An Introduction Guidelines Real World Evidence NICE 2019










- Slides: 15

An Introduction: Guidelines & ‘Real World’ Evidence - © NICE 2019. All rights reserved. Subject to notice of rights.

Disclosure of Interests I certify that, to the best of my knowledge, no aspect of my current personal or professional situation might reasonably be expected to affect significantly my views on the subject on which I am presenting, other than the following: • Employment with the National Institute of Health and Care Excellence (NICE) • Academic interest

What is ‘real world’ evidence? Evidence derived from the analysis and/or synthesis of real-world data (RWD). RWD is an overarching term for data on the effects of health interventions that are not collected in the context of RCTs.

Who is using ‘real world’ evidence? 1. Regulators 2. HTA agencies/Guideline developers/ HC providers/insurers 3. Life sciences industry

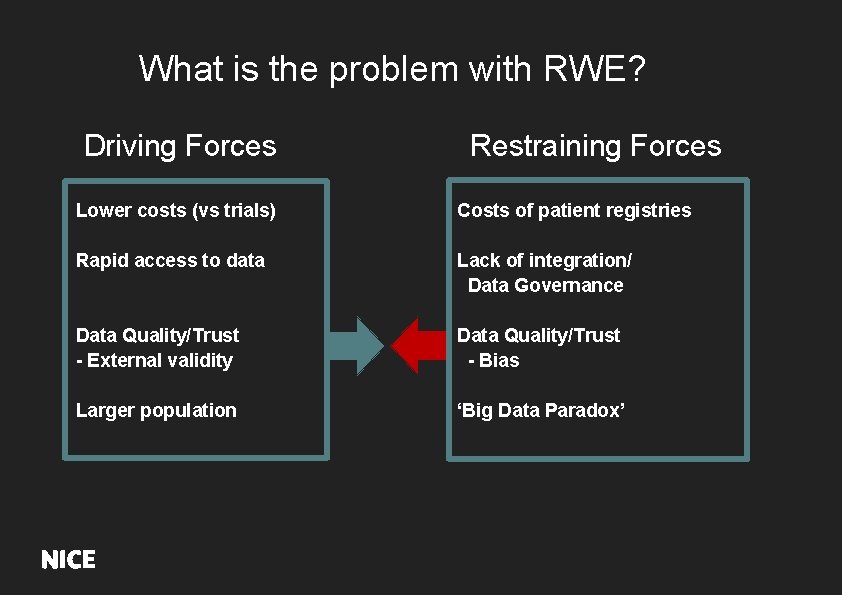
What is the problem with RWE? Driving Forces Restraining Forces Lower costs (vs trials) Costs of patient registries Rapid access to data Lack of integration/ Data Governance Data Quality/Trust - External validity Data Quality/Trust - Bias Larger population ‘ ‘Big Data Paradox’

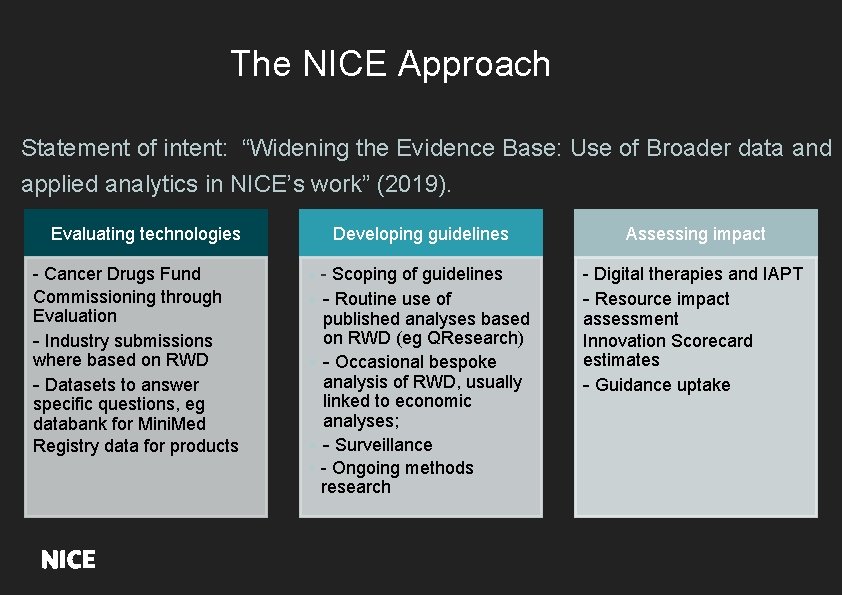
The NICE Approach Statement of intent: “Widening the Evidence Base: Use of Broader data and applied analytics in NICE’s work” (2019). Evaluating technologies - Cancer Drugs Fund Commissioning through Evaluation - Industry submissions where based on RWD - Datasets to answer specific questions, eg databank for Mini. Med Registry data for products Developing guidelines Assessing impact · - Scoping of guidelines · - Routine use of published analyses based on RWD (eg QResearch) · - Occasional bespoke analysis of RWD, usually linked to economic analyses; · - Surveillance · - Ongoing methods research - Digital therapies and IAPT - Resource impact assessment Innovation Scorecard estimates - Guidance uptake

Use of broader data and analytics in NICE Guidelines Data Sources Identified at scoping stage - Presentation on Saturday am (Clare Wohlgemuth) Guideline Development - NICE Cancer Service Guidance (1996 -2006) - Data sources- large audits (regional, national) combined with routine NHS data (HES and cancer registration and survival data) - Analyses of the relationship between hospital volume & outcomes; clinician volume & outcomes

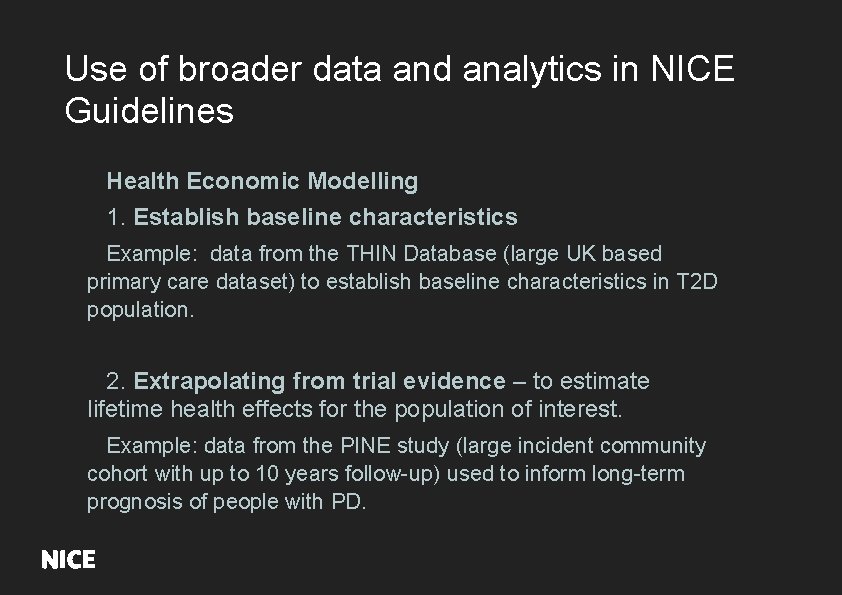
Use of broader data and analytics in NICE Guidelines Health Economic Modelling 1. Establish baseline characteristics Example: data from the THIN Database (large UK based primary care dataset) to establish baseline characteristics in T 2 D population. 2. Extrapolating from trial evidence – to estimate lifetime health effects for the population of interest. Example: data from the PINE study (large incident community cohort with up to 10 years follow-up) used to inform long-term prognosis of people with PD.

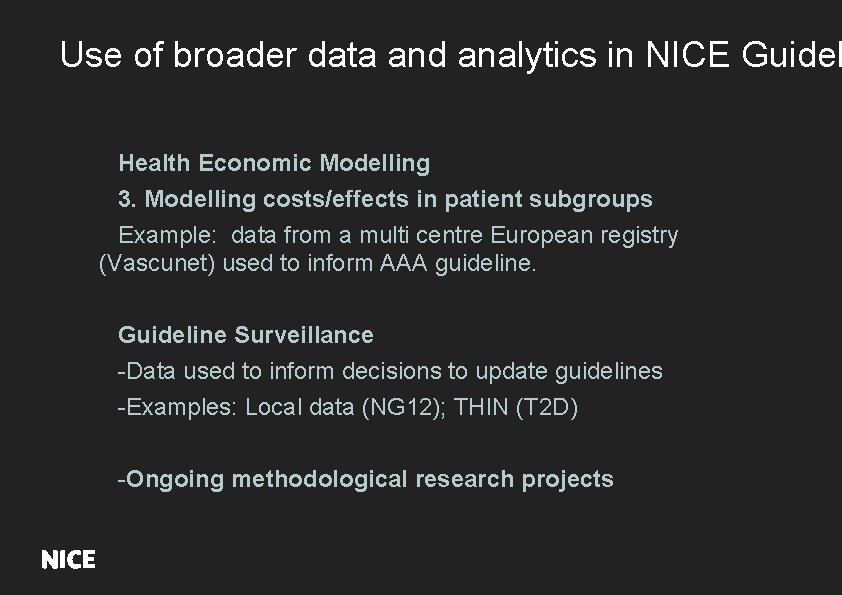
Use of broader data and analytics in NICE Guidel Health Economic Modelling 3. Modelling costs/effects in patient subgroups Example: data from a multi centre European registry (Vascunet) used to inform AAA guideline. Guideline Surveillance -Data used to inform decisions to update guidelines -Examples: Local data (NG 12); THIN (T 2 D) -Ongoing methodological research projects

Constraints to using broader sources of evidence in NICE Guidelines 1. Data Quality 2. Data sources/ Accessibility 3. Time

Future Vision Rapidly evolving: - Investment in improving standards and practice for the collection, synthesis, analysis and interpretation of ‘real world data’ -> € 730 M invested across 65 initiatives 1 - Investment in digital health and social care transformation programmes across the HC evidence ecosystem 1 Plueschke K, et al. . BMJ Open 2018; 8: e 021864.

Managing expectations Optimal treatment choices should be guided by strict requirements of organizations such as the FDA, demanding treatment effects to be estimated under actual conditions of use. Various improvements in design and analysis are recommended for future randomised controlled T 3/T 4 combination trials. - Patient Advocacy Group, Thyroid Disease (2017) We disagree with the committee’s view on sole inclusion of RCTs. It is our view that excluding all non-RCTs, in particular with reference to new technology, significantly restricts the available data on which to make a balanced and informed decision. - Industry, Cataracts (NG 77; 2017) This pathway provides local areas with information on the case for change and best practice for conditions alongside real world case studies. - HCP Organisation, Hypertension (NG 136; 2019) We ask you include the real world data provided by the analysis of IAPT MDS scores that showed equivalence between Cf. D and CBT. - Academic Group, Depression in Adults (2018) - Need for guideline developers to communicate the ‘warning label’ with use of data from non-RCT sources

“But I see a paradise, or even paradises, gained if there is a sufficient number of us who can engage in what we have advertised to be the hallmark of our discipline, that is, principled thinking and methodology development for dealing with uncertainty. ” Meng XL. . The Annals of Applied Statistics. 2018; 12(2): 685 -726.

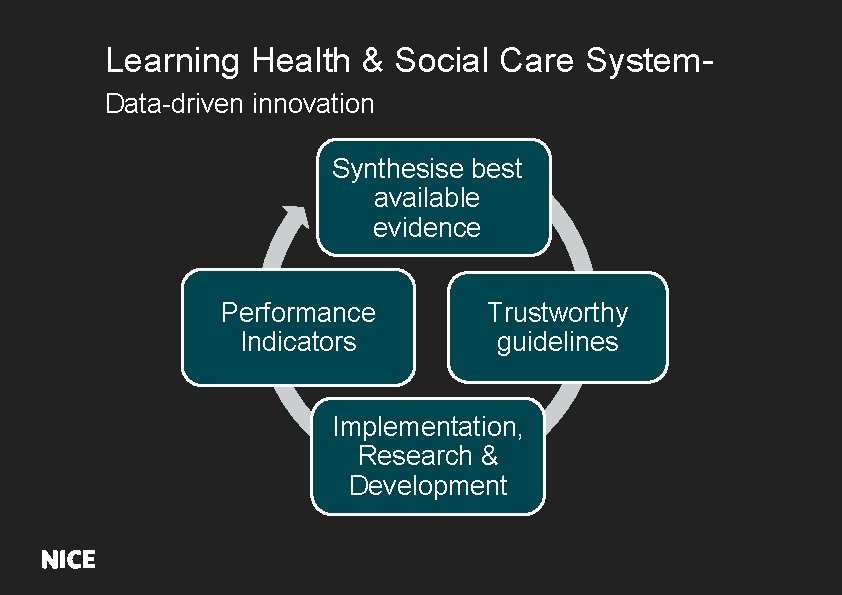
Learning Health & Social Care System. Data-driven innovation Synthesise best available evidence Performance Indicators Trustworthy guidelines Implementation, Research & Development

Acknowledgements Gabriel Rogers, Methods and Economics Team, NICE Adrian Jonas, Data Analytics Team, NICE Kay Nolan, Juliana Sanabria, Charlotte Haynes, Surveillance Team, NICE Methods and Economics Team, NICE