An Improved Method for Assessing ASR Potential in






















- Slides: 58

An Improved Method for Assessing ASR Potential in Concrete Enamur R Latifee Ph. D student, Clemson University, SC, USA Assistant Professor, Ahsanullah University of Science and Technology Dhaka, Bangladesh June, 2010

Some Case Histories Buck Hydroelectric plant on New River (Virginia, US) Arch dam in California crown deflection of 127 mm in 9 years Railroad Canyon Dam Morrow Point Dam, Colorado, USA Stewart Mountain Dam, Arizona Parker Dam (Arizona) expansion in excess of 0. 1 percent ASU/ACS/99

Case Study: Parker Dam, California Alkali-Aggregate Reactions in Hydroelectric Plants and Dams: http: //www. acres. com/aar/ Hydroelectric dam built in 1938 180 mm of arch deflection due to alkali silica gel expansion Cracking and gel flow in concrete

Case Study: I-85 - Atlanta, Georgia Possible ASR damage on concrete retaining wall picture taken 1/2002

ASR? What is Alkali Silica Reaction? Alkali-silica reaction (ASR) is a heterogeneous chemical reaction between alkali ions (Na+ and K+) and hydroxide ions (OH-) in the concrete pore solution, generally derived from the Portland cement, and forms of reactive silica (Si. O 2) in the aggregate (eg: chert, quartzite, opal, strained quartz crystals). The reaction produces a hydrous alkali-silica gel. Formation of the gel alone does not cause cracking, rather cracking occurs when the gel adsorbs water and swells. The swelling causes expansion. It often results in pressures greater than the concrete can withstand so produces cracks in the concrete.

ASR Aggregate reactivity depends directly on the alkalinity (typically expressed as p. H) of the solution in the concrete pores. This alkalinity generally primarily reflects the level of water-soluble alkalis (sodium and potassium) in the concrete. These alkalis are typically derived from the Portland cement.

ASR explanation ASR can be explained as the situation where cement alkalis react with certain forms of silica in the aggregate component of a concrete, forming an alkali-silica gel at the aggregates surface. This formation, often referred to as “reaction rim” has a very strong affinity for water, and thus has a tendency to swell. These expanding compounds can cause internal pressures sufficiently strong to cause cracking of the paste matrix, which can then result in a compromised concrete with an open door to an increasing rate of deterioration.

ASR is the most common form of alkali-aggregate reaction (AAR) in concrete; the other, much less common, form is alkali-carbonate reaction (ACR). ASR is a chemical process in which alkalis, usually predominantly from the cement, combine with certain types of silica in the aggregate when moisture is present. This reaction produces an alkali-silica gel that can absorb water and expand to cause cracking and disruption of the concrete. For damaging reaction to take place the following need to be present in sufficient quantities. High alkali cement Reactive aggregate Moisture [above 75%RH within the concrete]

Chemistry of Alkali Silica Reaction Cement production involves raw materials that contain alkalis in the range of 0. 2 to 1. 5 percent of Na 2 O This generates a pore fluid with high p. H (12. 5 to 13. 5) Strong alkalinity causes the acidic siliceous material to react ASU/ACS/99

ASTM specification ASTM C 150 designates cements with more than 0. 6 percent of Na 2 O as highalkali cements Even with low alkali content, but sufficient amount of cement, alkali-silica reactions can occur ASU/ACS/99

Other sources of alkali Even if alkali content is small, there is a chance of alkali-silica reaction due to alkaline admixtures aggregates that are contaminated penetration of seawater deicing solutions ASU/ACS/99

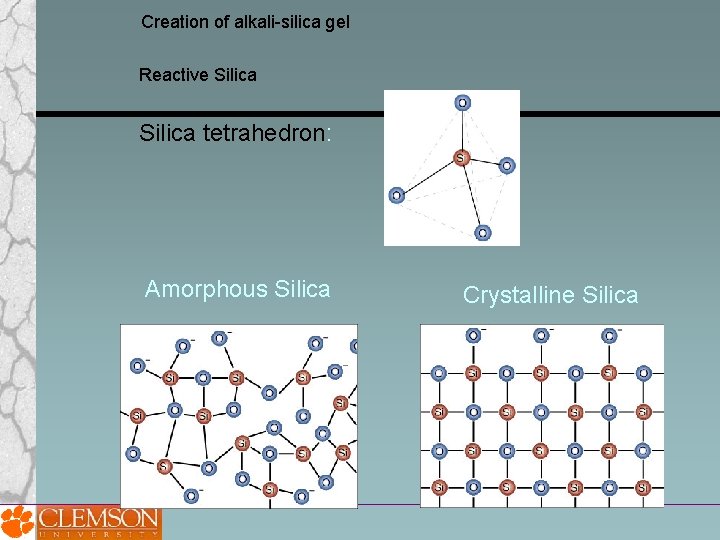
Creation of alkali-silica gel Reactive Silica tetrahedron: Amorphous Silica Crystalline Silica

Creation of alkali-silica gel Reactive Silica Amorphous or disordered silica = most chemically reactive Common reactive minerals: strained quartz opal obsidian cristobalite tridymite chelcedony cherts cryptocrystalline volcanic rocks

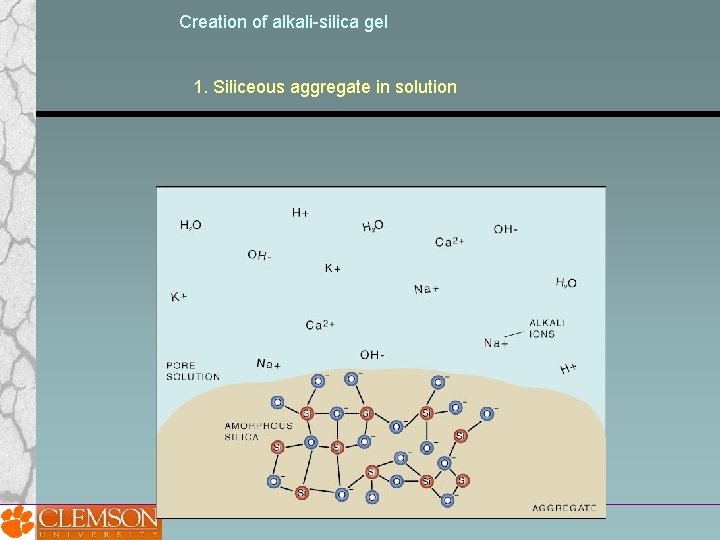
Creation of alkali-silica gel 1. Siliceous aggregate in solution

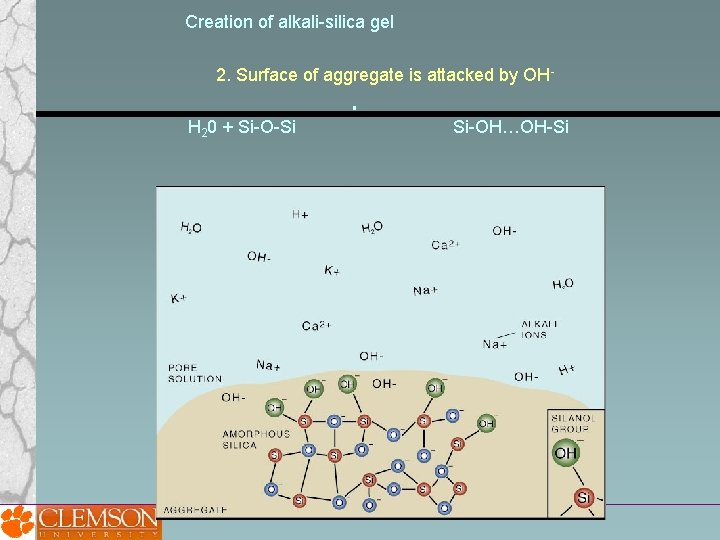
Creation of alkali-silica gel 2. Surface of aggregate is attacked by OHH 20 + Si-O-Si Si-OH…OH-Si

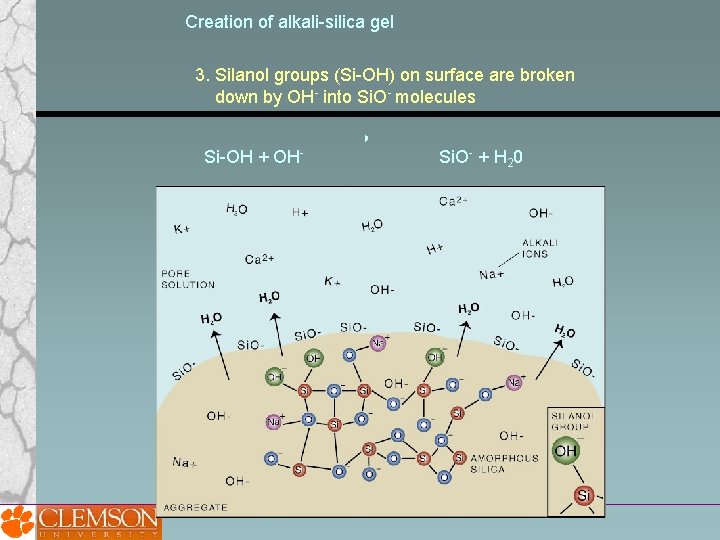
Creation of alkali-silica gel 3. Silanol groups (Si-OH) on surface are broken down by OH- into Si. O- molecules Si-OH + OH- Si. O- + H 20

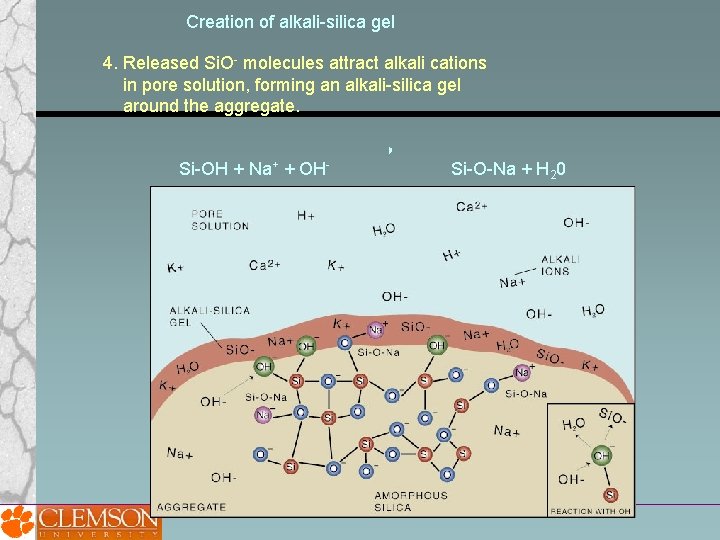
Creation of alkali-silica gel 4. Released Si. O- molecules attract alkali cations in pore solution, forming an alkali-silica gel around the aggregate. Si-OH + Na+ + OH- Si-O-Na + H 20

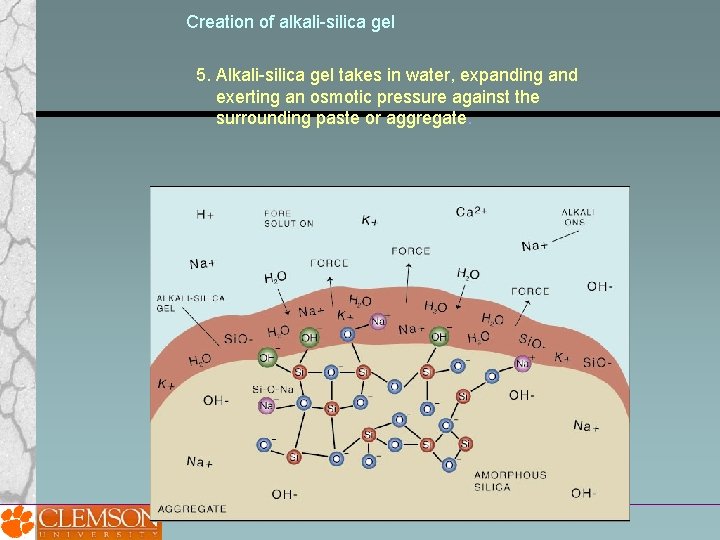
Creation of alkali-silica gel 5. Alkali-silica gel takes in water, expanding and exerting an osmotic pressure against the surrounding paste or aggregate.

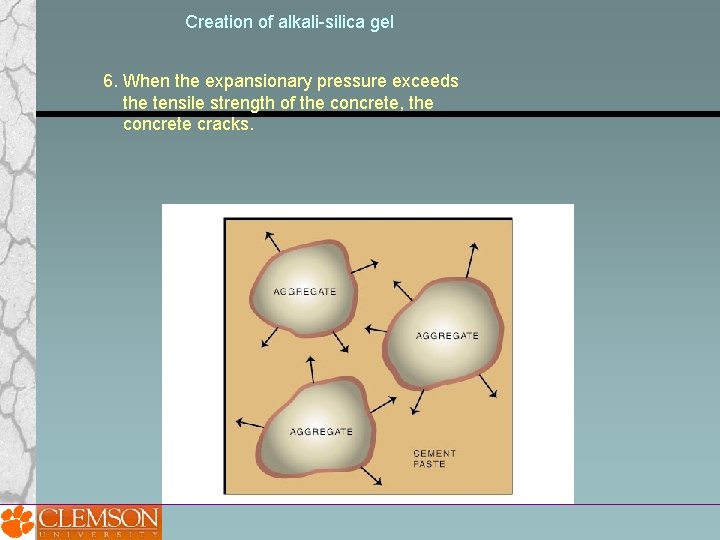
Creation of alkali-silica gel 6. When the expansionary pressure exceeds the tensile strength of the concrete, the concrete cracks.

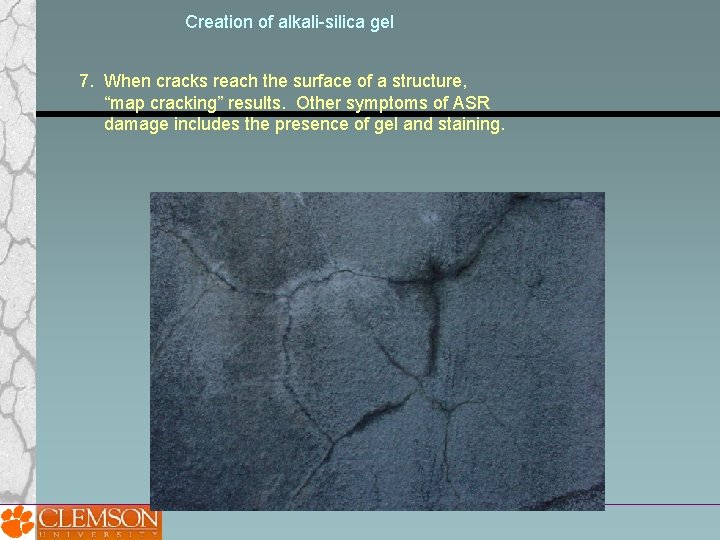
Creation of alkali-silica gel 7. When cracks reach the surface of a structure, “map cracking” results. Other symptoms of ASR damage includes the presence of gel and staining.

Creation of alkali-silica gel 8. Once ASR damage has begun: Expansion and cracking of concrete Increased permeability More water and external alkalis penetrate concrete Increased ASR damage

Measures for prevention Low alkali content cement and mildly reactive aggregate Sweetening of aggregate using limestone Control of access of water to concrete Replacing part of cement by pozzolanic admixtures Mg. O content should not exceed 6 percent (ASTM C 150 -83) Lithium nitrate can be used to control expansion in new concrete, ASU/ACS/99

ACR Alkali-carbonate reactions (ACR) are observed with certain dolomitic rocks. Dedolomitization, the breaking down of dolomite, is normally associated with expansion.

DEF Delayed ettringite formation (DEF) is a special case of internal sulfate attack.

List of Reactive Silica Minerals and Rocks Andesites, Argillites, Certain Siliceous Limestones & Dolomites, Chalcedonic Cherts, Chalcedony Cristobalite Dacites Glassy or Cryptocrystalline volcanics Foliated Gneiss Granite Gneiss Graywackes, Metagraywackes Siltstones Opal, Opaline Shales Phylites, Quartzites Quartzoses Cherts, Flint Rhyolites, Schists Siliceous Shales, Strained quartz Other forms of quartz, Synthetic and Natural siliceous glass Tridymite

SILICA MINERALS IN ORDER OF DECREASING REACTIVITY 1. # Amorphous silica: sedimentary or volcanic glass (a volcanic glass that is devitrified and/or mostly recrystallized may still be reactive) 2. # Opal 3. # Unstable crystalline silica (tridymite and cristobalite) 4. # Chert 5. # Chalcedony 6. # Other cryptocrystalline forms of silica 7. # Metamorphically granulated and distorted quartz 8. # Stressed quartz 9. # Imperfectly crystallized quartz 10. # Pure quartz occurring in perfect crystals

ROCKS IN ORDER OF DECREASING REACTIVITY 1. # Volcanic glasses, including tuffs (especially highly siliceous ones) 2. # Metaquartzites metamorphosed sandstones) 3. # Highly granulated granite gneisses 4. # Highly stressed granite gneisses 5. # Other silica-bearing metamorphic rocks 6. # Siliceous and micaceous schists and phyllites 7. # Well-crystallized igneous rock 8. # Pegmatitic (coarsely crystallized) igneous rock 9. # Nonsiliceous rock

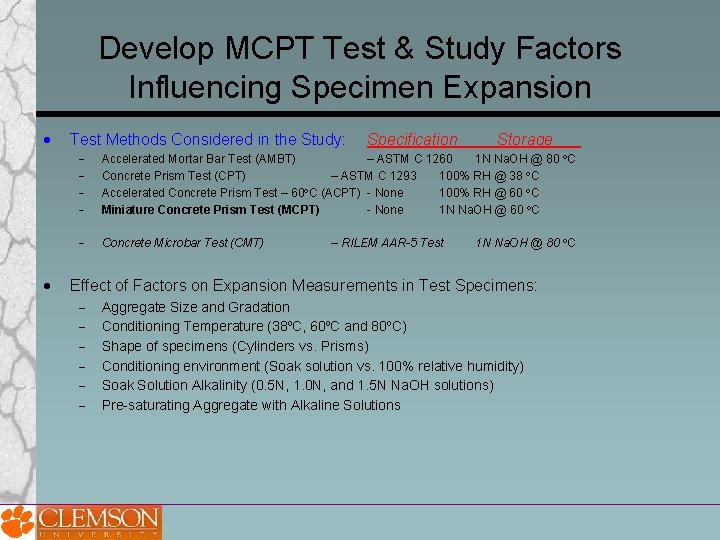
Develop MCPT Test & Study Factors Influencing Specimen Expansion Test Methods Considered in the Study: Specification Storage___ Accelerated Mortar Bar Test (AMBT) – ASTM C 1260 1 N Na. OH @ 80 C Concrete Prism Test (CPT) – ASTM C 1293 100% RH @ 38 C Accelerated Concrete Prism Test – 60 C (ACPT) - None 100% RH @ 60 C Miniature Concrete Prism Test (MCPT) - None 1 N Na. OH @ 60 C Concrete Microbar Test (CMT) – RILEM AAR-5 Test 1 N Na. OH @ 80 C Effect of Factors on Expansion Measurements in Test Specimens: Aggregate Size and Gradation Conditioning Temperature (38⁰C, 60⁰C and 80⁰C) Shape of specimens (Cylinders vs. Prisms) Conditioning environment (Soak solution vs. 100% relative humidity) Soak Solution Alkalinity (0. 5 N, 1. 0 N, and 1. 5 N Na. OH solutions) Pre-saturating Aggregate with Alkaline Solutions

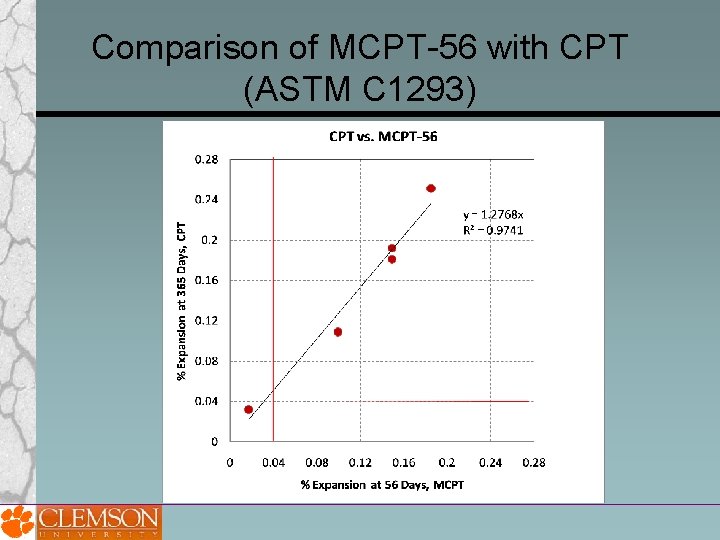
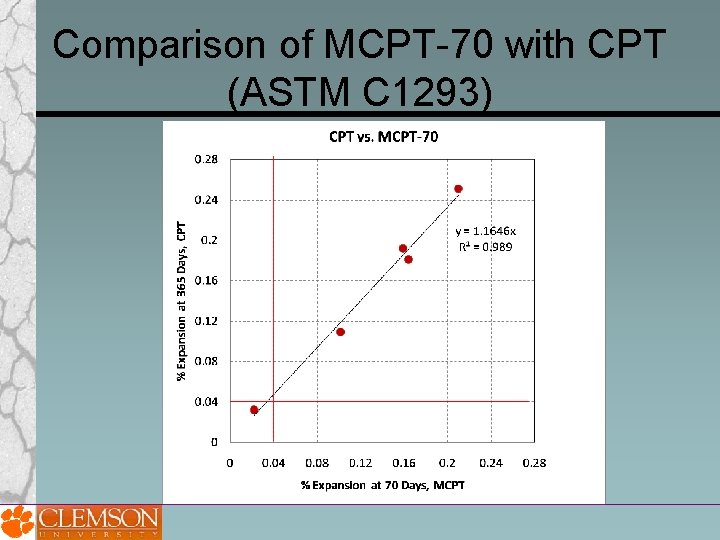
Drawbacks of ASTM C 1260 and 1293 The major drawback to ASTM C 1293 is its long duration (1 or 2 years). One technical deficiency of ASTM C 1293 is that it is not wellsuited for determining the effects of cement alkalinity on expansion, most likely because of leac One downside of ASTM C 1260 is that it tends to be overly severe when testing some aggregates, resulting in expansions exceeding the failure limit, even though these aggregates pass the concrete prism test and perform well in field applications.

Need An Improved Method Miniature Concrete Prism Test Developing at Clemson University, South Carolina, USA No need to wait for one year (ASTM C 1293) Do not have to modify the aggregates (ASTM C 1260)

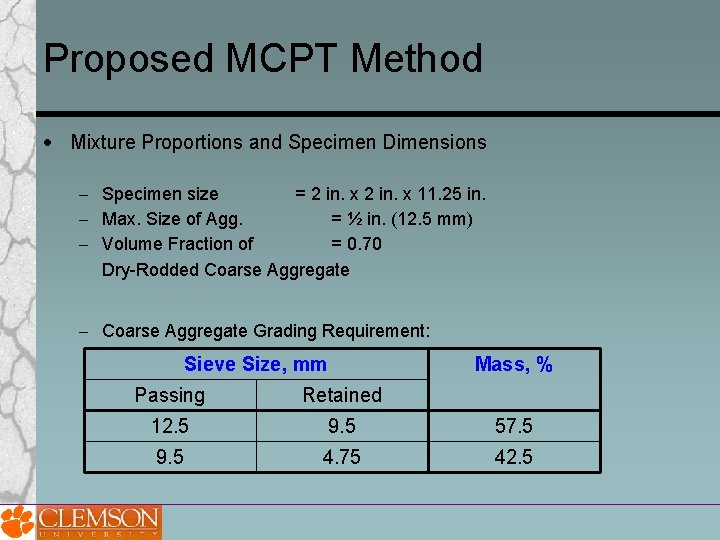
Proposed MCPT Method Mixture Proportions and Specimen Dimensions Specimen size = 2 in. x 11. 25 in. Max. Size of Agg. = ½ in. (12. 5 mm) Volume Fraction of = 0. 70 Dry-Rodded Coarse Aggregate Grading Requirement: Sieve Size, mm Mass, % Passing Retained 12. 5 9. 5 57. 5 9. 5 4. 75 42. 5

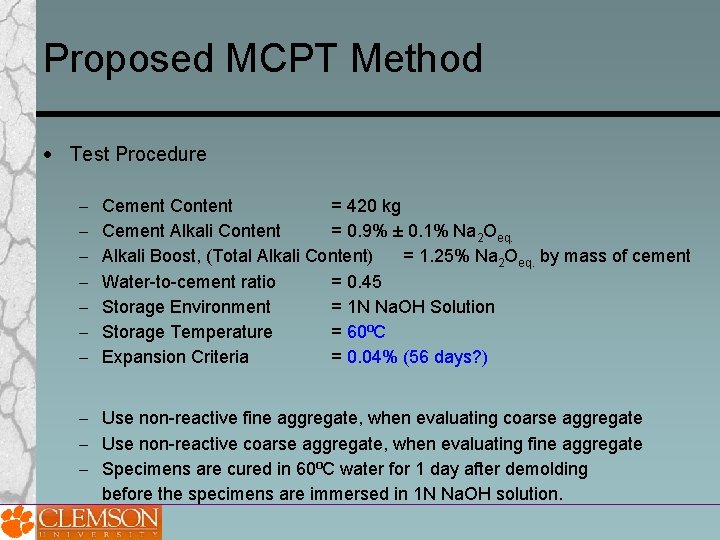
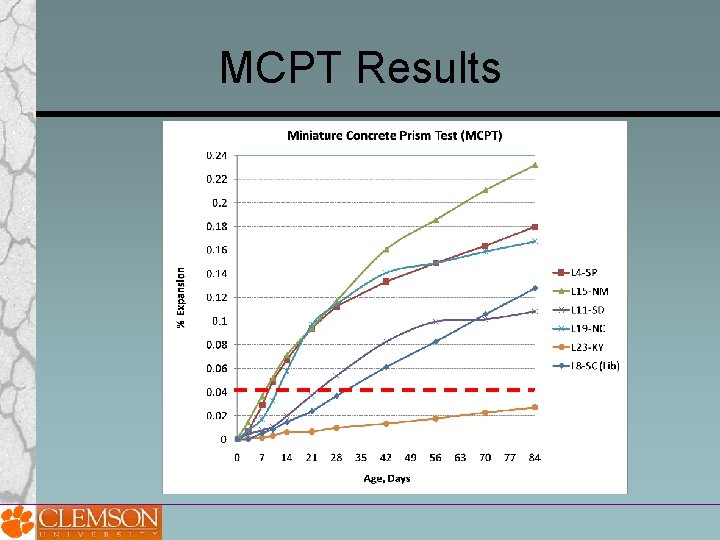
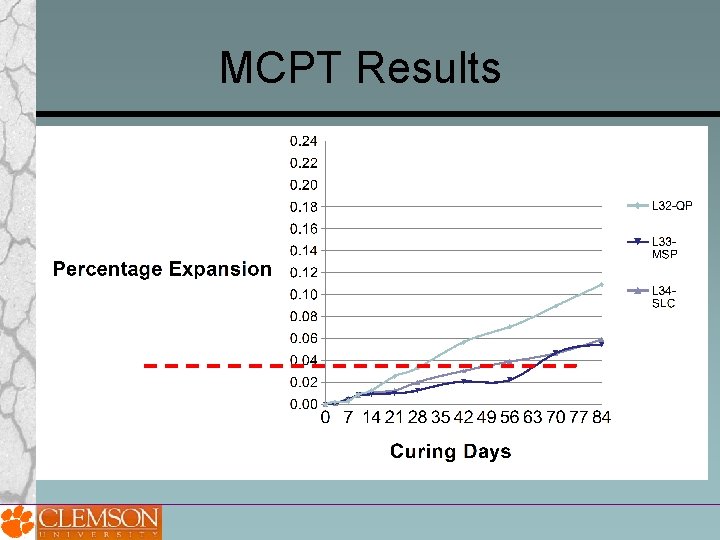
Proposed MCPT Method Test Procedure Cement Content = 420 kg Cement Alkali Content = 0. 9% ± 0. 1% Na 2 Oeq. Alkali Boost, (Total Alkali Content) = 1. 25% Na 2 Oeq. by mass of cement Water-to-cement ratio = 0. 45 Storage Environment = 1 N Na. OH Solution Storage Temperature = 60⁰C Expansion Criteria = 0. 04% (56 days? ) Use non-reactive fine aggregate, when evaluating coarse aggregate Use non-reactive coarse aggregate, when evaluating fine aggregate Specimens are cured in 60⁰C water for 1 day after demolding before the specimens are immersed in 1 N Na. OH solution.

Proposed MCPT Method 33/38

Length comparator with Reference Bar

Length comparator with MCPT Cample

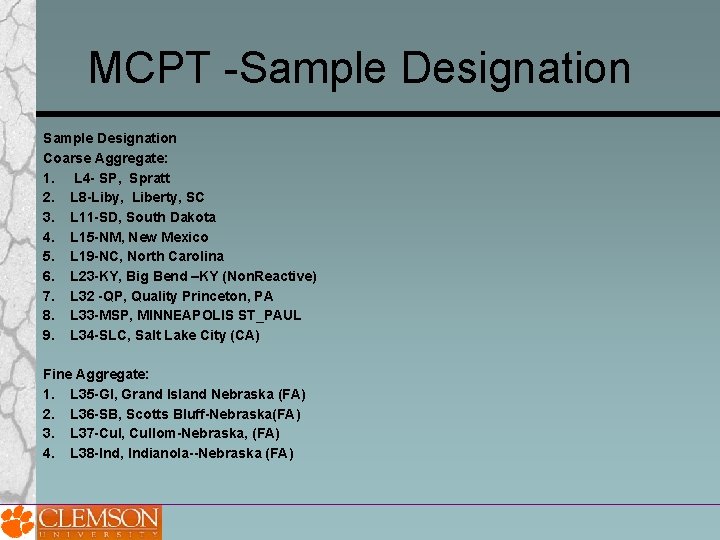
MCPT -Sample Designation Coarse Aggregate: 1. L 4 - SP, Spratt 2. L 8 -Liby, Liberty, SC 3. L 11 -SD, South Dakota 4. L 15 -NM, New Mexico 5. L 19 -NC, North Carolina 6. L 23 -KY, Big Bend –KY (Non. Reactive) 7. L 32 -QP, Quality Princeton, PA 8. L 33 -MSP, MINNEAPOLIS ST_PAUL 9. L 34 -SLC, Salt Lake City (CA) Fine Aggregate: 1. L 35 -GI, Grand Island Nebraska (FA) 2. L 36 -SB, Scotts Bluff-Nebraska(FA) 3. L 37 -Cul, Cullom-Nebraska, (FA) 4. L 38 -Ind, Indianola--Nebraska (FA)

MCPT Results

MCPT Results

Comparison of MCPT-56 with CPT (ASTM C 1293)

Comparison of MCPT-70 with CPT (ASTM C 1293)

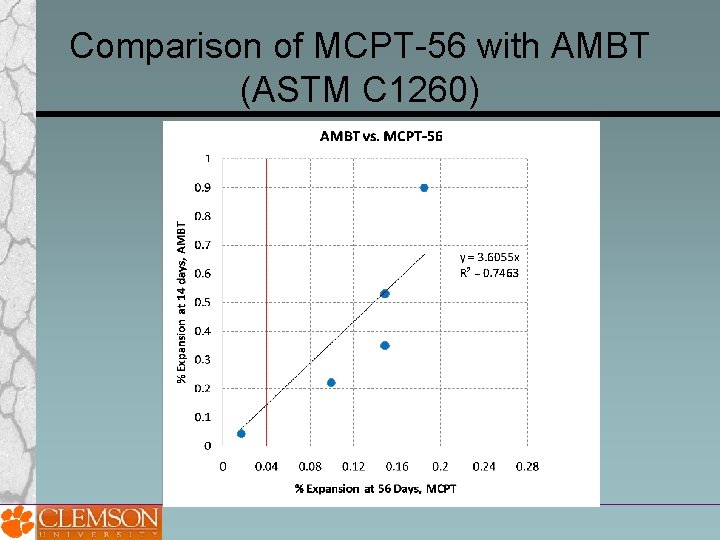
Comparison of MCPT-56 with AMBT (ASTM C 1260)

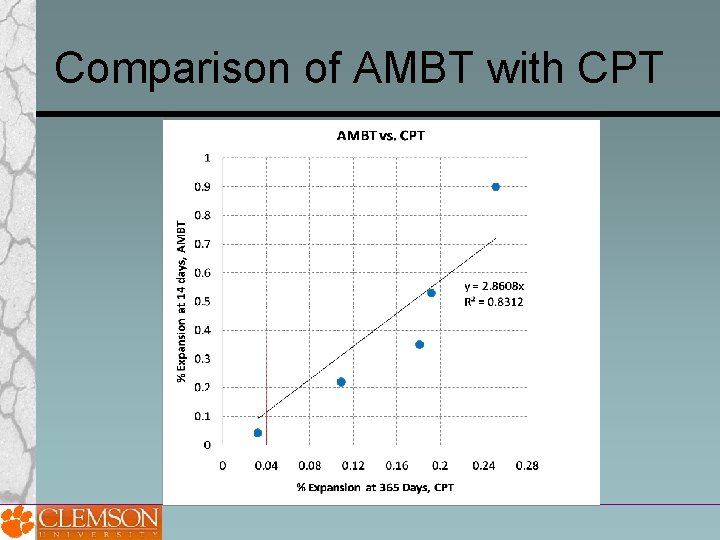
Comparison of AMBT with CPT

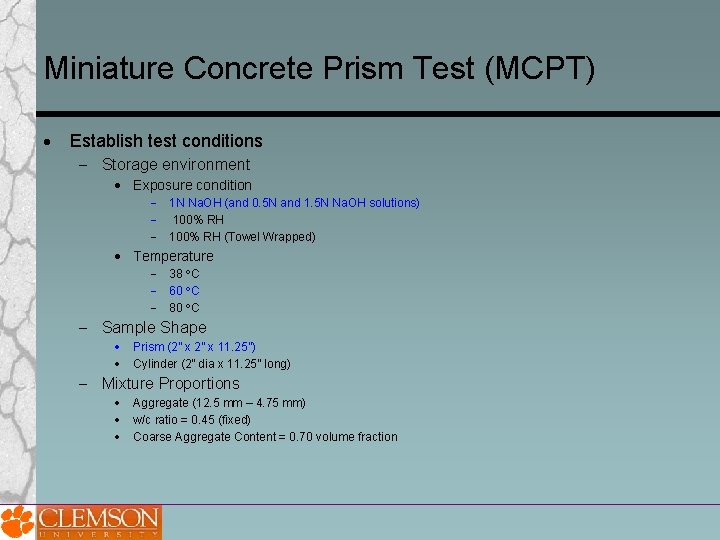
Miniature Concrete Prism Test (MCPT) Establish test conditions Storage environment Exposure condition 1 N Na. OH (and 0. 5 N and 1. 5 N Na. OH solutions) 100% RH (Towel Wrapped) Temperature 38 C 60 C 80 C Sample Shape Prism (2” x 11. 25”) Cylinder (2” dia x 11. 25” long) Mixture Proportions Aggregate (12. 5 mm – 4. 75 mm) w/c ratio = 0. 45 (fixed) Coarse Aggregate Content = 0. 70 volume fraction

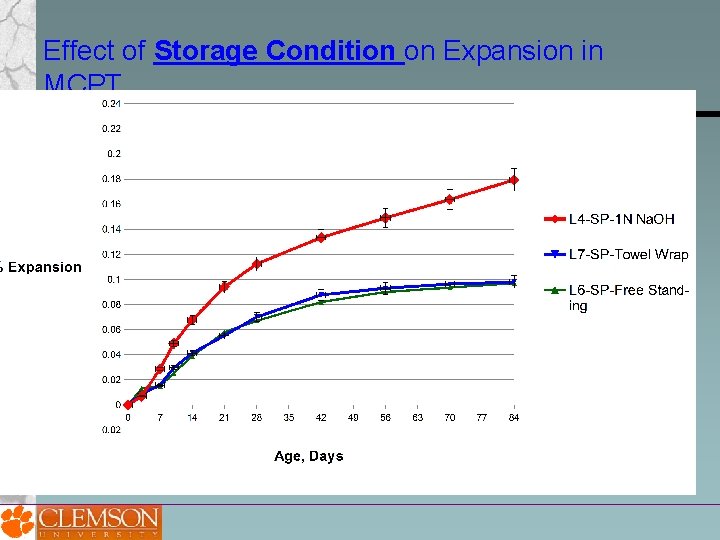
Effect of Storage Condition 100% RH, Free standing 1 N Na. OH Soak Solution 100% RH, Towel Wrapped

Effect of Storage Condition on Expansion in MCPT

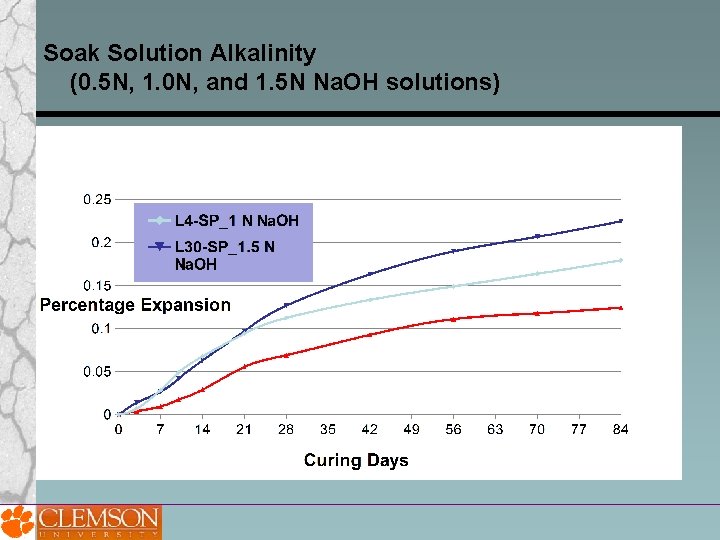
Soak Solution Alkalinity (0. 5 N, 1. 0 N, and 1. 5 N Na. OH solutions)

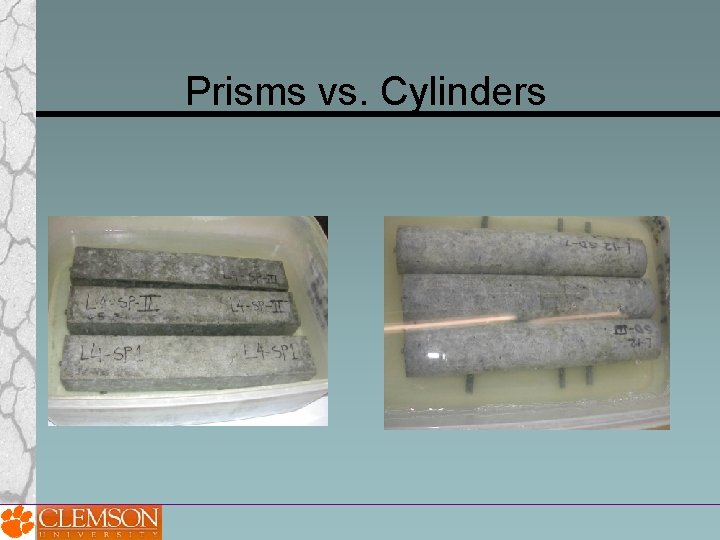
Prisms vs. Cylinders

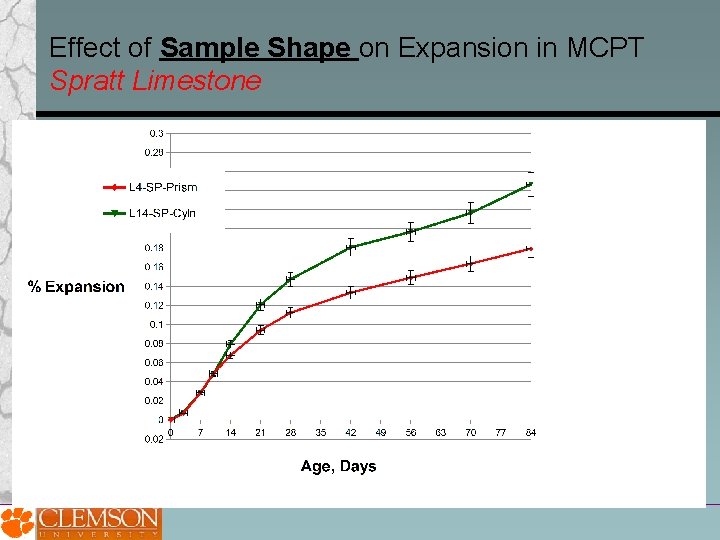
Effect of Sample Shape on Expansion in MCPT Spratt Limestone

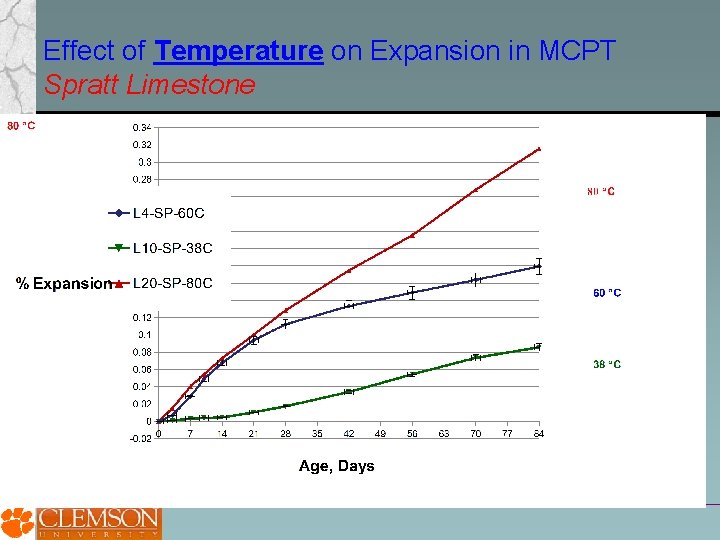
Effect of Temperature on Expansion in MCPT Spratt Limestone

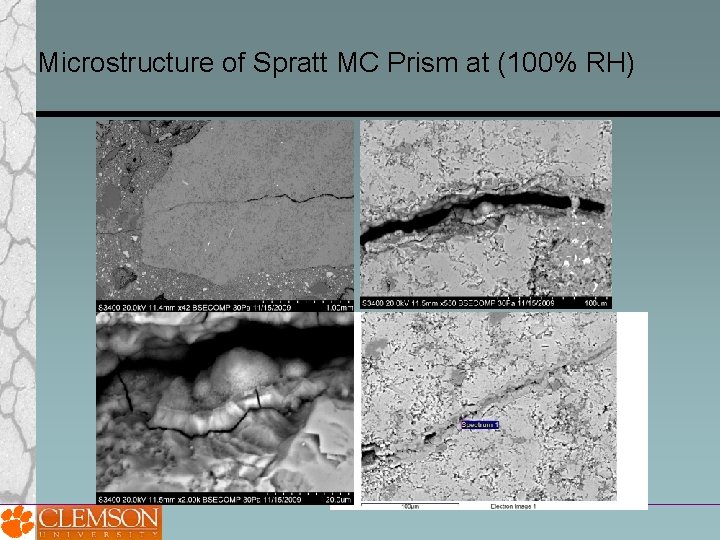
Microstructure of Spratt MC Prism at (100% RH)

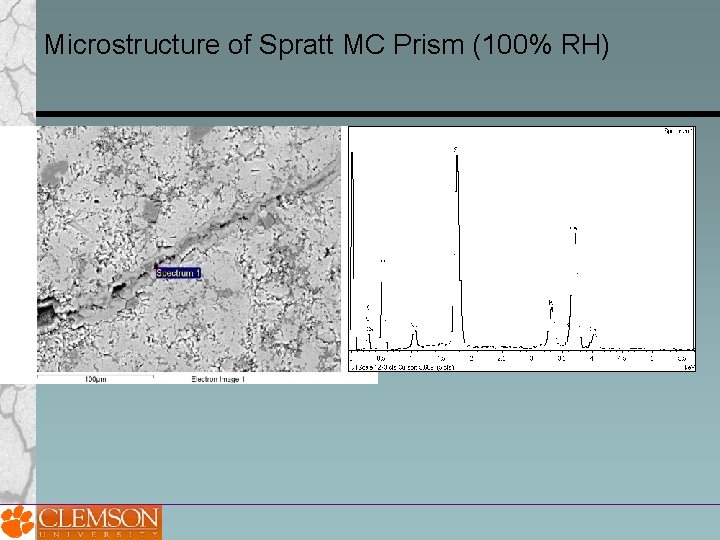
Microstructure of Spratt MC Prism (100% RH)

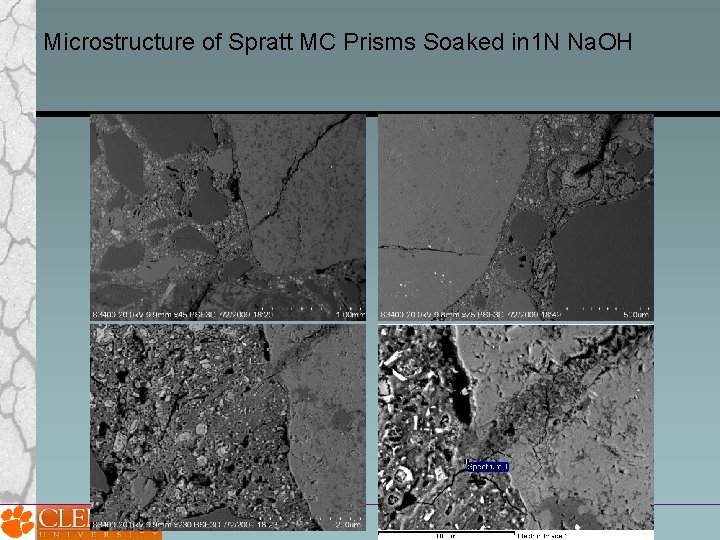
Microstructure of Spratt MC Prisms Soaked in 1 N Na. OH

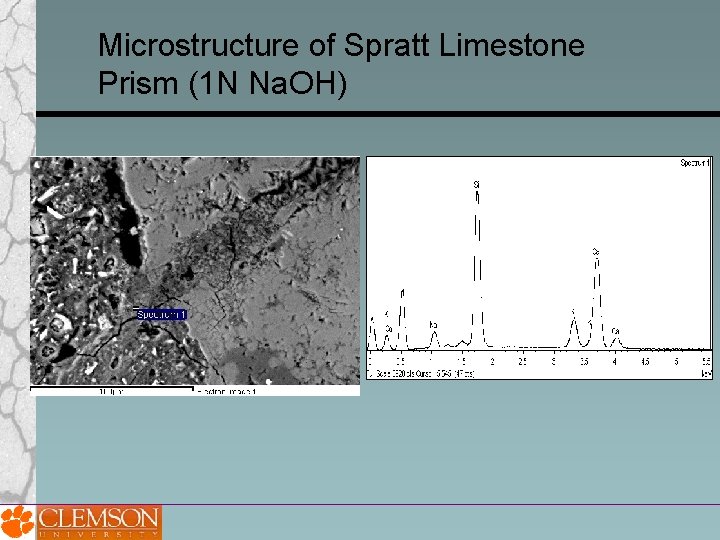
Microstructure of Spratt Limestone Prism (1 N Na. OH)

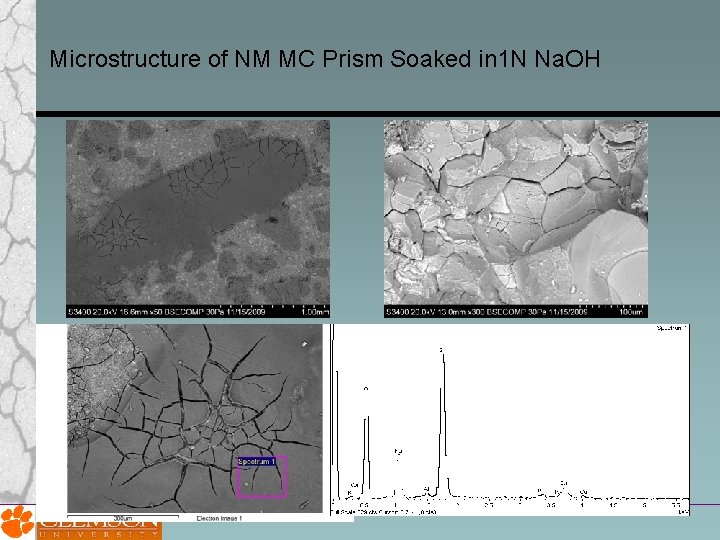
Microstructure of NM MC Prism Soaked in 1 N Na. OH

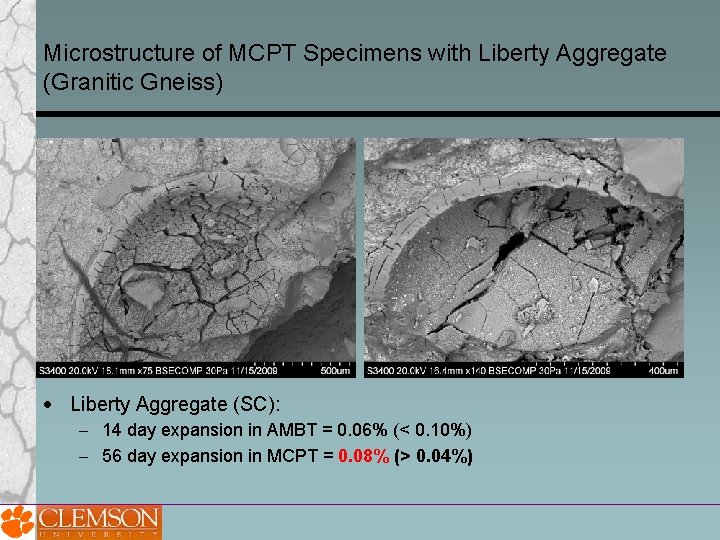
Microstructure of MCPT Specimens with Liberty Aggregate (Granitic Gneiss) Liberty Aggregate (SC): 14 day expansion in AMBT = 0. 06% (< 0. 10%) 56 day expansion in MCPT = 0. 08% (> 0. 04%)

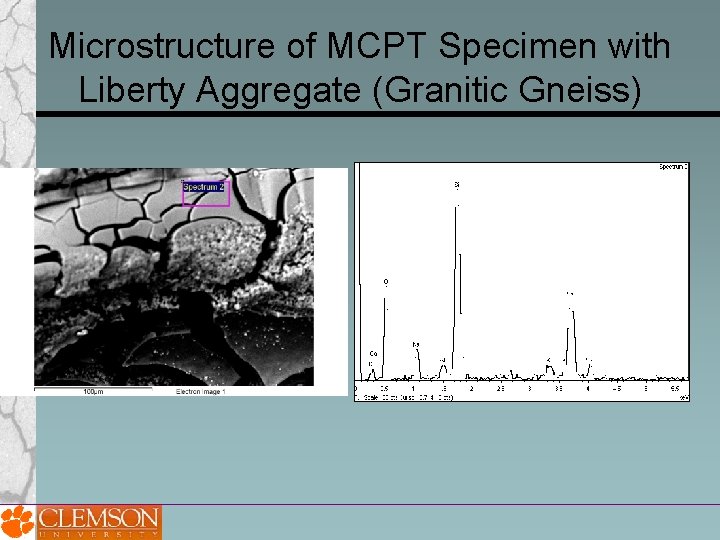
Microstructure of MCPT Specimen with Liberty Aggregate (Granitic Gneiss)

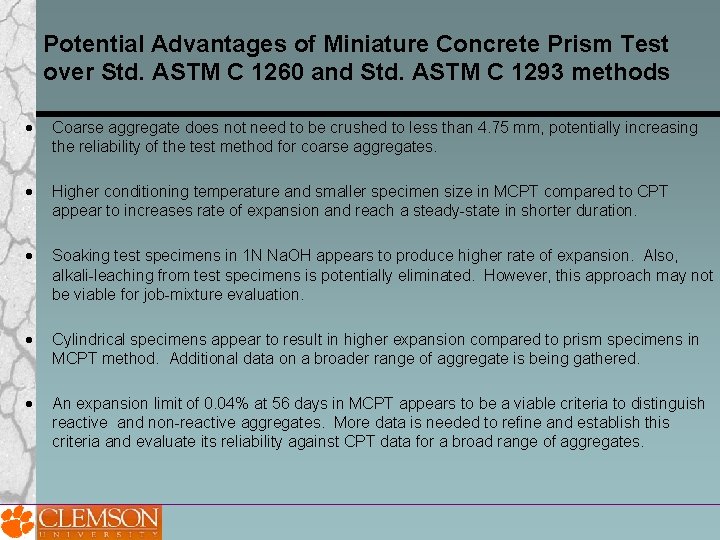
Potential Advantages of Miniature Concrete Prism Test over Std. ASTM C 1260 and Std. ASTM C 1293 methods Coarse aggregate does not need to be crushed to less than 4. 75 mm, potentially increasing the reliability of the test method for coarse aggregates. Higher conditioning temperature and smaller specimen size in MCPT compared to CPT appear to increases rate of expansion and reach a steady-state in shorter duration. Soaking test specimens in 1 N Na. OH appears to produce higher rate of expansion. Also, alkali-leaching from test specimens is potentially eliminated. However, this approach may not be viable for job-mixture evaluation. Cylindrical specimens appear to result in higher expansion compared to prism specimens in MCPT method. Additional data on a broader range of aggregate is being gathered. An expansion limit of 0. 04% at 56 days in MCPT appears to be a viable criteria to distinguish reactive and non-reactive aggregates. More data is needed to refine and establish this criteria and evaluate its reliability against CPT data for a broad range of aggregates.

Questions? elatife@clemson. edu