AMS Weather Studies Introduction to Atmospheric Science 4


























- Slides: 35

AMS Weather Studies Introduction to Atmospheric Science, 4 th Edition Chapter 2 Atmosphere: Origin, Composition, and Structure © AMS 1

Case-in-Point § Weather and climatic issues in one part of the world can affect those in another part § North African dust storms can affect the weather and air quality of the southeastern U. S. § Dust can harbor microscopic disease-causing organisms § This dust may be harming coral reefs in the Caribbean § This dust may increase the frequency of red tides © AMS 2

Driving Question § What is the composition and structure of the atmosphere? – This chapter covers: § Evolution of the atmosphere § How meteorologists monitor the atmosphere – At the surface – Upper-air § The temperature profile of the atmosphere § The thermal subdivisions of the atmosphere § Electromagnetic characteristics of the upper atmosphere © AMS 3

Atmosphere, Weather, and Climate § Atmosphere – – – Gases and suspended particles ½ of the mass is found in the lower 5500 m (18, 000 ft) 99% of the mass is below 32 km (20 miles) § Weather – The state of the atmosphere at a given time and place – Variables – temperature, humidity, cloudiness, precipitation, wind speed, wind direction § Climate – Weather conditions at a location over a specified time period plus weather extremes at that locale – Computed over a 30 year period, beginning with the 1 st year of a decade § Climatology is the study of climate, climate controls, variability (change) over space & time © AMS 4

Evolution of the Atmosphere § Primeval phase – Gases surrounding Earth were primarily helium plus hydrogen compounds § Methane and ammonia § Eventually, these escaped to space – 4. 4 billion years ago, there was enough gravity to retain an atmosphere – Rocks outgassed as they solidified and cooled § Primarily carbon dioxide, nitrogen, and water vapor § Trace amounts – methane, ammonia, sulfur dioxide – Water vapor was broken into hydrogen and oxygen by UV radiation © AMS 5

Evolution of the Atmosphere § Primeval phase, continued – 4. 5 – 2. 5 billion years ago, sun was much fainter § Earth was not cooler due to CO 2 (greenhouse gas) – CO 2 combined with rainwater to form carbonic acid § This reacted with rocks, locked carbon in the rocks, so there was less in the atmosphere § Living organisms took CO 2 out of the atmosphere via photosynthesis, locking carbon into carbohydrates – Oxygen became the 2 nd most abundant gas in the atmosphere § Nitrogen is 1 st. It is an out-gassing product that is relatively inert – CO 2 has been a minor component of the atmosphere for the last 2. 5 billion years § Fluctuations play important roles in climate change © AMS 6

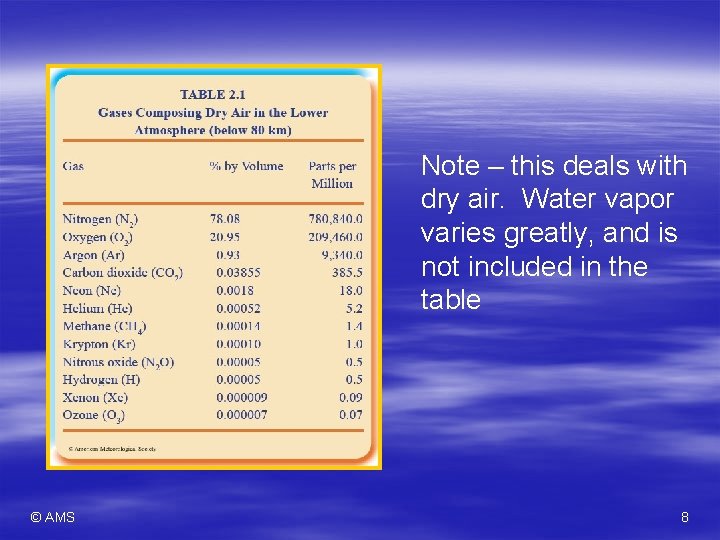
Evolution of the Atmosphere § Modern phase – Lower atmosphere (to 80 km or 50 miles) circulates and maintains uniform ratios of gasses § Homosphere – Above this, gases separate based on weight § Results in stratified layers § Heterosphere – Nitrogen ≈ 78%, Oxygen ≈ 21% of the homosphere § Argon < 1% § CO 2 <. 04% © AMS 7

Note – this deals with dry air. Water vapor varies greatly, and is not included in the table © AMS 8

Evolution of the Atmosphere § § § Modern phase, continued O 2 in the homosphere O in the heterosphere – 150 km (95 miles) above Earth’s surface – UV radiation splits O 2 § Other planets’ atmospheres are much different § May have started the same § Earth’s atmosphere also has aerosols – Liquid and solid particles – Sources § Wind erosion of soil § Volcanic eruptions © AMS ■ Forest fires ■ Ocean spray ■ Agricultural & industrial activities 9

Evolution of the Atmosphere § Modern phase, continued – Water vapor § By volume, < 4% of the lowest 1 km of the atmosphere § Necessary for clouds and precipitation – CO 2 – even though <. 04%: § Required for essential function to all life – photosynthesis – Both CO 2 and water vapor absorb and emit infrared radiation § Keeps the lower atmosphere warm § Allows for life to exist © AMS 10

Air Pollution § An air pollutant is an aerosol or gas that occurs at a concentration that threatens the well-being of living organisms – Most are human-made, some are natural § Dust storms, volcanoes, pollen, decay of plants/animals § Primary air pollutants – Harmful immediately as emitted § Secondary air pollutants – Harmful after combination with one or more substances – Photochemical smog © AMS 11

Air Pollution § The Environmental Protection Agency (EPA) – Standards for 6 air pollutants § carbon monoxide § nitrogen oxides lead ■ particulates ■ ozone ■ sulfur dioxide ■ – Primary air quality standards § Maximum exposure levels humans can tolerate without ill effects – Secondary air quality standards § Maximum exposure levels allowable to minimize the impact on crops, visibility, personal comfort, and climate – Compliance with standards © AMS § Attainment areas – geographic regions where standards are met or below § Non-attainment areas – geographic regions where the primary standard is not met 12

Investigating the Atmosphere § Scientists (including meteorologists) use the scientific method – Identify questions related to the problem – Propose an answer to one of the questions § This is an educated guess – State the educated guess in a manner that can be tested § This is the hypothesis – Predict the outcome as if the hypothesis were correct – Test the hypothesis to see if the prediction is correct – Reject or revise the hypothesis if the prediction is wrong § An hypothesis that stands the test of time is a scientific theory © AMS 13

Atmospheric models § Scientific models are approximations or simulations of a real system § The Earth-atmosphere system can be scientifically modeled – A conceptual model is an abstract idea that represents some fundamental law or relationship § Example – the geostrophic wind model – A graphical model compiles and displays data in a format that readily conveys meaning § Example – a weather map – A physical model is a miniaturized version of a system © AMS § Example – a tornado vortex chamber – next slide 14

Tornado Vortex Chamber © AMS 15

Atmospheric Models § Meteorologists today use numerical models rather than physical models – Mathematical equations represent relationships among system variables § Example – a global climate model and rising CO 2 § All other climate variables are held constant § CO 2 is increased § Results are noted – All have inherent errors § Accuracy of component equations may be a problem © AMS 16

Monitoring the Atmosphere § Surface observations – Systematic observations in some areas as far back as 1644 -45 in North America § Old Swedes Fort (Wilmington, DE) had 1 st systematic observations – Philadelphia began in 1731 – Charleston, SC – 1738 – Cambridge, MA – 1753 – New Haven, CT – 1781 – uninterrupted to today © AMS 17

Monitoring the Atmosphere § Surface observations, continued – – – Army monitored weather to compare with troop health Mid-1800 s – national network of volunteer observers 1849 – telegraph companies transmitted weather conditions free of charge – 1860 s – loss of ships in Great Lakes § Government took a greater role in forecasting – 1870 – President Ulysses S. Grant established 24 stations under the auspices of the U. S. Army Signal Corps – 1891 – transferred from military to civilian hands © AMS § New weather bureau under U. S. Department of Agriculture 18

Monitoring the Atmosphere § Surface observations, continued – Transferred to Commerce Department in 1940 – 1965, Weather Bureau reorganized into the National Weather Service (NWS) § Under Environmental Science Services Administration (ESSA), which became National Oceanic and Atmospheric Administration (NOAA) – 1990 s – NWS modernized and expanded © AMS § Today, 123 NWS Forecast Offices (see next slide) § Added Automated Surface Observing Systems (ASOS) 19

NWS Forecast Offices © AMS 20

Automated Surface Observing System (ASOS) © AMS 21

Cooperative Observer Network § The NWS also has a Cooperative Observer Network – Member stations record daily precipitation and max/min temperatures for hydrologic, agricultural, and climatic purposes © AMS 22

Upper Air Observations § Kites were used early on – 1749, Glasgow, Scotland, Alexander Wilson – 1752, Benjamin Franklin, demonstrated electrical nature of lightning § Balloons – Manned balloon, 1804, Gay-Lussac & Biot § Air samples taken, measured temperature and humidity up to 7, 000 m (23, 000 ft) – Manned balloon, 1862, Glaisher & Coxwell § Weather measurements to 9000 m (29, 500 ft) § Nearly perished from cold and oxygen deprivation § Kites carried the first thermograph aloft in 1894 § 1907 -1933 – box kites with meteorographs, up to 3000 m (10, 000 ft) © AMS 23

Upper Air Observations § Radiosondes – Invented in late 1920 s – Transmits altitude readings (soundings) of: § Temperature ■ Dewpoint ■ Air pressure – Data is received immediately § No need to recover equipment – Rawinsonde § § § A radiosonde that is tracked by direction-finding antennas Provides data on wind direction and speed Dropwindsonde is not launched with a balloon § It is dropped from an aircraft on a parachute – These devices are launched simultaneously worldwide © AMS § Launched at 0000 Z and 1200 Z § Only 20% of the devices are recovered 24

Upper Air Observations Launching a radiosonde © AMS radiosonde 25

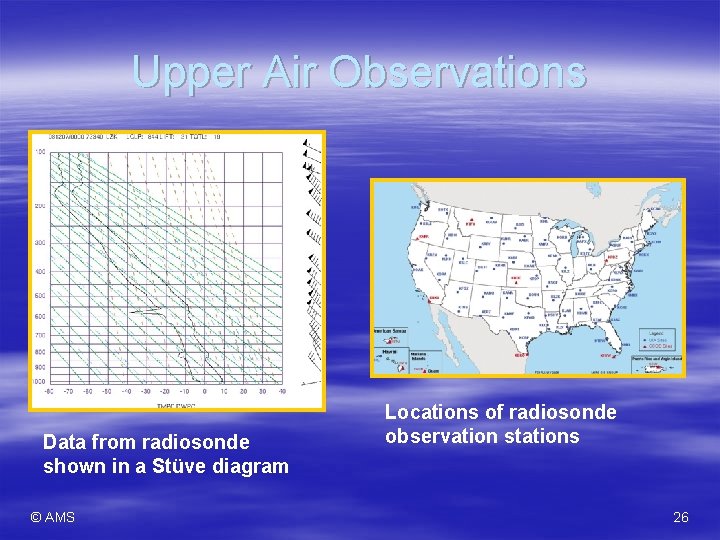
Upper Air Observations Data from radiosonde shown in a Stüve diagram © AMS Locations of radiosonde observation stations 26

Remote Sensing § Measurement of environmental conditions by processing signals that are either emitted by an object or reflected back to a signal source – Radar – Satellites © AMS 27

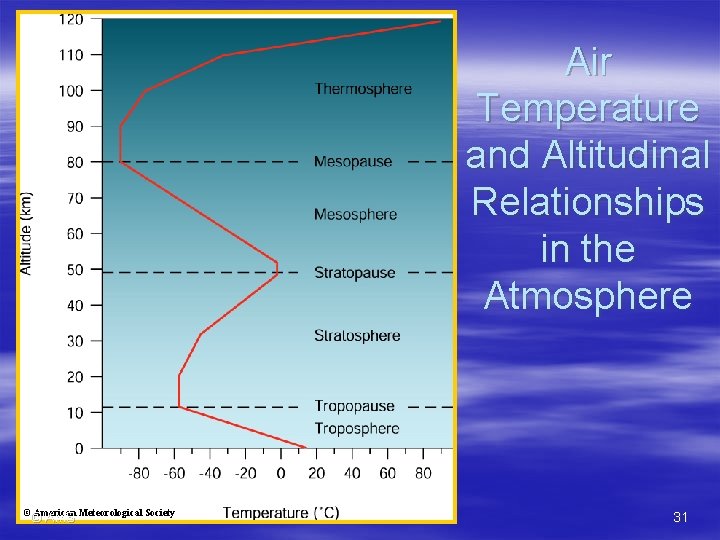
Temperature Profile of the Atmosphere § Troposphere – Lowest layer – Where almost all weather occurs – Temperature decreases with altitude § Generally, but with frequent exceptions (e. g. , inversion, isothermal layer) § Average temperature drop is 6. 5 °C/1000 m (3. 5 °F/1000 ft) – ~ 6 km (3. 7 mi) thick at the poles, ~20 km (12 mi) thick at the equator – Upper boundary/transition zone to next layer is called the tropopause © AMS 28

Temperature Profile of the Atmosphere § Stratosphere – – – Next higher level Goes from troposphere up to ~ 50 km (30 mi) In lower stratosphere, temperature is constant § This is an isothermal condition – Above 20 km (12 mi), temperature increases with increasing altitude § Stratosphere is warmed by the energy released by ozone absorbing UV radiation – Stable conditions are ideal for jet aircraft travel, but can cause trapping of pollutants (e. g. from volcanic eruptions) in lower stratosphere – Upper boundary/transition zone to next layer is called © AMS the stratopause 29

Temperature Profile of the Atmosphere § Mesosphere – – – Next higher level Goes from stratopause up to about 80 km (50 mi) Temperature once again decreases with increasing altitude in this layer § Thermosphere – Next higher level – Temperatures are isothermal (constant temperature condition) in the lower thermosphere – Temperatures rise rapidly above that § Air temperature particularly sensitive to incoming solar radiation § Temperature is more variable than in other regions © AMS 30

Air Temperature and Altitudinal Relationships in the Atmosphere © American Meteorological Society © AMS 31

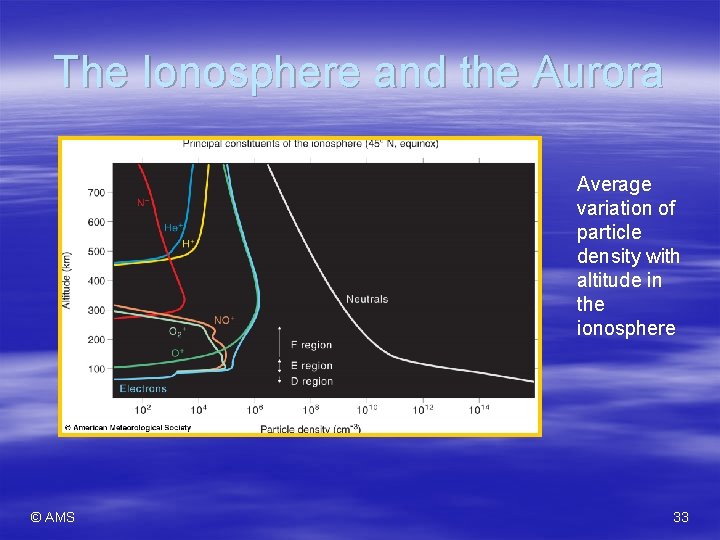
The Ionosphere and the Aurora § The ionosphere is located mostly in thermosphere – High concentration of ions and electrons § Electrically-charged atomic-scale particles § Caused by solar energy stripping electrons from oxygen and nitrogen atoms and molecules – Leaves a positive charge – Auroras are found in the ionosphere § Caused by solar wind – Sub-atomic, super-hot, electrically charged particles § Earth’s magnetic field deflects the solar wind – Makes a teardrop-shaped cavity known as the magnetosphere © AMS § Auroras are only visible at the higher latitudes 32

The Ionosphere and the Aurora Average variation of particle density with altitude in the ionosphere © AMS 33

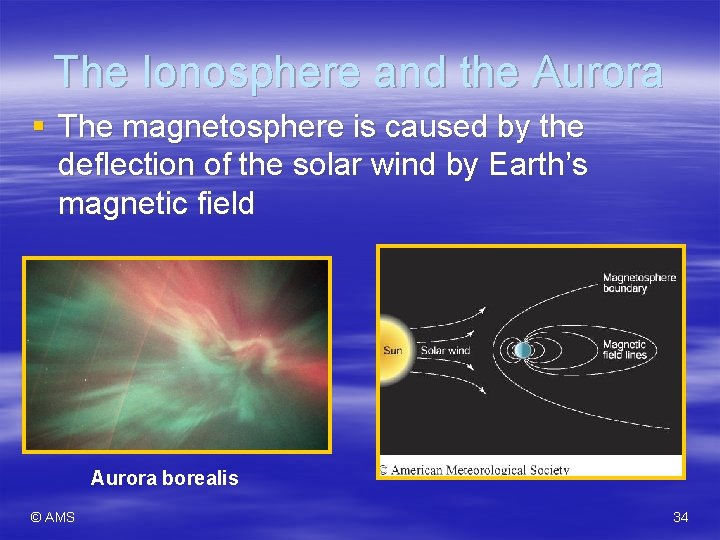
The Ionosphere and the Aurora § The magnetosphere is caused by the deflection of the solar wind by Earth’s magnetic field Aurora borealis © AMS 34

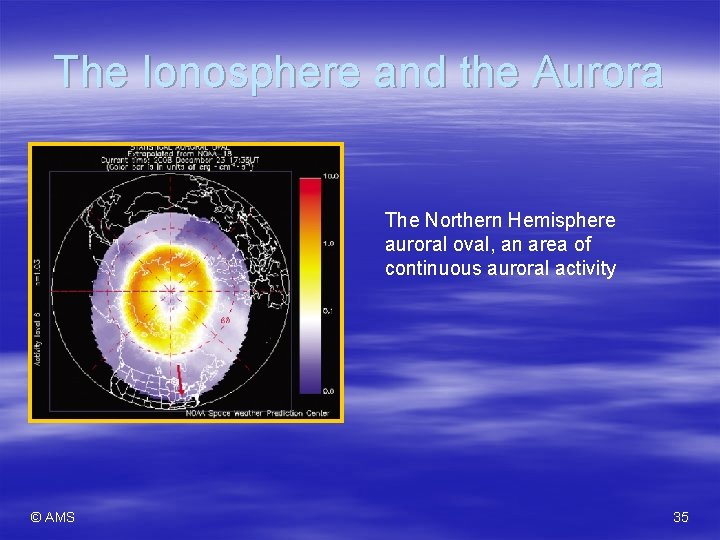
The Ionosphere and the Aurora The Northern Hemisphere auroral oval, an area of continuous auroral activity © AMS 35
 Weather studies introduction to atmospheric science
Weather studies introduction to atmospheric science American meteorological society
American meteorological society Ams modern studies
Ams modern studies Ams realtime weather maps central
Ams realtime weather maps central What are weather variables
What are weather variables Penn state atmospheric science
Penn state atmospheric science Lightning elves
Lightning elves My favourite subject science
My favourite subject science Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Think central science fusion
Think central science fusion Weather model symbols
Weather model symbols Whether the weather be cold
Whether the weather be cold Poems about seasons changing
Poems about seasons changing Windy
Windy Whether the weather is fine
Whether the weather is fine Heavy weather by weather report
Heavy weather by weather report Capital weather gang weather wall
Capital weather gang weather wall Ams 4:23
Ams 4:23 Ams letter format
Ams letter format Ams filing requirements
Ams filing requirements Ams bestätigung vorstellungsgespräch vorlage
Ams bestätigung vorstellungsgespräch vorlage Simplified letter format
Simplified letter format Come back eingliederungsbeihilfe
Come back eingliederungsbeihilfe Tithi bhojan scheme
Tithi bhojan scheme Ams(https://mdm.gujarat.gov.in
Ams(https://mdm.gujarat.gov.in Ibm ams
Ibm ams Ams material
Ams material Transition veriloga
Transition veriloga Ams torrent
Ams torrent Ams trd
Ams trd Bme: ams
Bme: ams Ams
Ams Audit ams
Audit ams Ams konto
Ams konto Ams designer
Ams designer Ams csru
Ams csru

























































