An introduction The element song Periodic Table A

An introduction……. The element song
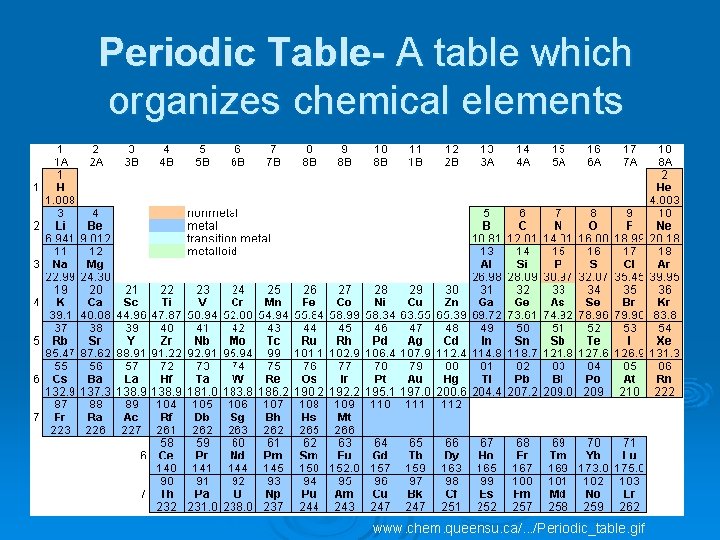
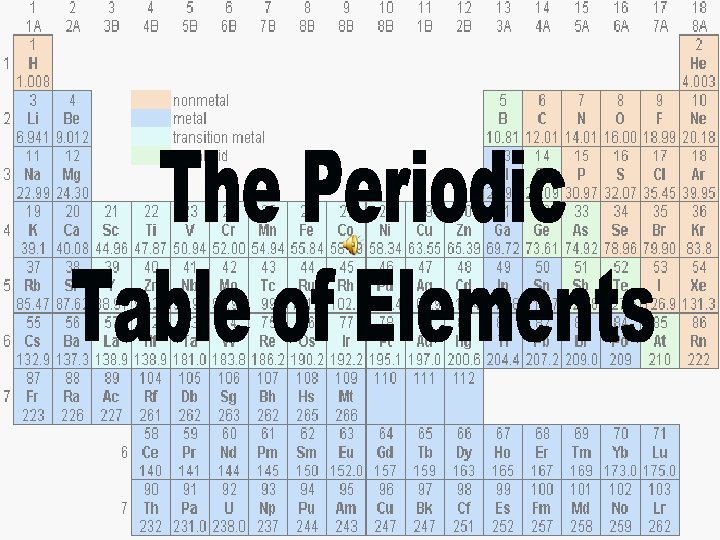
Periodic Table- A table which organizes chemical elements www. chem. queensu. ca/. . . /Periodic_table. gif
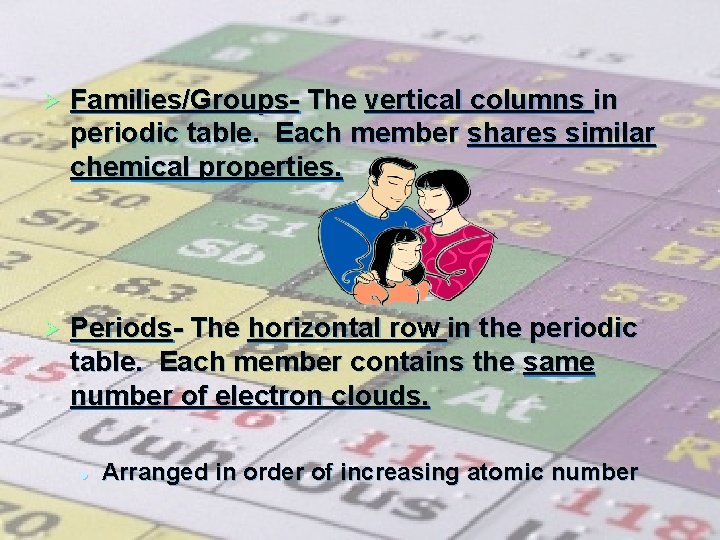
Ø Families/Groups- The vertical columns in periodic table. Each member shares similar chemical properties. Ø Periods- The horizontal row in the periodic table. Each member contains the same number of electron clouds. l Arranged in order of increasing atomic number
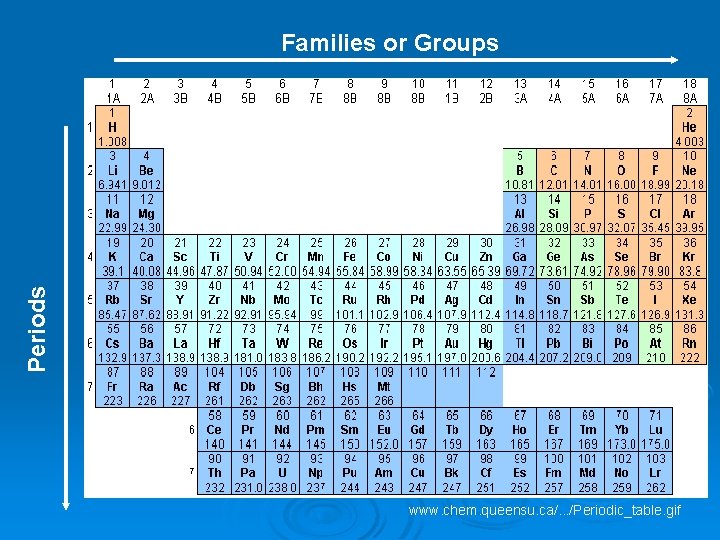
Periods Families or Groups www. chem. queensu. ca/. . . /Periodic_table. gif
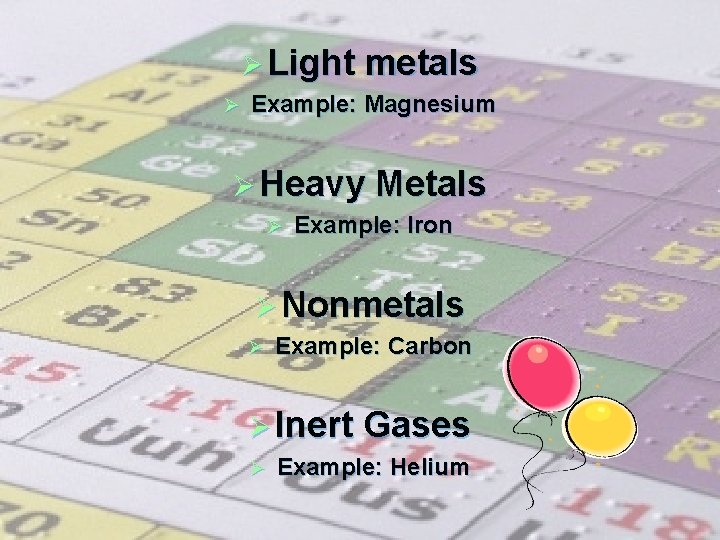
Ø Light metals Ø Example: Magnesium Ø Heavy Metals Ø Example: Iron Ø Nonmetals Ø Example: Carbon Ø Inert Gases Ø Example: Helium
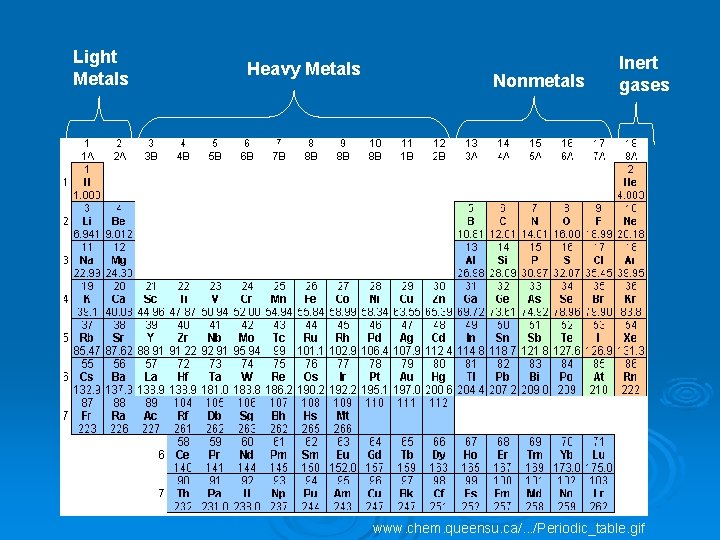
Light Metals Heavy Metals Nonmetals Inert gases www. chem. queensu. ca/. . . /Periodic_table. gif
An Element (A substance consisting of a single atom) The periodic table consists of many elements.
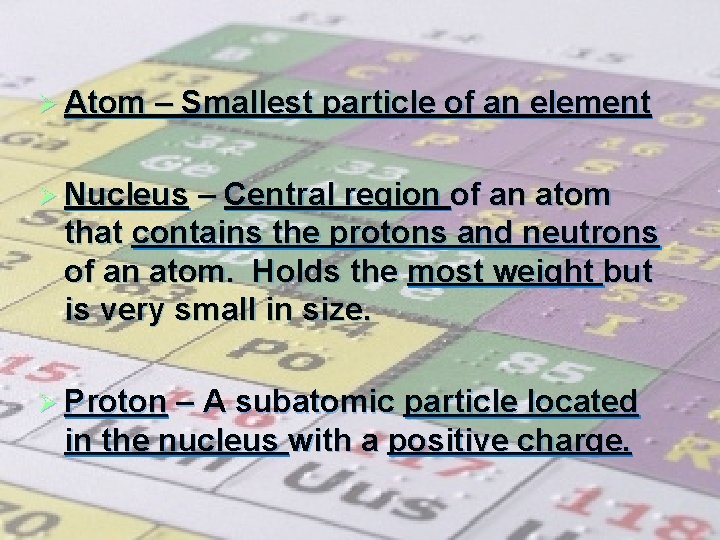
Ø Atom – Smallest particle of an element Ø Nucleus – Central region of an atom that contains the protons and neutrons of an atom. Holds the most weight but is very small in size. Ø Proton – A subatomic particle located in the nucleus with a positive charge.
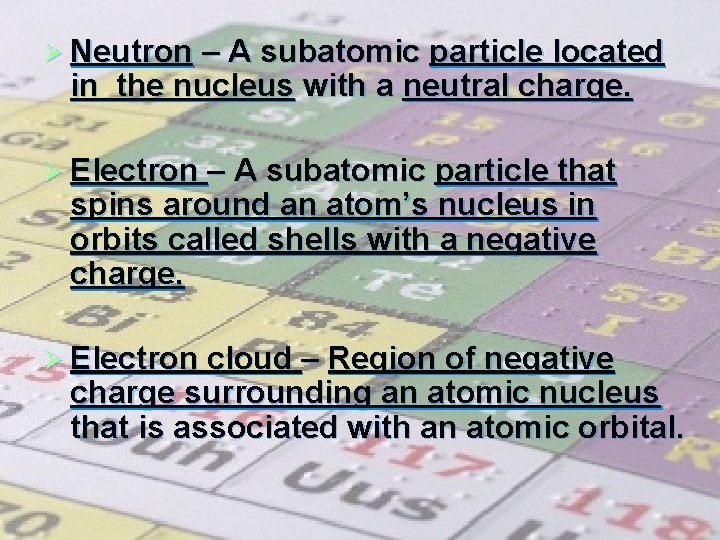
Ø Neutron – A subatomic particle located in the nucleus with a neutral charge. Ø Electron – A subatomic particle that spins around an atom’s nucleus in orbits called shells with a negative charge. Ø Electron cloud – Region of negative charge surrounding an atomic nucleus that is associated with an atomic orbital.
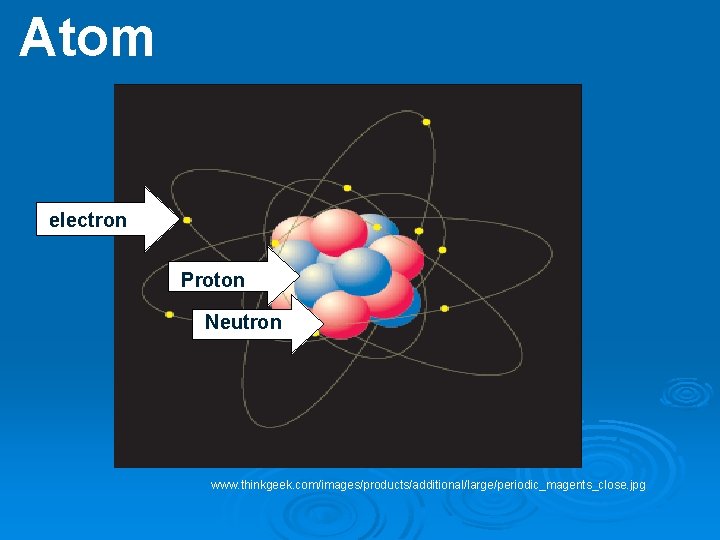
Atom electron Proton Neutron www. thinkgeek. com/images/products/additional/large/periodic_magents_close. jpg
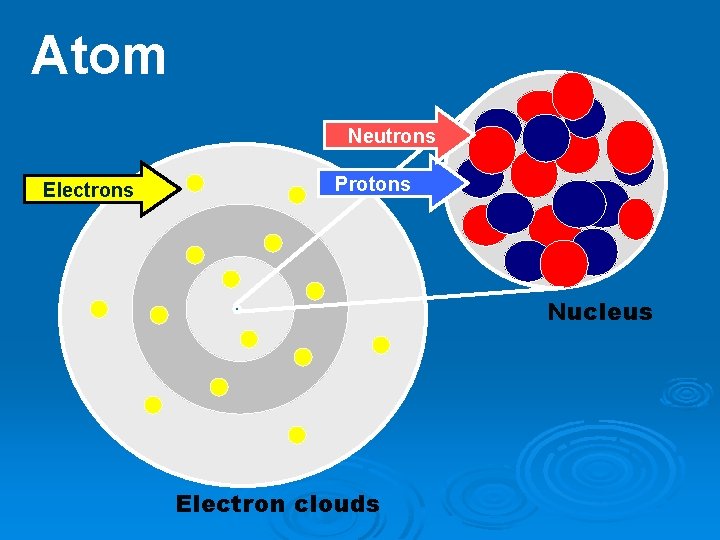
Atom Neutrons Electrons Protons Nucleus Electron clouds
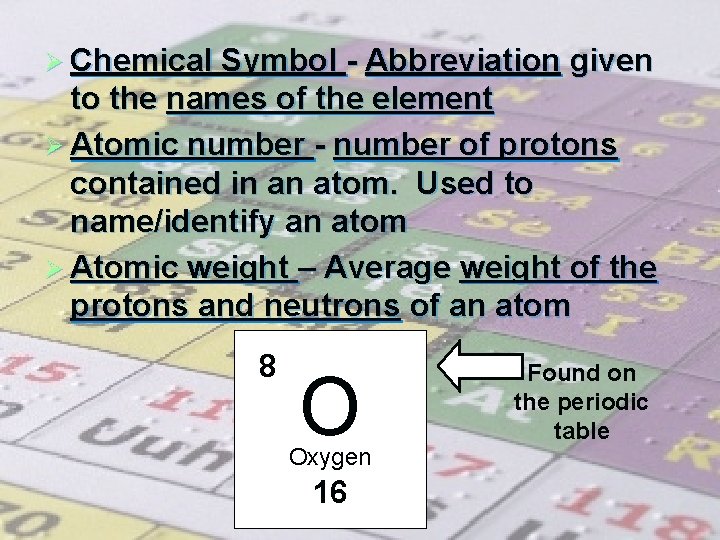
Ø Chemical Symbol - Abbreviation given to the names of the element Ø Atomic number - number of protons contained in an atom. Used to name/identify an atom Ø Atomic weight – Average weight of the protons and neutrons of an atom 8 O Oxygen 16 Found on the periodic table
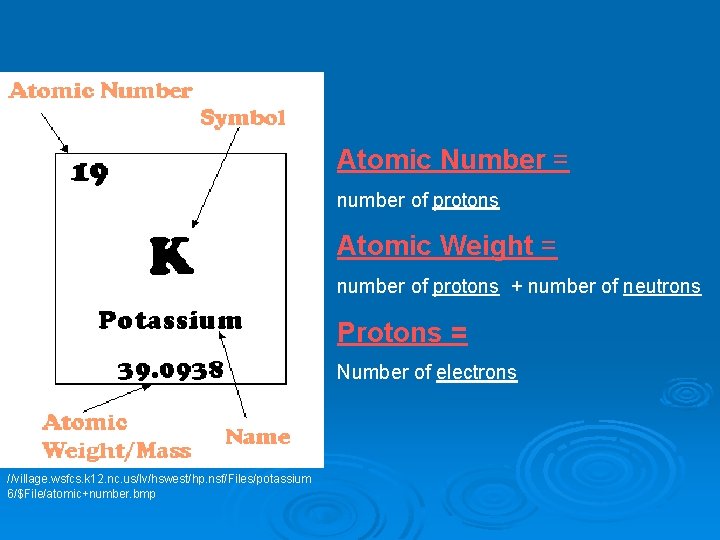
Atomic Number = number of protons Atomic Weight = number of protons + number of neutrons Protons = Number of electrons //village. wsfcs. k 12. nc. us/lv/hswest/hp. nsf/Files/potassium 6/$File/atomic+number. bmp
Ø How can we determine the number of neutrons of a particular element from the information given on the periodic table? Atomic weight (or mass) = number of protons + number of neutrons www. nrc. gov/reading-rm/basicref/students/images/ask. gif
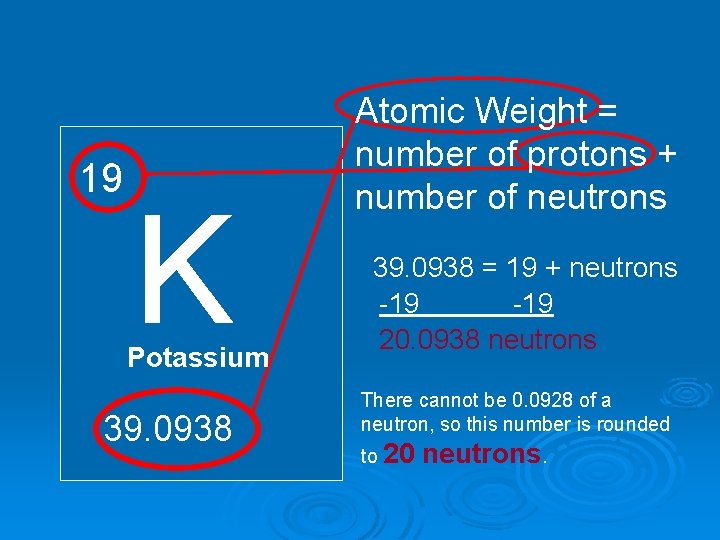
19 K Potassium 39. 0938 Atomic Weight = number of protons + number of neutrons 39. 0938 = 19 + neutrons -19 20. 0938 neutrons There cannot be 0. 0928 of a neutron, so this number is rounded to 20 neutrons.
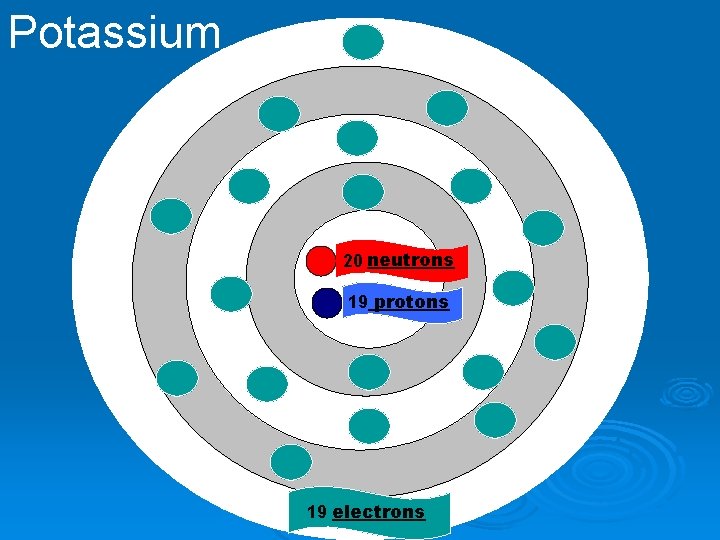
Potassium 20 neutrons 19 protons 19 electrons

Atoms can lose or gain electrons, if they do, they are called IONS Loses electrons = positively charged ion Gaines electrons = negatively charged ion WHY? Electrons are negatively charged.
Chemical Bonds- force holding two atoms or ions together Ionic bonds- force of attraction between oppositely charged ions. One atom gives their electrons and the other takes them which gives them a full outer energy level Covalent bonds- results from atoms sharing their electrons molecule- a group of atoms held together by covalent bonds
- Slides: 19