An idea for classifying EVs and HEVs Kazuyuki

- Slides: 3

An idea for classifying EVs and HEVs Kazuyuki Narusawa

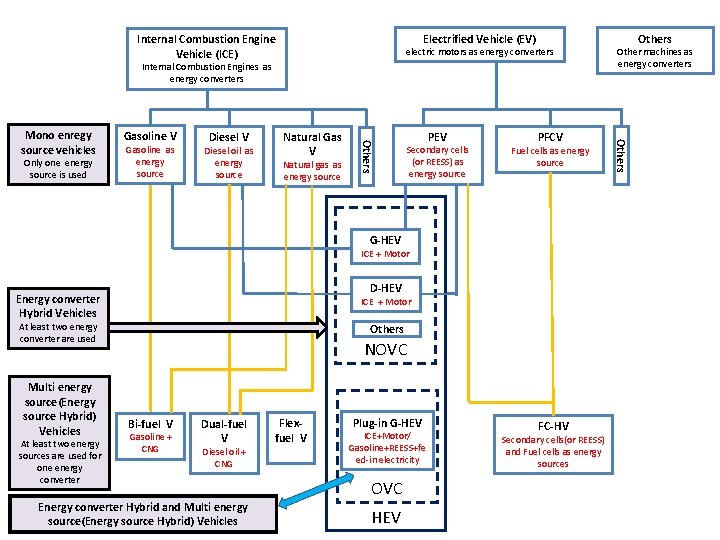
Electrified Vehicle (EV) Internal Combustion Engine Vehicle (ICE) electric motors as energy converters Internal Combustion Engines as energy converters Gasoline as energy source Diesel V Diesel oil as energy source Natural Gas V Natural gas as energy source PEV Secondary cells (or REESS) as energy source PFCV Fuel cells as energy source G-HEV ICE + Motor D-HEV Energy converter Hybrid Vehicles ICE + Motor At least two energy converter are used Multi energy source(Energy source Hybrid) Vehicles At least two energy sources are used for one energy converter Others NOVC Bi-fuel V Gasoline + CNG Dual-fuel V Diesel oil + CNG Energy converter Hybrid and Multi energy source(Energy source Hybrid) Vehicles Flexfuel V Plug-in G-HEV ICE+Motor/ Gasoline+REESS+fe ed-in electricity OVC HEV FC-HV Secondary cells(or REESS) and Fuel cells as energy sources Others Only one energy source is used Gasoline V Others Mono enregy source vehicles Other machines as energy converters

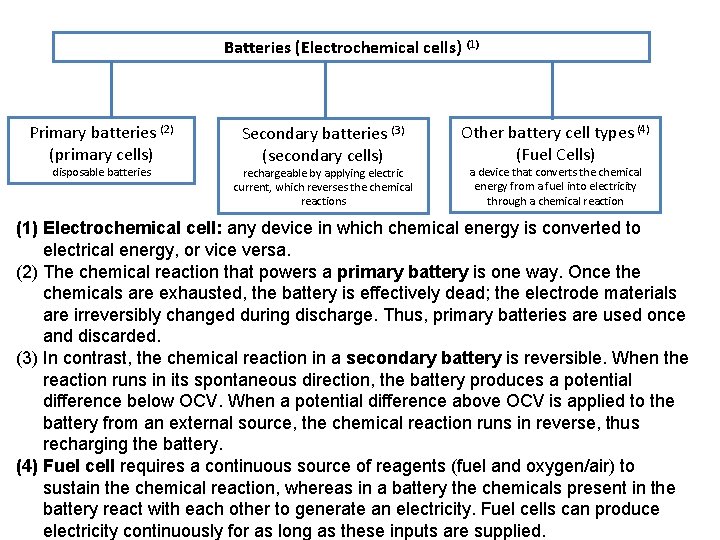
Batteries (Electrochemical cells) (1) Primary batteries (2) (primary cells) disposable batteries Secondary batteries (3) (secondary cells) rechargeable by applying electric current, which reverses the chemical reactions Other battery cell types (4) (Fuel Cells) a device that converts the chemical energy from a fuel into electricity through a chemical reaction (1) Electrochemical cell: any device in which chemical energy is converted to electrical energy, or vice versa. (2) The chemical reaction that powers a primary battery is one way. Once the chemicals are exhausted, the battery is effectively dead; the electrode materials are irreversibly changed during discharge. Thus, primary batteries are used once and discarded. (3) In contrast, the chemical reaction in a secondary battery is reversible. When the reaction runs in its spontaneous direction, the battery produces a potential difference below OCV. When a potential difference above OCV is applied to the battery from an external source, the chemical reaction runs in reverse, thus recharging the battery. (4) Fuel cell requires a continuous source of reagents (fuel and oxygen/air) to sustain the chemical reaction, whereas in a battery the chemicals present in the battery react with each other to generate an electricity. Fuel cells can produce electricity continuously for as long as these inputs are supplied.