An exercise in using the Notre Dame Kinetics




- Slides: 29

An exercise in using the Notre Dame Kinetics Database in fitting transient absorption data Dave Bartels Tim Marin Irek Janik $$$ DOE – BES Chemical Sciences, NE/NERI

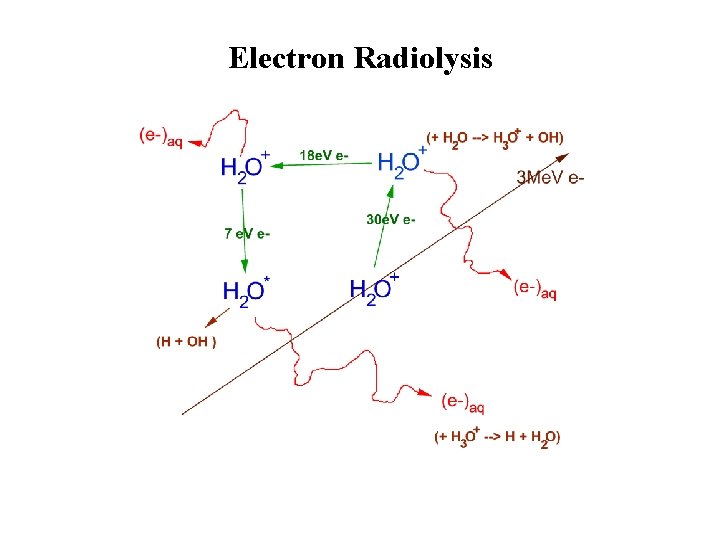
Electron Radiolysis

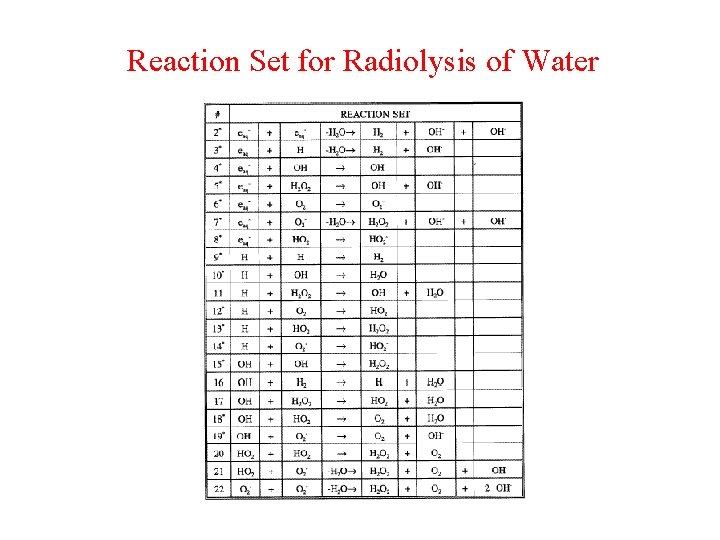
Reaction Set for Radiolysis of Water

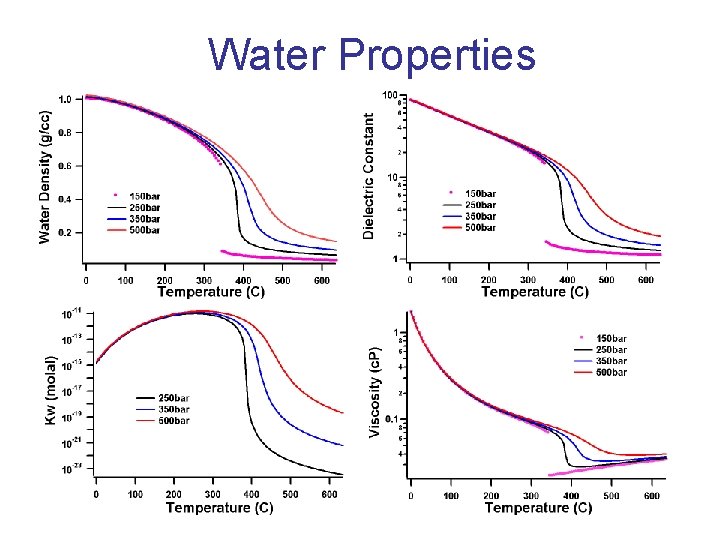
Water Properties

Apparatus for Supercritical Water Radiolysis Studies

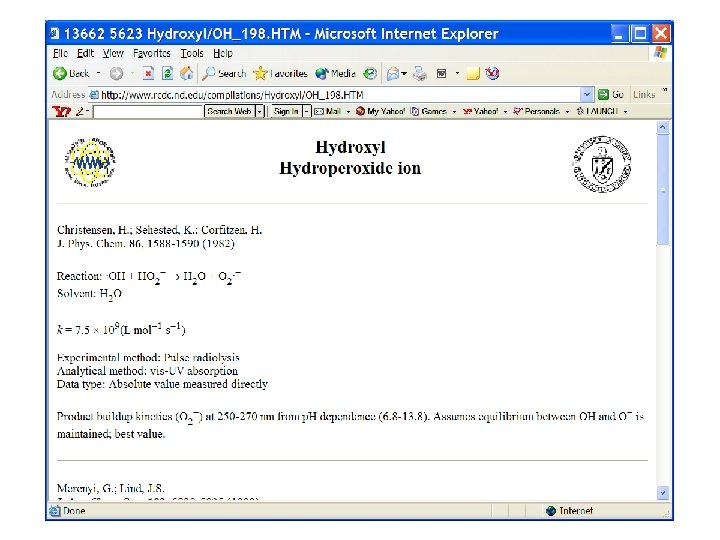
Reaction of OH Radical with H 2 Why should this “simple” reaction slow down at higher temperature?


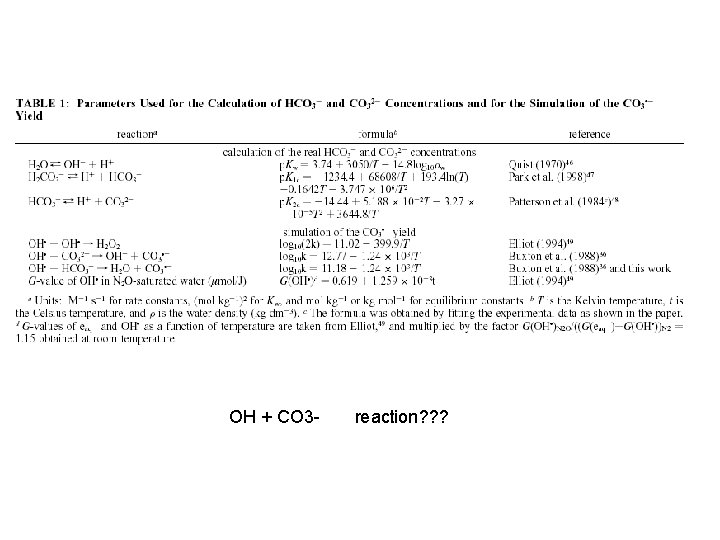
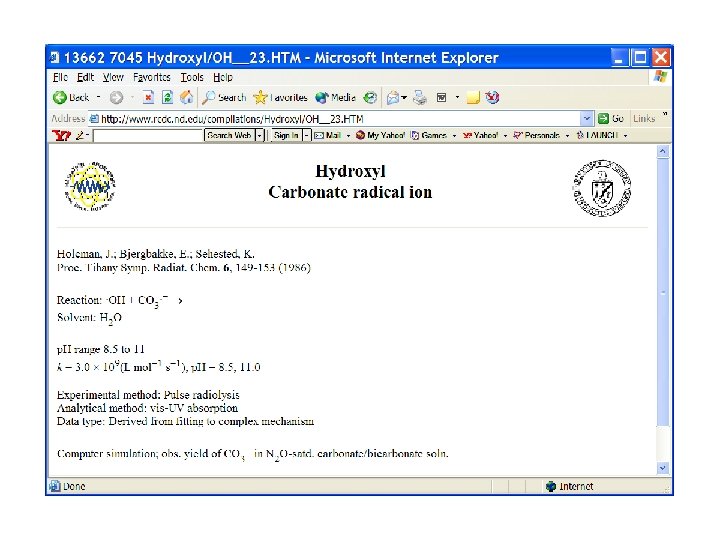
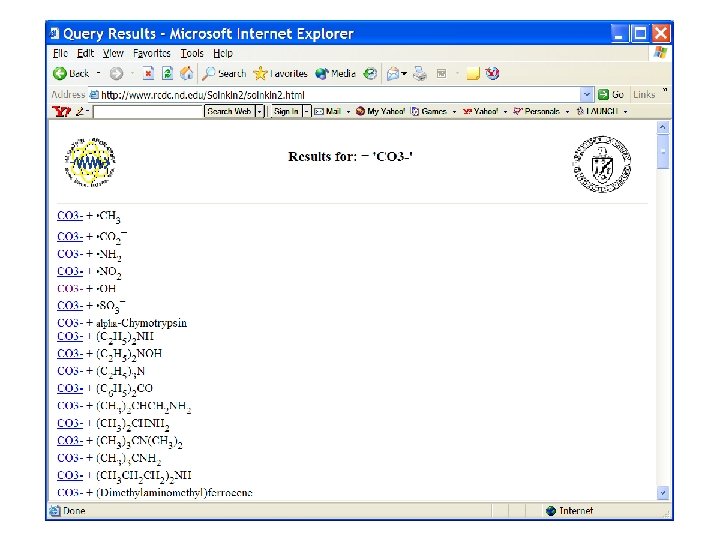
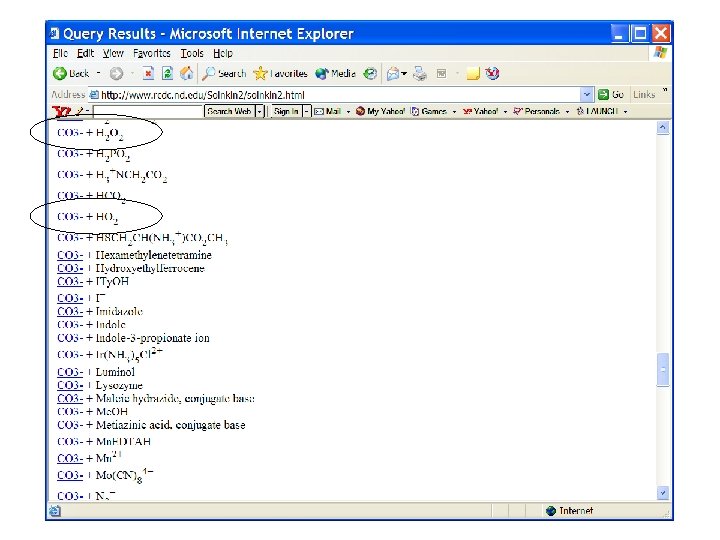
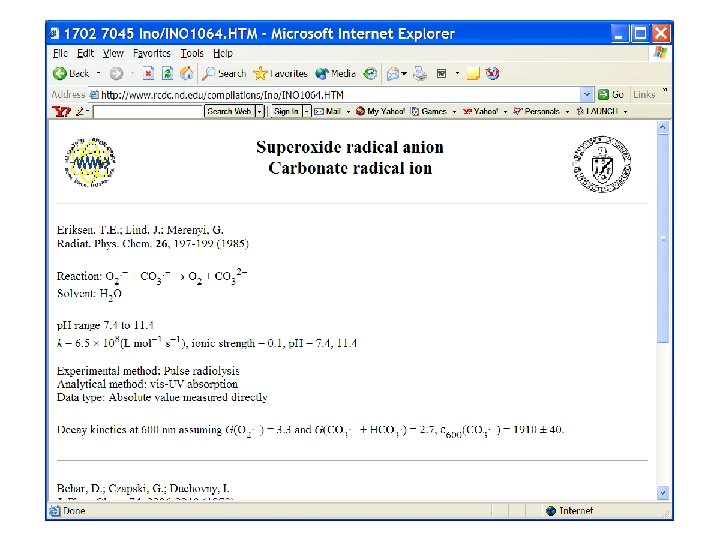
OH + CO 3 - reaction? ? ?

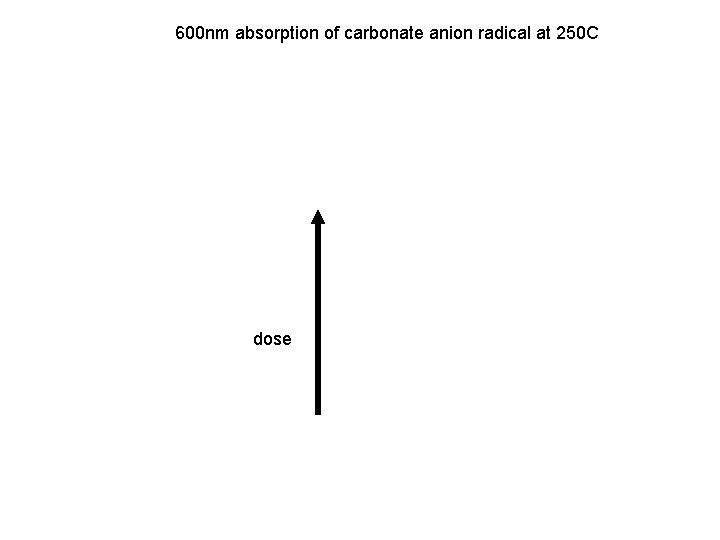
600 nm absorption of carbonate anion radical at 250 C dose

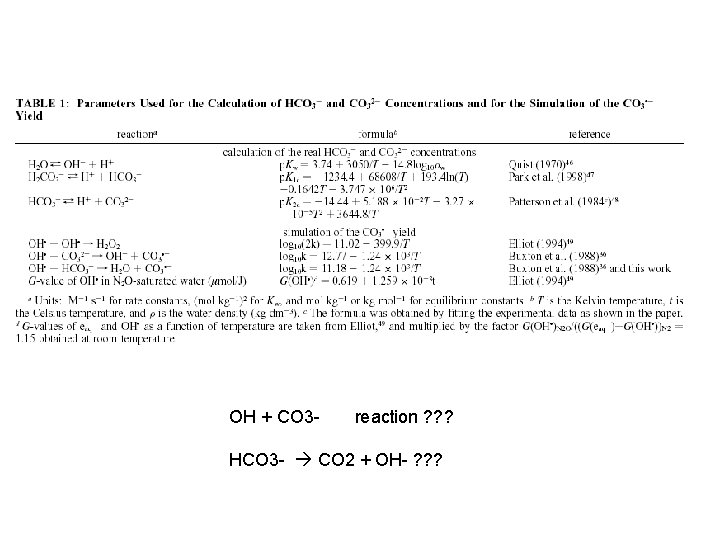
OH + CO 3 - reaction ? ? ? HCO 3 - CO 2 + OH- ? ? ?

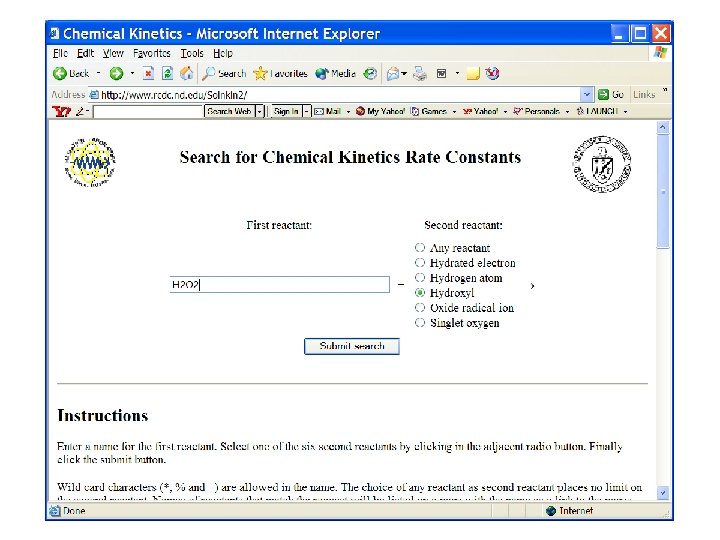
Search on carbonate radical reactions



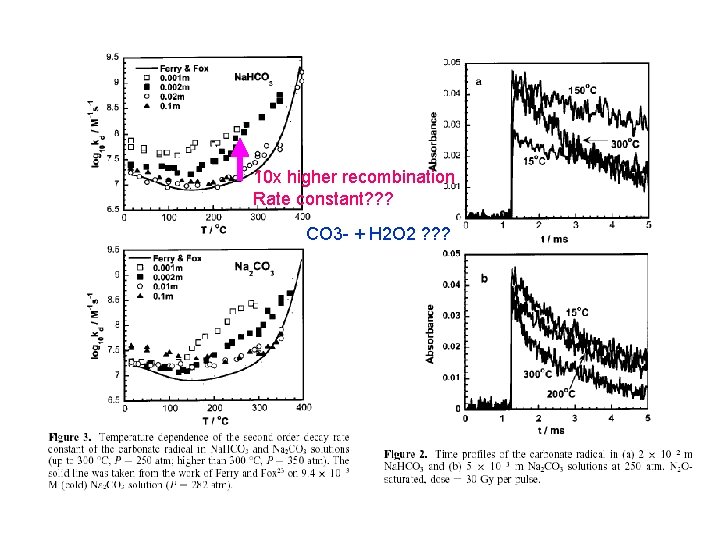
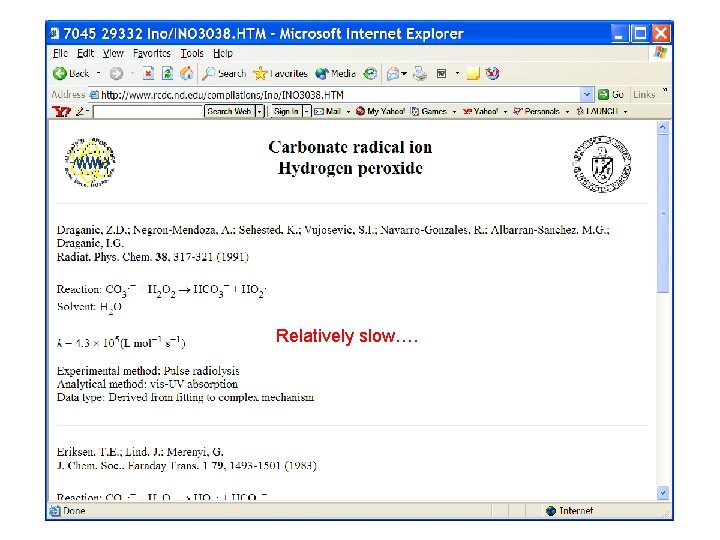
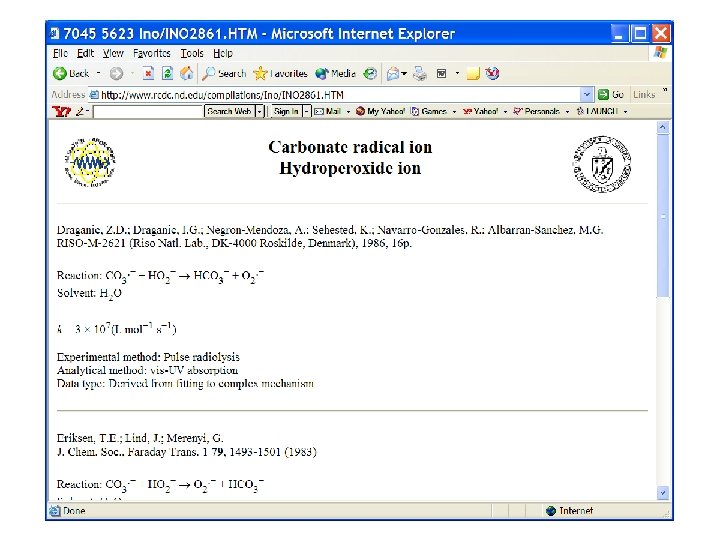
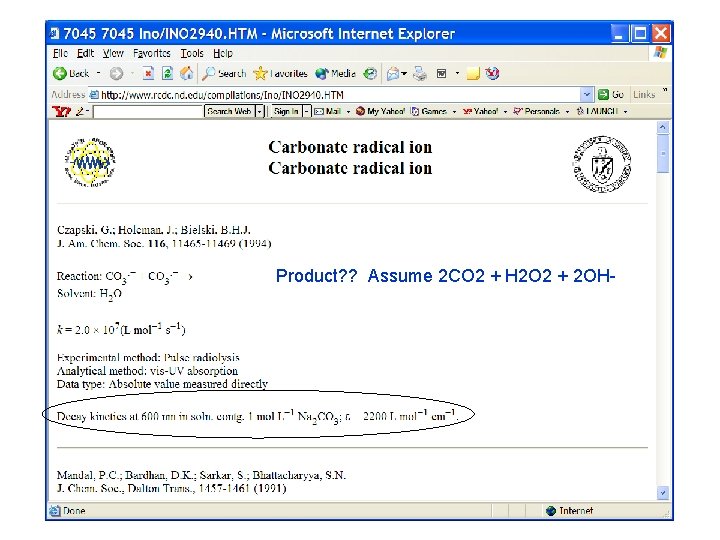
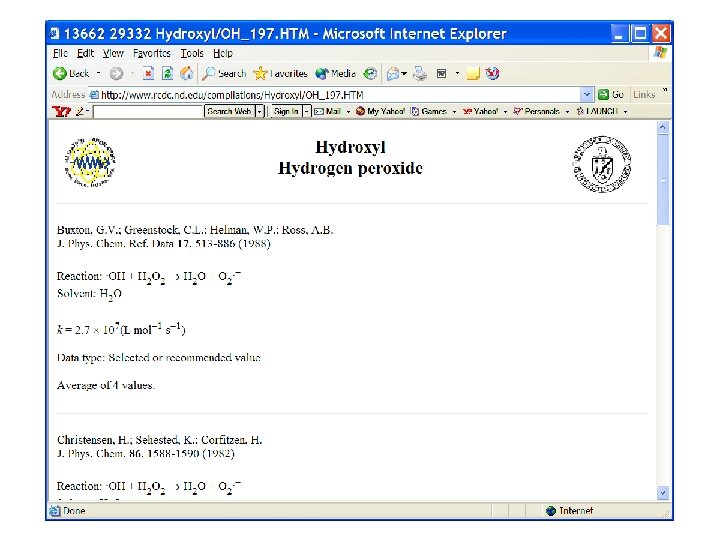
10 x higher recombination Rate constant? ? ? CO 3 - + H 2 O 2 ? ? ?




Relatively slow….



Product? ? Assume 2 CO 2 + H 2 O 2 + 2 OH-

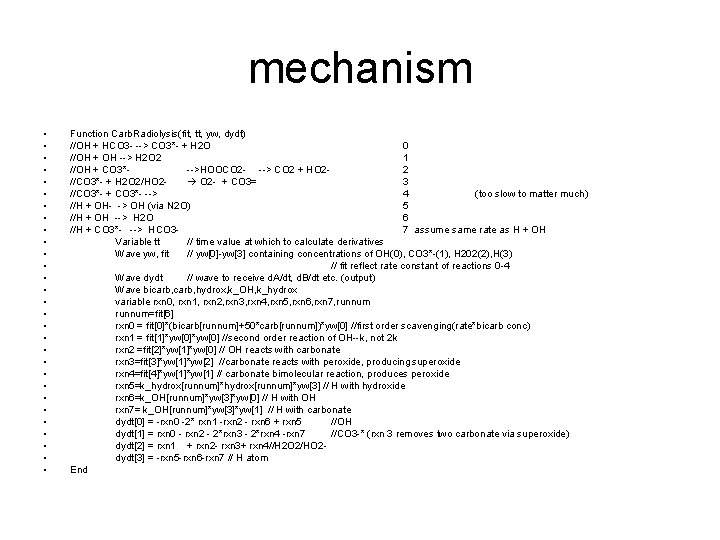
mechanism • • • • • • • • Function Carb. Radiolysis(fit, tt, yw, dydt) //OH + HCO 3 - --> CO 3*- + H 2 O 0 //OH + OH --> H 2 O 2 1 //OH + CO 3*-->HOOCO 2 - --> CO 2 + HO 22 //CO 3*- + H 2 O 2/HO 2 O 2 - + CO 3= 3 //CO 3*- + CO 3*- --> 4 (too slow to matter much) //H + OH- -> OH (via N 2 O) 5 //H + OH --> H 2 O 6 //H + CO 3*- --> HCO 37 assume same rate as H + OH Variable tt // time value at which to calculate derivatives Wave yw, fit // yw[0]-yw[3] containing concentrations of OH(0), CO 3*-(1), H 202(2), H(3) // fit reflect rate constant of reactions 0 -4 Wave dydt // wave to receive d. A/dt, d. B/dt etc. (output) Wave bicarb, hydrox, k_OH, k_hydrox variable rxn 0, rxn 1, rxn 2, rxn 3, rxn 4, rxn 5, rxn 6, rxn 7, runnum=fit[6] rxn 0 = fit[0]*(bicarb[runnum]+50*carb[runnum])*yw[0] //first order scavenging(rate*bicarb conc) rxn 1 = fit[1]*yw[0] //second order reaction of OH--k, not 2 k rxn 2 =fit[2]*yw[1]*yw[0] // OH reacts with carbonate rxn 3=fit[3]*yw[1]*yw[2] //carbonate reacts with peroxide, producing superoxide rxn 4=fit[4]*yw[1] // carbonate bimolecular reaction, produces peroxide rxn 5=k_hydrox[runnum]*yw[3] // H with hydroxide rxn 6=k_OH[runnum]*yw[3]*yw[0] // H with OH rxn 7= k_OH[runnum]*yw[3]*yw[1] // H with carbonate dydt[0] = -rxn 0 -2* rxn 1 -rxn 2 - rxn 6 + rxn 5 //OH dydt[1] = rxn 0 - rxn 2 - 2*rxn 3 - 2*rxn 4 -rxn 7 //CO 3 -* (rxn 3 removes two carbonate via superoxide) dydt[2] = rxn 1 + rxn 2 - rxn 3+ rxn 4//H 2 O 2/HO 2 dydt[3] = -rxn 5 -rxn 6 -rxn 7 // H atom End

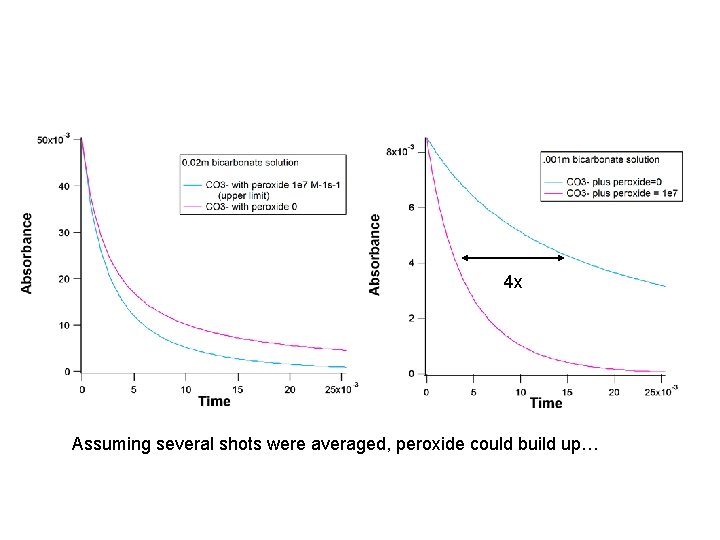
4 x Assuming several shots were averaged, peroxide could build up…

…Yes

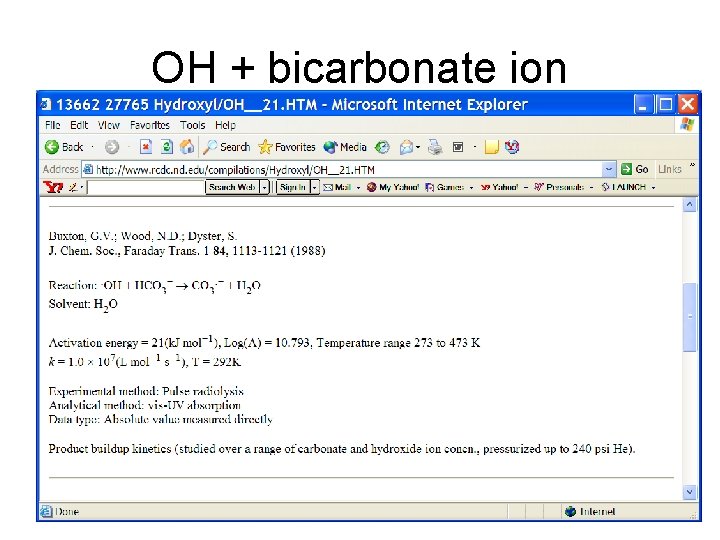
OH + bicarbonate ion

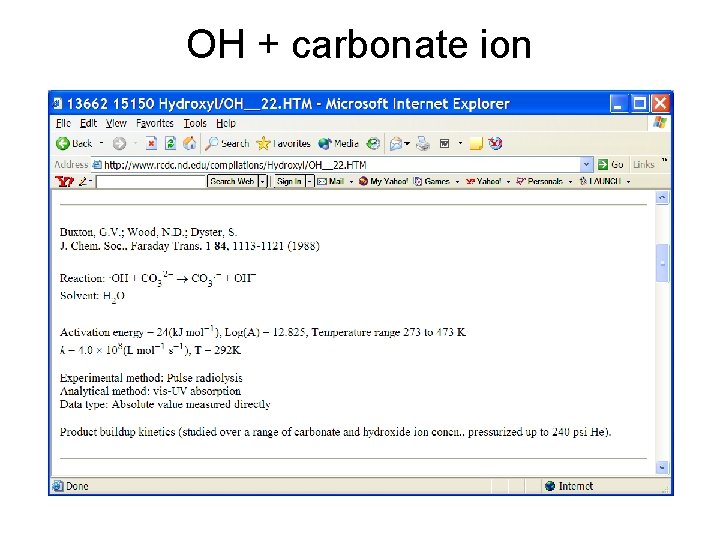
OH + carbonate ion


