing authorisation (MA), including: – Manufacturing, non-clinical and clinical trials, risk-management plans, ways to develop generics, hybrids and biosimilars, significant benefit for orphan medicines, development in children etc. – Post-licencing extension of indication to different age groups / stages of the disease / different conditions, evidence generation on safety aspects etc. • Prospective in nature - focusing on development strategies rather than pre-evaluation of data to support a MAA. 5 An introduction to EMA’s support for medicines development 14 March 2019

Qualification of novel methodologies and biomarkers Framework to guide the development of new more efficient ways to develop drugs, e. g. development of new endpoints for clinical trials Aim: Speed up/optimise drug development and utilisation, improve public health Examples: • • • 6 Methods to predict toxicity; strategies to enrich a patient population for a clinical trial Surrogate clinical endpoints Patient and caregiver reported outcomes, patient registries An introduction to EMA’s support for medicines development 14 March 2019

PRIME scheme To foster the development of medicines with major public health interest ? Reinforce scientific and regulatory advice ! § Foster and facilitate early interaction § Raise awareness of requirements earlier in development Optimise development for robust data generation § Focus efficient development § Promote generation of robust and high quality data Enable accelerated assessment § Promote generation of high quality data § Facilitated by knowledge gained throughout development 7 An introduction to EMA’s support for medicines development 14 March 2019

Special populations: Paediatrics and orphan diseases Paediatric Investigation Pan • • Basis for development and Orphan designation - related incentives (such as development paediatric population subsets. support, fee reduction, market exclusivity) Includes details of the timing and • Scientific advice for orphan demonstrate Quality, Safety and medicines is called protocol Efficacy assistance, also covering Binding on company compliance check (as a basis for the incentives e. g. SPC extension) 8 • authorisation of a medicine for all the measures proposed, to • Orphan Designation An introduction to EMA’s support for medicines development questions on studies needed to demonstrate the significant benefit of the medicine SPC = Supplementary Protection Certificate 14 March 2019

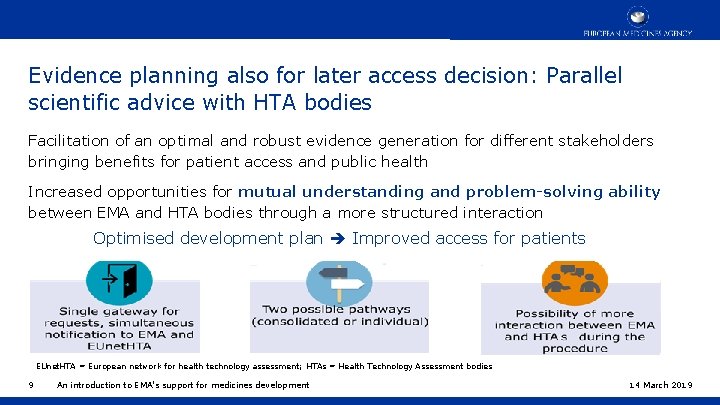
Evidence planning also for later access decision: Parallel scientific advice with HTA bodies Facilitation of an optimal and robust evidence generation for different stakeholders bringing benefits for patient access and public health Increased opportunities for mutual understanding and problem-solving ability between EMA and HTA bodies through a more structured interaction Optimised development plan Improved access for patients EUnet. HTA = European network for health technology assessment; HTAs = Health Technology Assessment bodies 9 An introduction to EMA’s support for medicines development 14 March 2019

Providing specific support for SMEs SME Briefing meetings to discuss regulatory strategy and navigate the range of procedures and incentives available Multidisciplinary group (scientific advice, paediatrics, orphans, regulatory affairs, etc); open to medicinal products for human and veterinary use; fee reductions for EMA procedures Certification of quality and non-clinical data for ATMPs Scientific evaluation of quality data and, when available, non-clinical data that SMEs have generated at any stage of the ATMP development process. Aims to identify any potential issues to be addressed early on, prior to the submission of MAA 10 An introduction to EMA’s support for medicines development 14 March 2019

Take home messages • EMA is open to discuss scientific, regulatory and technical aspects of medicines developments • Different platforms depending on scope, timing and contributors to such engagement on R&D activities • Multiple engagement opportunities to support developers and investors in their decision making to mitigate the risk of failures Overview: EMA Supporting medicine developers 11 An introduction to EMA’s support for medicines development 14 March 2019

Thank you for your attention Further information: List of available guidance and opportunities for interaction Michael. Berntgen@ema. europa. eu European Medicines Agency 30 Churchill Place • Canary Wharf • London E 14 5 EU • United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www. ema. europa. eu/contact Follow us on @EMA_News