An Alternative Semiconductor Definition What is a Semiconductor






- Slides: 29

An Alternative Semiconductor Definition!

What is a Semiconductor? B - Ch 1, Y - Ch 1, S - Ch 1 Conductivity/Resistivity Definition (σ = conductivity, ρ = resistivity) Metals: Good Conductors! 103 ≤ σ ≤ 108 (Ω-cm)-1; 10 -8 ≤ ρ ≤ 10 -3 Ω-cm Semiconductors & Semimetals: 10 -8 ≤ σ ≤ 103 (Ω-cm)-1; 10 -3 ≤ ρ ≤ 108 Ω-cm NOTE THE HUGE RANGE!! Insulators: σ ≤ 10 -8 (Ω-cm)-1; ρ ≥ 108 Ω-cm Actually, there are no rigid boundaries!

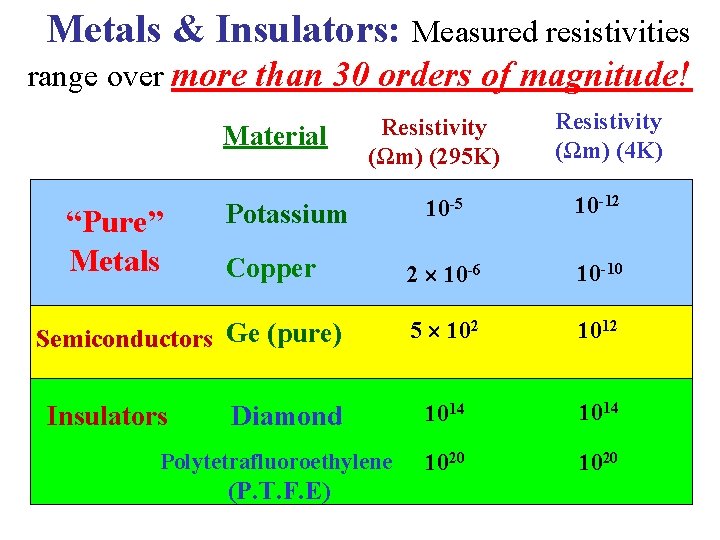
Metals & Insulators: Measured resistivities range over more than 30 orders of magnitude! Material Resistivity (Ωm) (295 K) Resistivity (Ωm) (4 K) 10 -5 10 -12 “Pure” Metals Potassium Copper 2 10 -6 10 -10 Semiconductors Ge (pure) 5 102 1012 Diamond 1014 1020 10 20 Insulators Polytetrafluoroethylene (P. T. F. E)

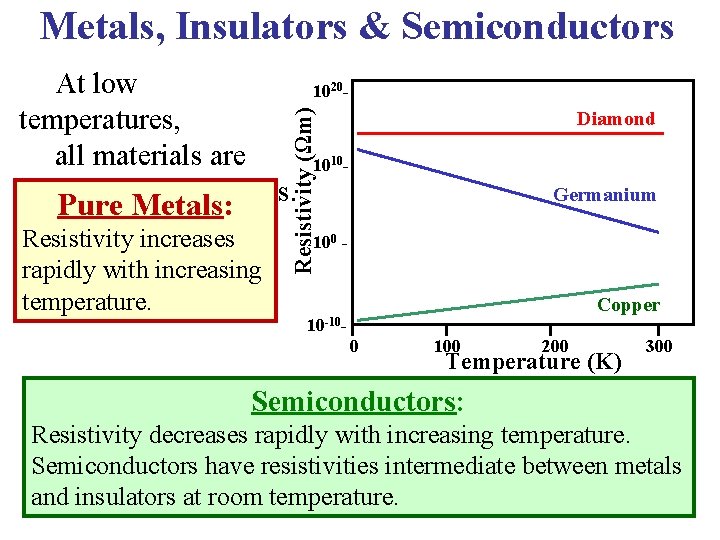
Metals, Insulators & Semiconductors Resistivity increases rapidly with increasing temperature. 1020 - Resistivity (Ωm) At low temperatures, all materials are insulators or metals. Pure Metals: Diamond 1010 - Germanium 100 - Copper 10 -100 200 Temperature (K) 300 Semiconductors: Resistivity decreases rapidly with increasing temperature. Semiconductors have resistivities intermediate between metals and insulators at room temperature.

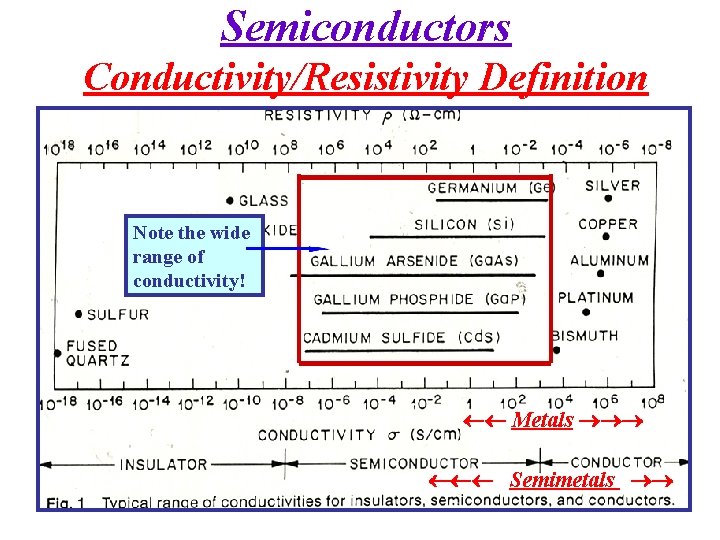
Semiconductors Conductivity/Resistivity Definition Metals Semimetals

Semiconductors Conductivity/Resistivity Definition Note the wide range of conductivity! Metals Semimetals

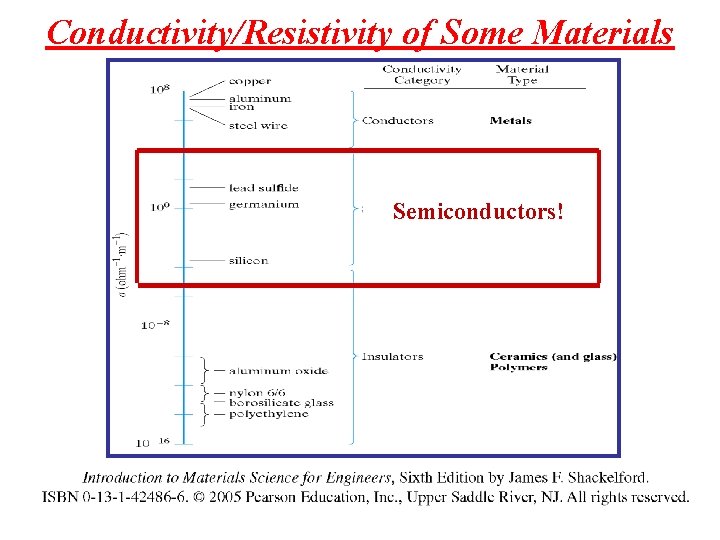
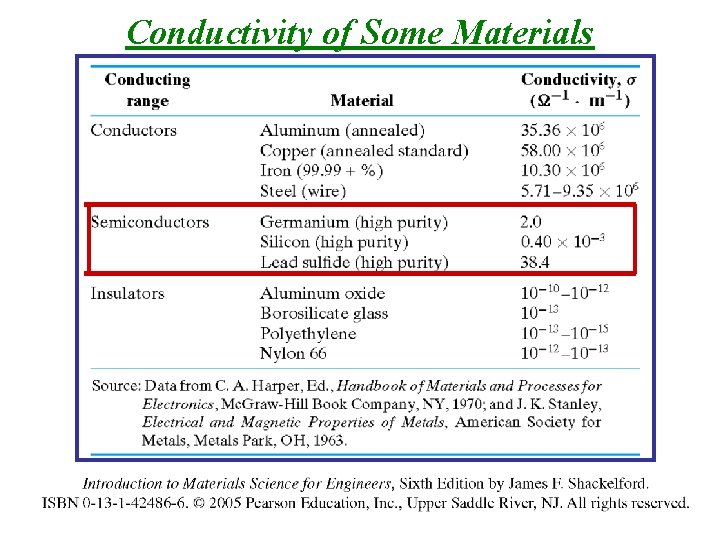
Conductivity/Resistivity of Some Materials Semiconductors!

Conductivity of Some Materials

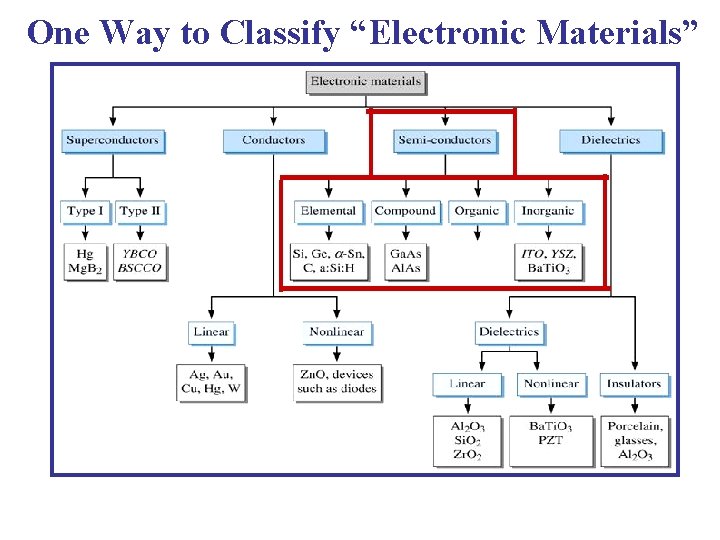
One Way to Classify “Electronic Materials”

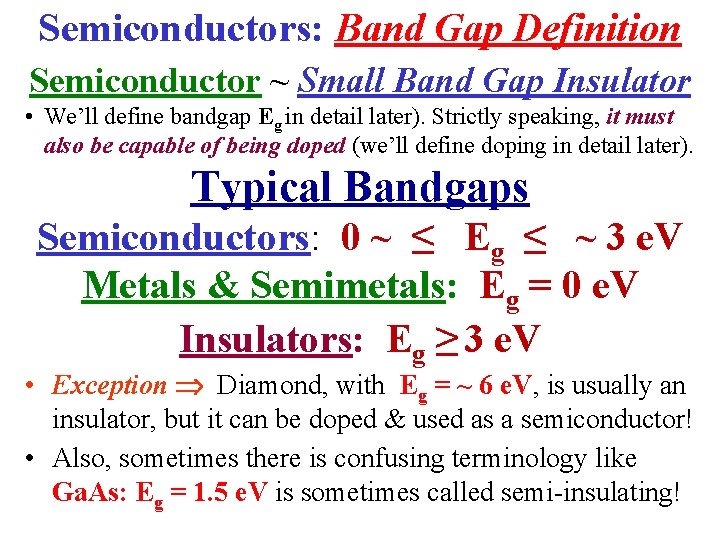
Semiconductors: Band Gap Definition Semiconductor ~ Small Band Gap Insulator • We’ll define bandgap Eg in detail later). Strictly speaking, it must also be capable of being doped (we’ll define doping in detail later). Typical Bandgaps Semiconductors: 0 ~ ≤ Eg ≤ ~ 3 e. V Metals & Semimetals: Eg = 0 e. V Insulators: Eg ≥ 3 e. V • Exception Diamond, with Eg = ~ 6 e. V, is usually an insulator, but it can be doped & used as a semiconductor! • Also, sometimes there is confusing terminology like Ga. As: Eg = 1. 5 e. V is sometimes called semi-insulating!

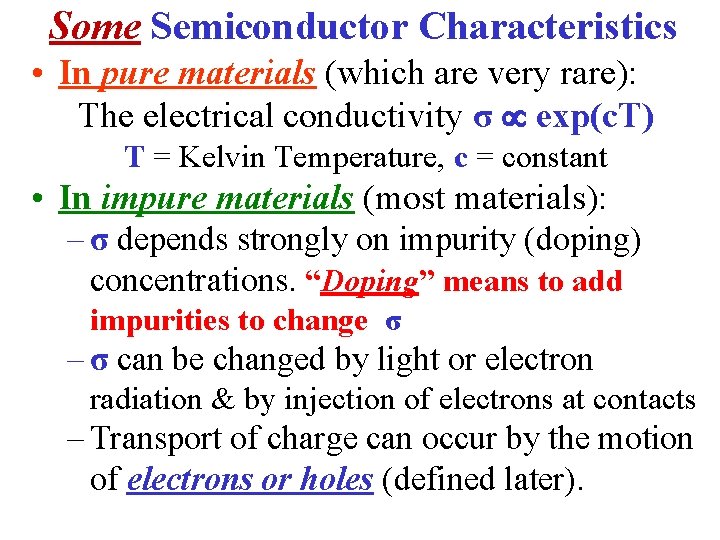
Some Semiconductor Characteristics • In pure materials (which are very rare): The electrical conductivity σ exp(c. T) T = Kelvin Temperature, c = constant • In impure materials (most materials): – σ depends strongly on impurity (doping) concentrations. “Doping” means to add impurities to change σ – σ can be changed by light or electron radiation & by injection of electrons at contacts – Transport of charge can occur by the motion of electrons or holes (defined later).

The Best Known Semiconductor is Silicon (Si) • But, there are HUNDREDS (THOUSANDS!) • • • of others! Elemental: Si, Ge, C (diamond) Binary Compounds: Ga. As, In. P, . Organic Compounds: (CH)n (polyacetyline) Magnetic Semiconductors: Cdx. Mn 1 -x. Te, … Ferroelectric Semiconductors: Sb. I, … Superconducting Compounds (!!) Ge. Te, Sr. Ti. O 3, . . ( “High Tc materials!” )

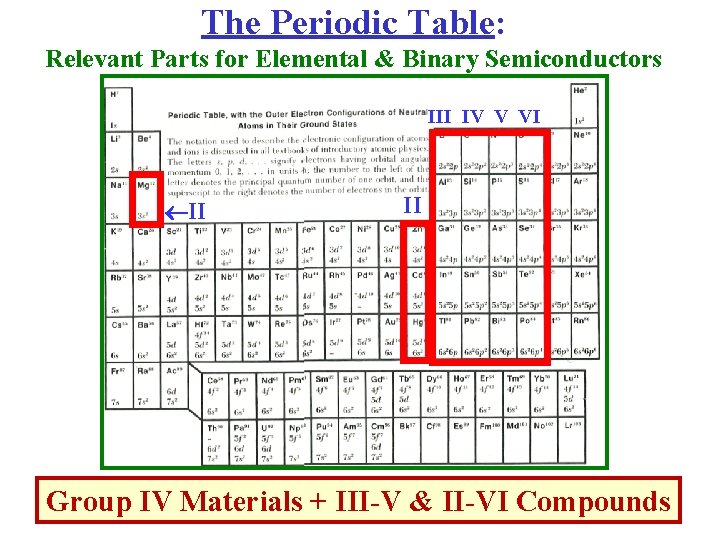
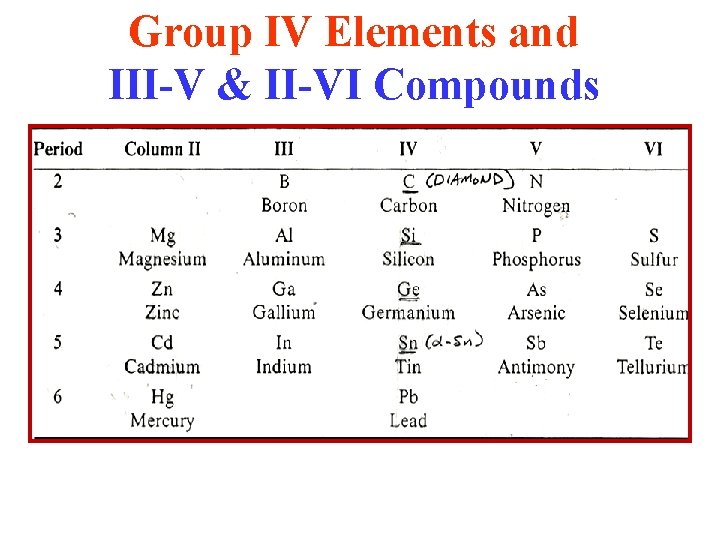
The Periodic Table: Relevant Parts for Elemental & Binary Semiconductors III IV V VI II II Group IV Materials + III-V & II-VI Compounds

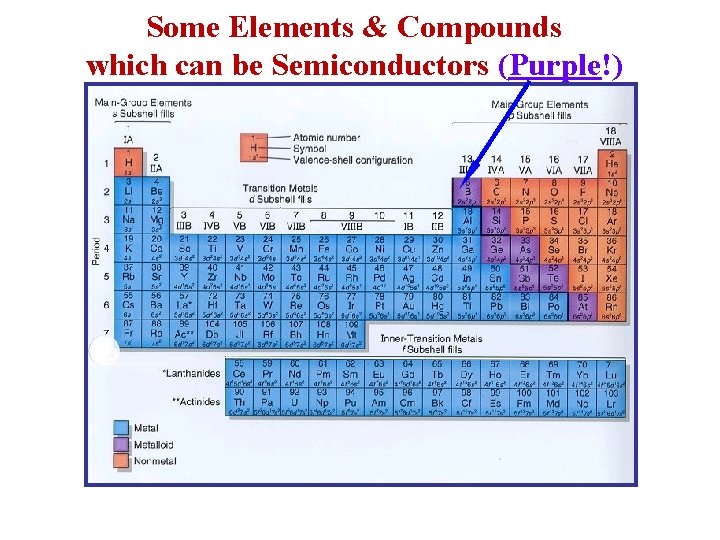
Some Elements & Compounds which can be Semiconductors (Purple!)

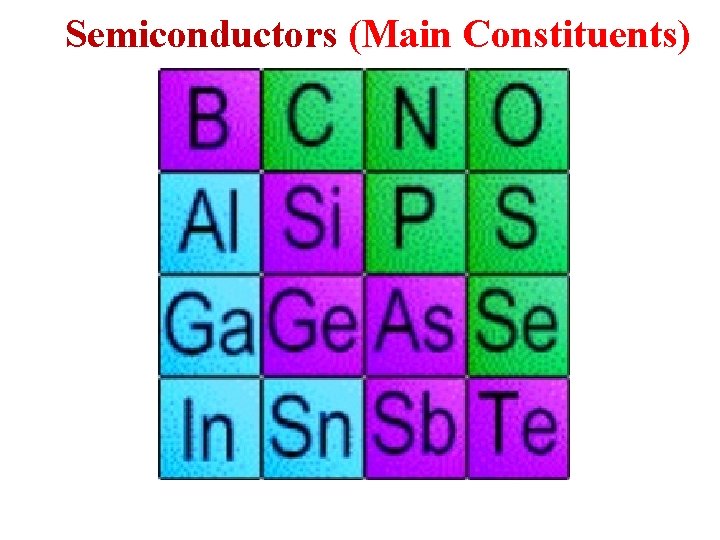
Semiconductors (Main Constituents)

The Periodic Table Cloth!

Group IV Elements and III-V & II-VI Compounds

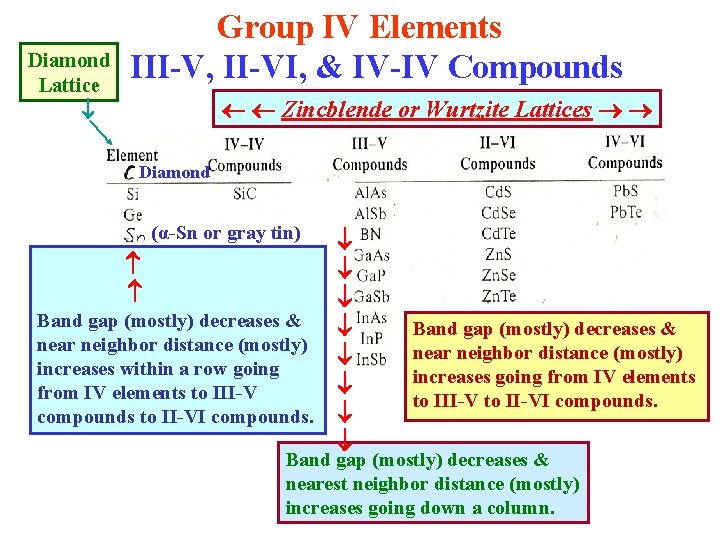
Group IV Elements Diamond III-V, II-VI, & IV-IV Compounds Lattice Zincblende or Wurtzite Lattices Diamond (α-Sn or gray tin) Band gap (mostly) decreases & near neighbor distance (mostly) increases within a row going from IV elements to III-V compounds to II-VI compounds. Band gap (mostly) decreases & near neighbor distance (mostly) increases going from IV elements to III-V to II-VI compounds. Band gap (mostly) decreases & nearest neighbor distance (mostly) increases going down a column.

Many Materials of Interest in This Course: Have Crystal Lattice Structures Diamond or Zincblende (These will be discussed in detail again later!) • In these structures, each atom is tetrahedrally coordinated with four (4) nearest-neighbors. • The bonding between neighbors is (mostly) sp 3 hybrid bonding (strongly covalent). There are 2 atoms/unit cell (repeated to form an infinite solid).

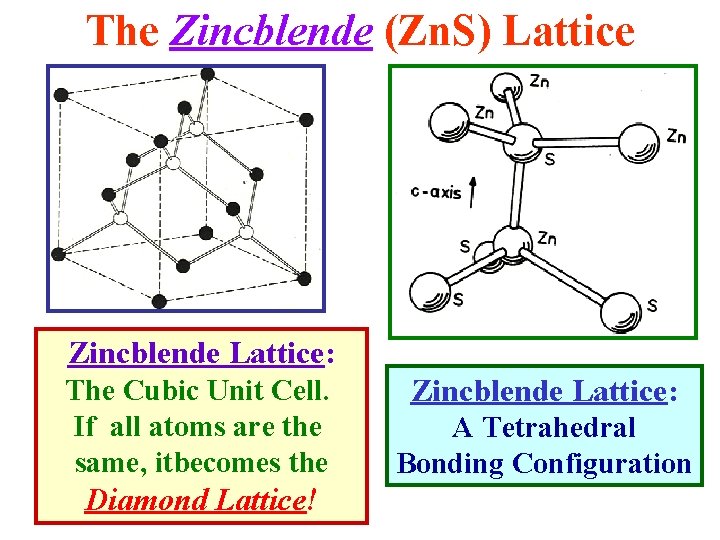
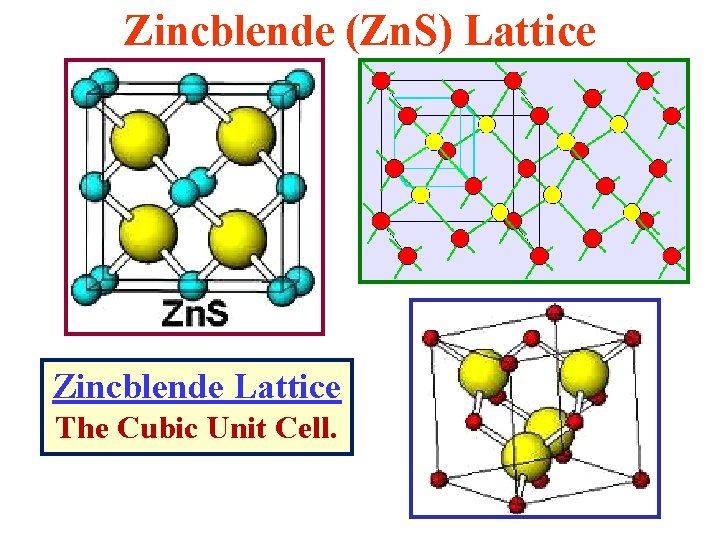
The Zincblende (Zn. S) Lattice Zincblende Lattice: The Cubic Unit Cell. If all atoms are the same, itbecomes the Diamond Lattice! Zincblende Lattice: A Tetrahedral Bonding Configuration

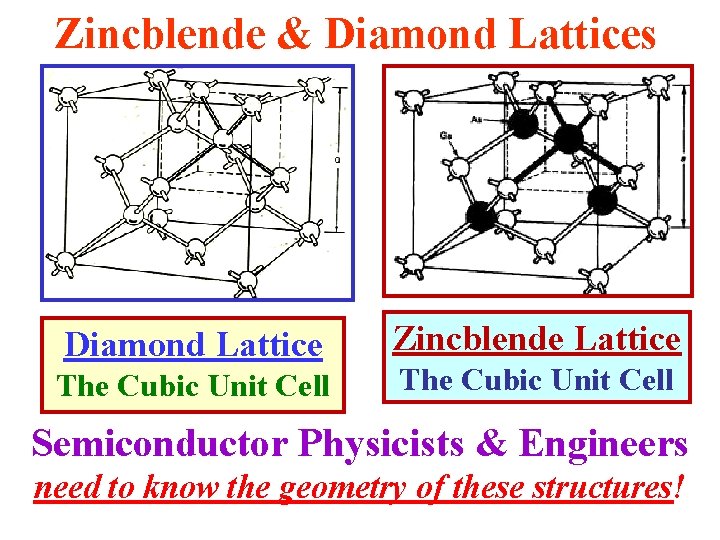
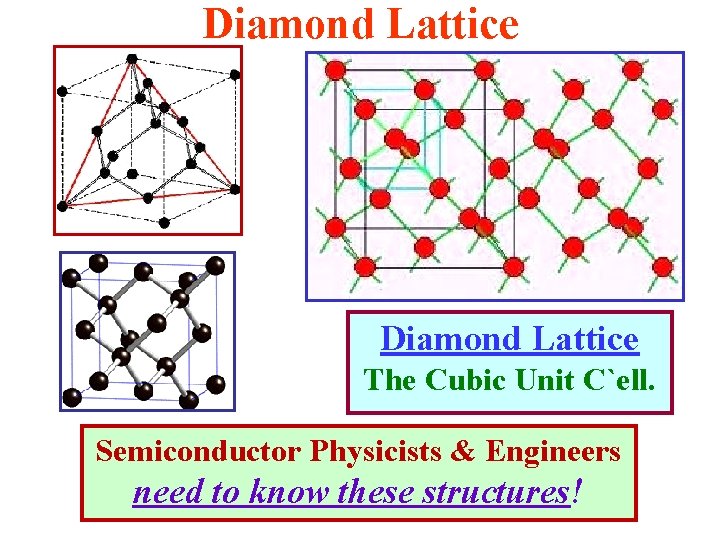
Zincblende & Diamond Lattices Diamond Lattice The Cubic Unit Cell Zincblende Lattice The Cubic Unit Cell Semiconductor Physicists & Engineers need to know the geometry of these structures!

Diamond Lattice The Cubic Unit C`ell. Semiconductor Physicists & Engineers need to know these structures!

Zincblende (Zn. S) Lattice Zincblende Lattice The Cubic Unit Cell.

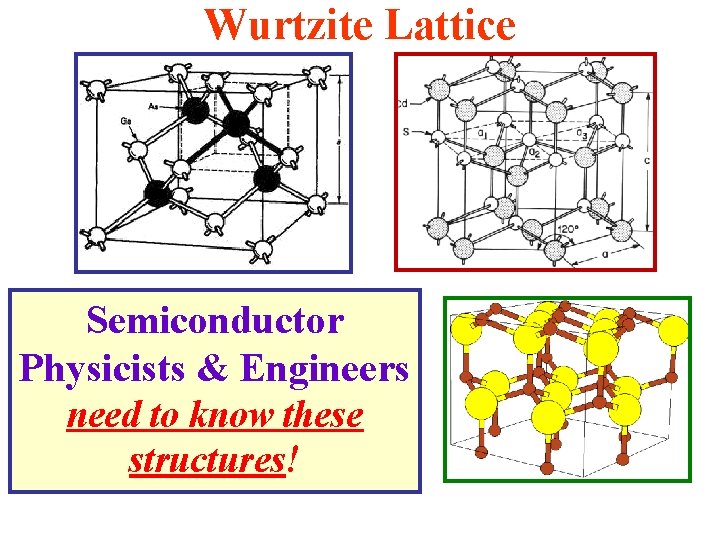
Some Materials of Interest in This Course Have Crystal Lattice Structures Wurtzite Structure (This will be discussed in detail again later!) • This is similar to the Zincblende structure, but it has hexagonal symmetry instead of cubic. • In these structures, each atom is tetrahedrally coordinated with four (4) nearest-neighbors. • The bonding between neighbors is (mostly) sp 3 hybrid bonding (strongly covalent). There are 2 atoms/unit cell (repeated to form an infinite solid).

Wurtzite Lattice Semiconductor Physicists & Engineers need to know these structures!

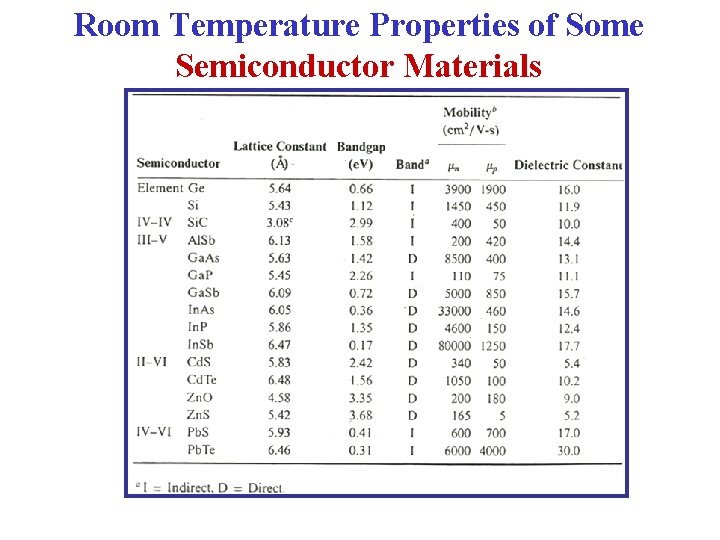
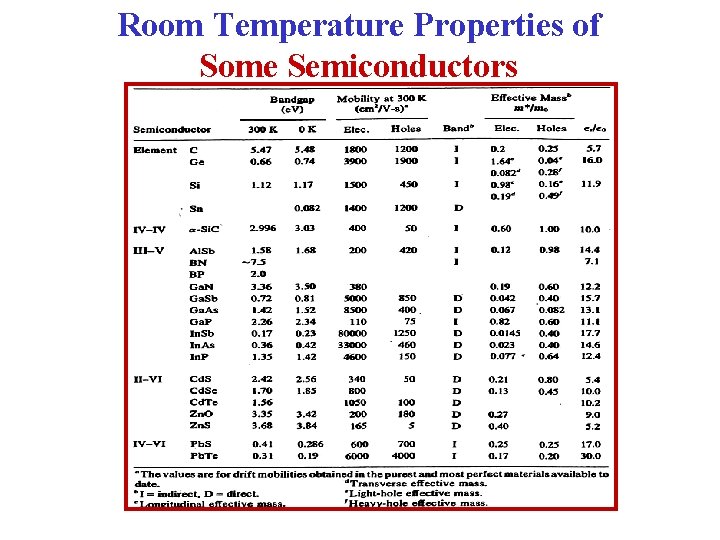
Room Temperature Properties of Some Semiconductor Materials

Room Temperature Properties of Some Semiconductors

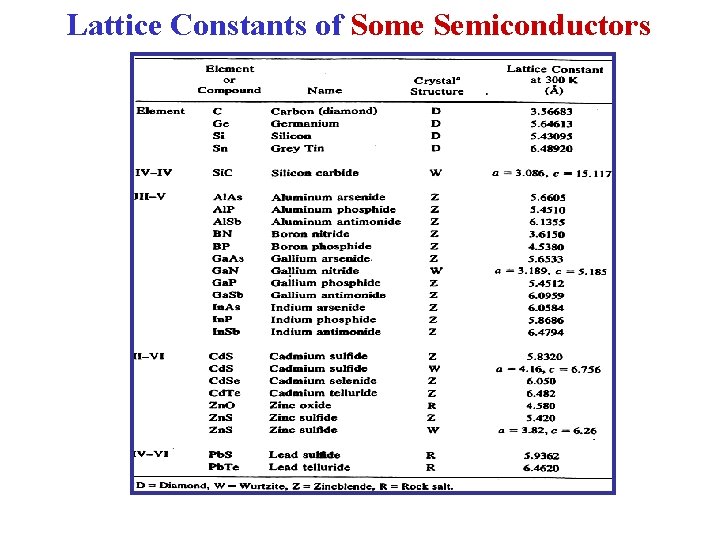
Lattice Constants of Some Semiconductors

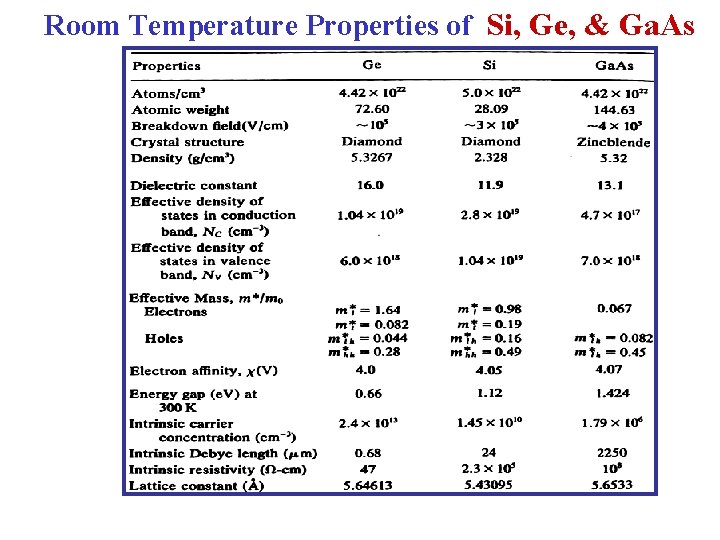
Room Temperature Properties of Si, Ge, & Ga. As