Amino Acids Peptides Proteins Functions of proteins Enzymes


- Slides: 24

Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses Control growth and differentiation Lens protein in eye Feathers Spider webs Horns Milk proteins Antibiotics Mushroom poison …. . Luciferin, luciferase Hemoglobin

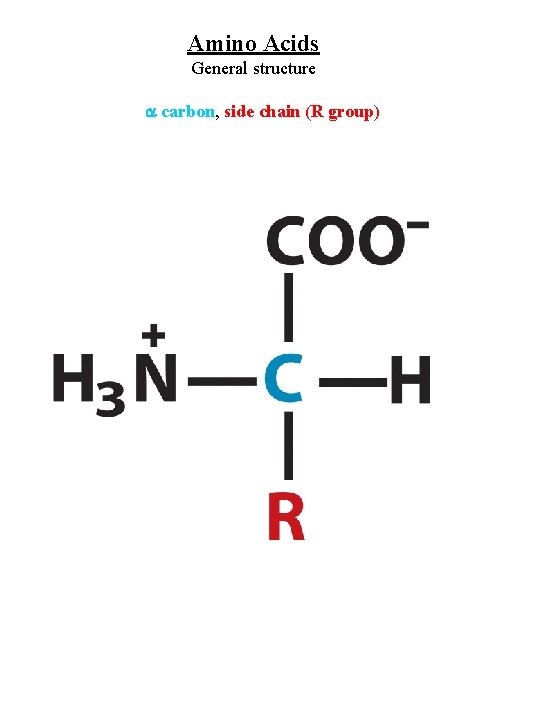
Amino Acids General structure carbon, side chain (R group)

Amino Acids

Amino Acids

Amino Acids

Amino Acids

Amino Acids

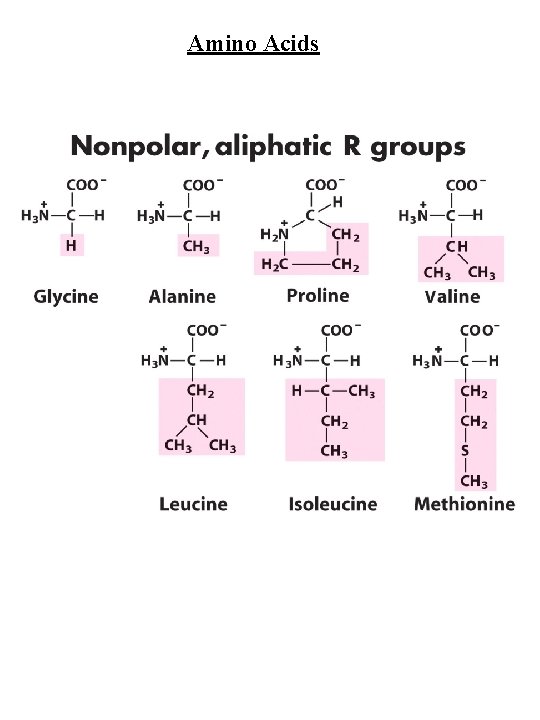
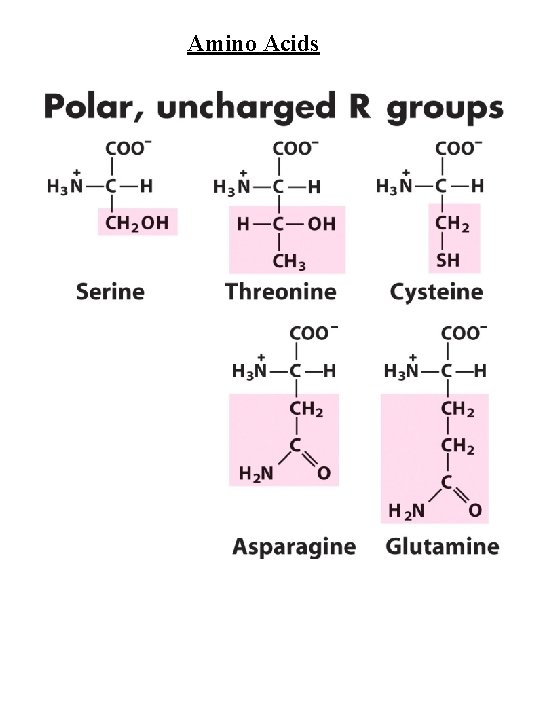
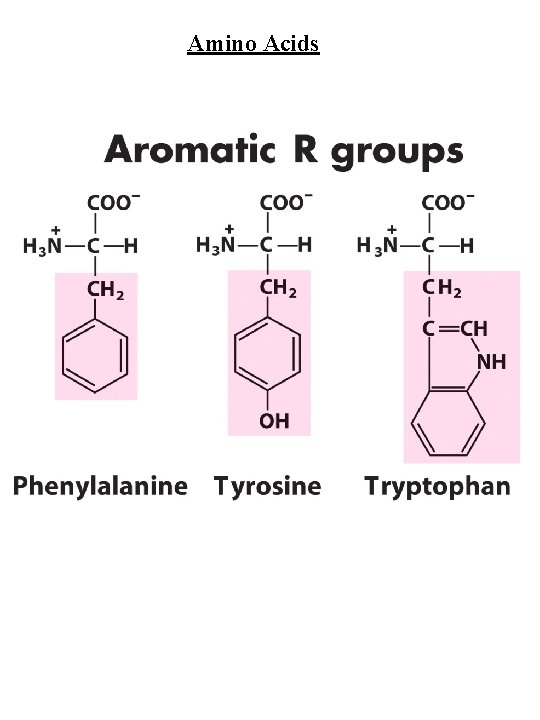
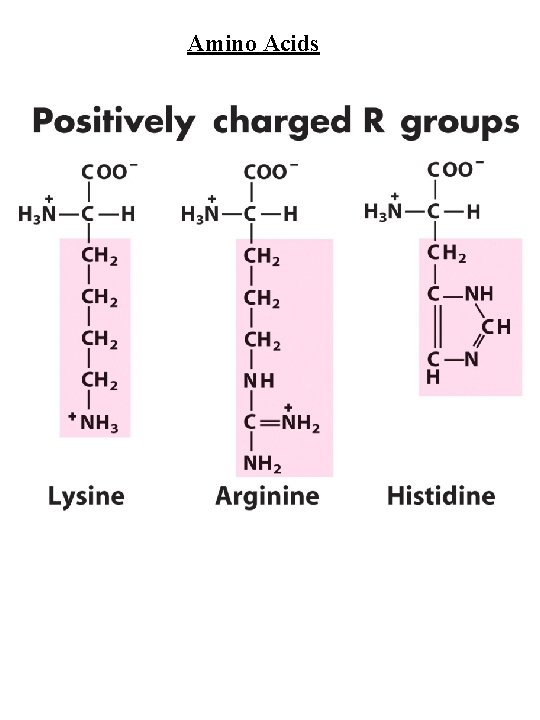
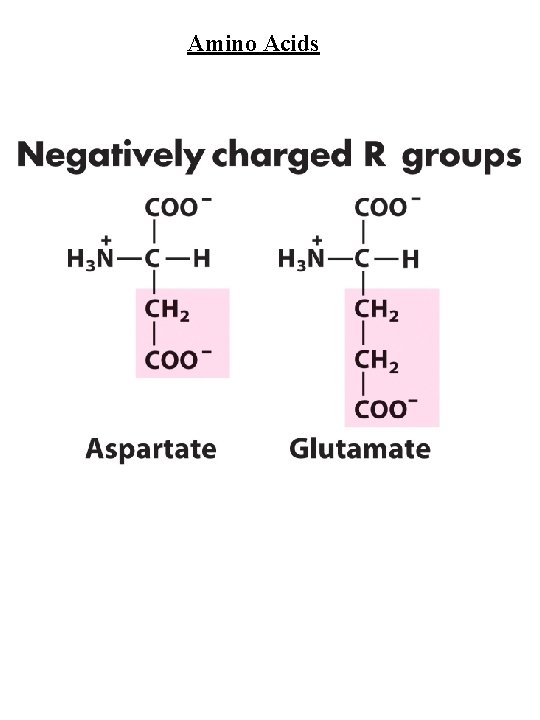
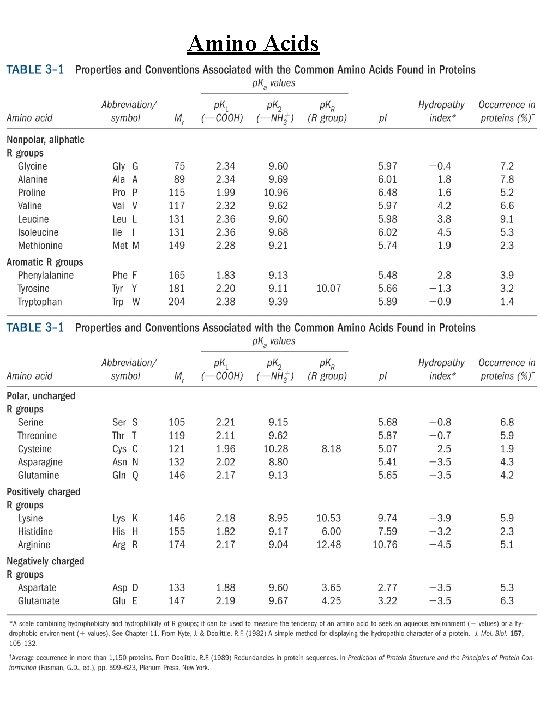
Amino Acids Amino Acid Abbreviations Nonpolar, aliphatic R group Glycine Alanine Proline Valine Leucine Isoleucine Methionine Gly Ala Pro Val Leu Ile Met G A P V L I M Aromatic R groups Phenylalanine Tyrosine Tryptophan Phe Tyr Trp F Y W Polar, uncharged R groups Serine Threonine Cysteine Asparagine Glutamine Ser Thr Cys Asn Gln S T C N Q Positively charge R groups Lysine Histidine Arginine Lys His Arg K H R Negatively charged R groups Aspartate Glutamate Asp Glu D E

Amino Acids

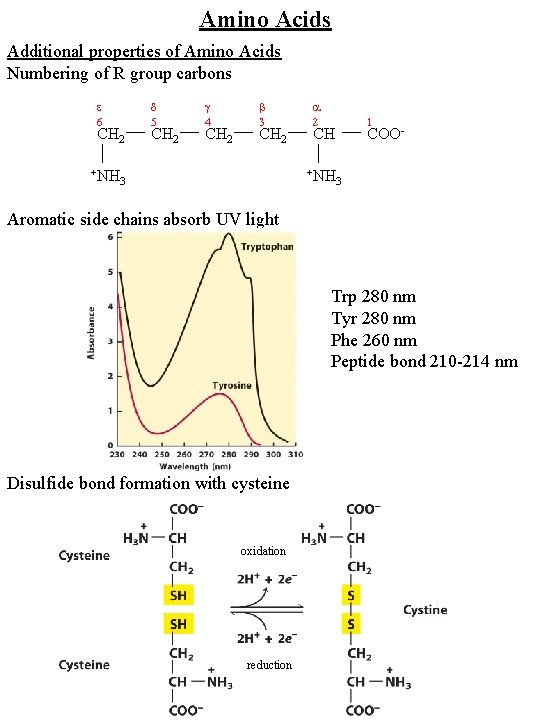
Amino Acids Additional properties of Amino Acids Numbering of R group carbons 6 CH 2 +NH 5 CH 2 4 CH 2 3 CH 2 2 +NH 3 1 CH COO 3 Aromatic side chains absorb UV light Trp 280 nm Tyr 280 nm Phe 260 nm Peptide bond 210 -214 nm Disulfide bond formation with cysteine oxidation reduction

Amino Acids Nonpolar, aliphatic R group Gly, Ala, Pro, Val, Leu, Ile, Met Gly - no steric hindrance Pro - hinders backbone flexibility hydrophobic core of soluble proteins found in transbilayer part of membrane proteins Aromatic R groups Phe, Tyr, Trp hydrophobic, Stacking Tyr/Trp - H-bonding Tyr - site of phosphorylation Polar, uncharged R groups Ser, Thr, Cys, Asn, Gln Ser/Thr - H-bonding; phosphorylated, glycosylated; enzyme active sites Cys - disulfide bonds; enzyme active sites; metal ion binding Asn/Gln - very polar, H-bonding Positively charge R groups Lys, His, Arg His - p. Ka close to neutrality (catalysis); ligand for metal ions (Zn 2+, Fe 2+) Negatively charged R groups Asp, Glu General acids/bases in catalysis (lysozyme) Chelate divalent metal ions (Mg, Ca, Mn, Zn)

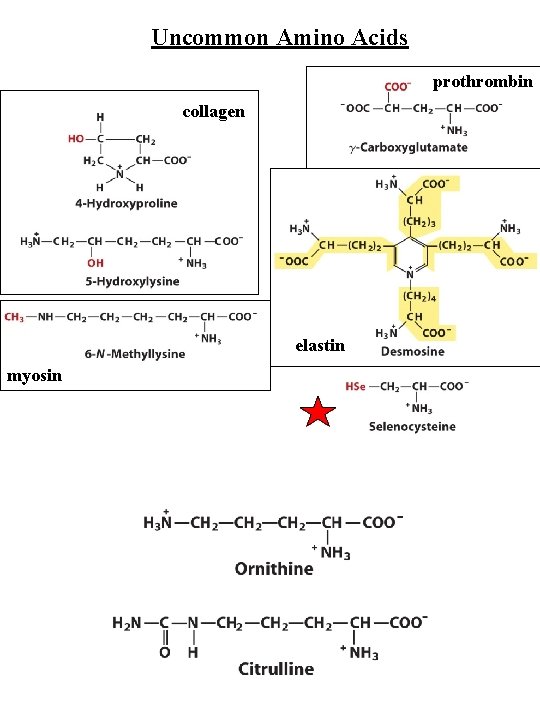
Uncommon Amino Acids prothrombin collagen elastin myosin

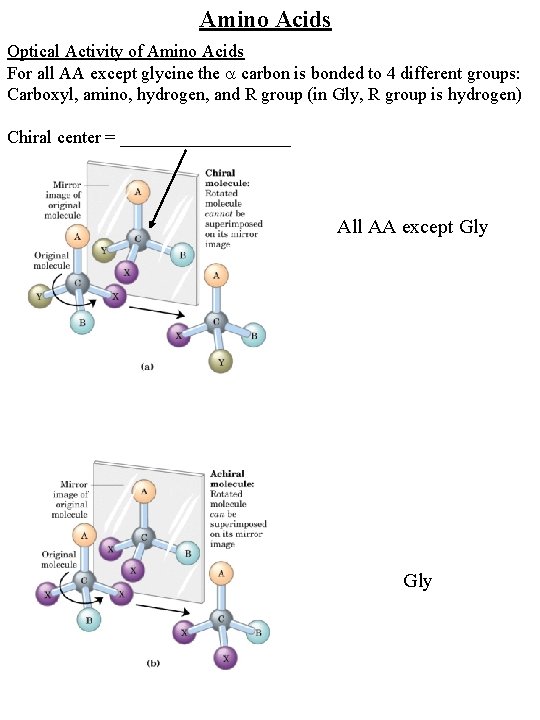
Amino Acids Optical Activity of Amino Acids For all AA except glycine the carbon is bonded to 4 different groups: Carboxyl, amino, hydrogen, and R group (in Gly, R group is hydrogen) Chiral center = __________ All AA except Gly

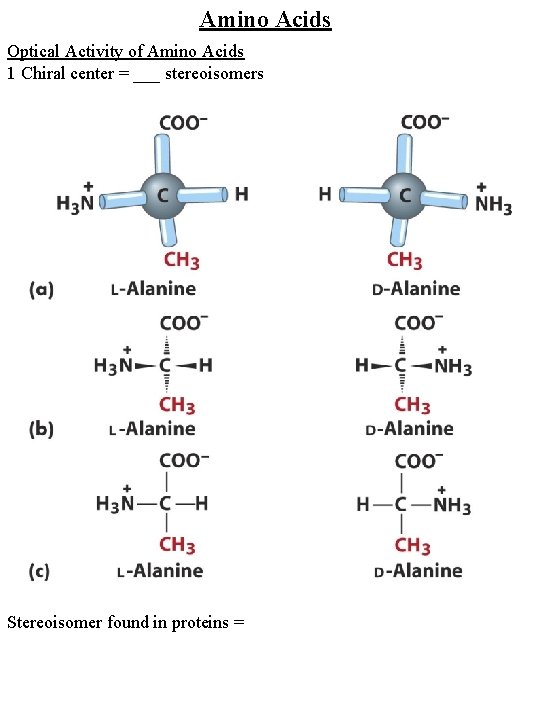
Amino Acids Optical Activity of Amino Acids 1 Chiral center = ___ stereoisomers Stereoisomer found in proteins =

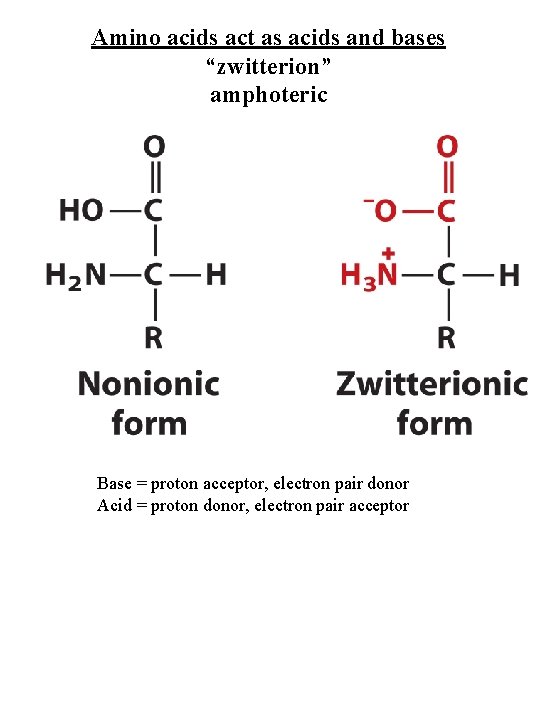
Amino acids act as acids and bases “zwitterion” amphoteric Base = proton acceptor, electron pair donor Acid = proton donor, electron pair acceptor

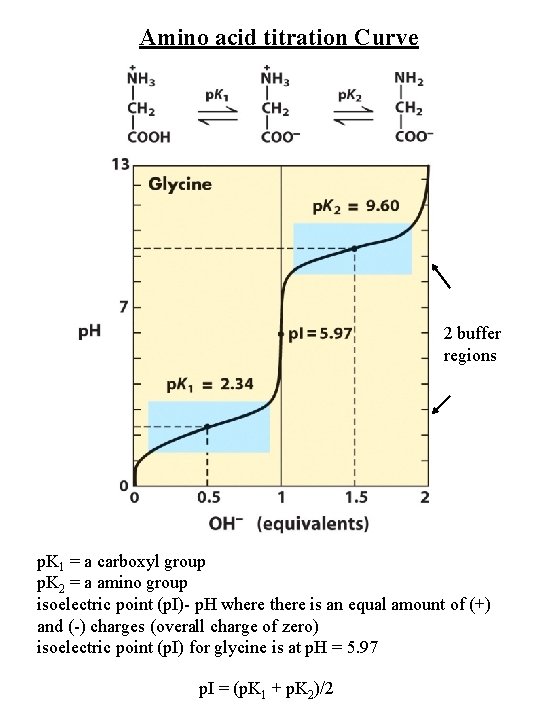
Amino acid titration Curve 2 buffer regions p. K 1 = a carboxyl group p. K 2 = a amino group isoelectric point (p. I)- p. H where there is an equal amount of (+) and (-) charges (overall charge of zero) isoelectric point (p. I) for glycine is at p. H = 5. 97 p. I = (p. K 1 + p. K 2)/2

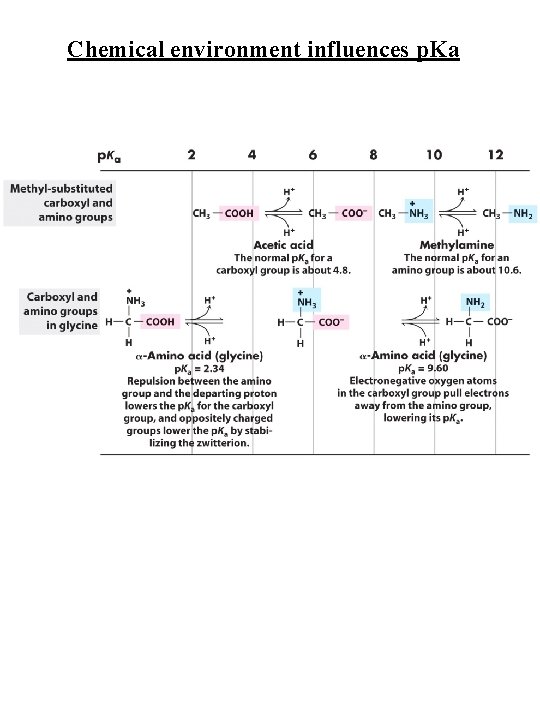
Chemical environment influences p. Ka

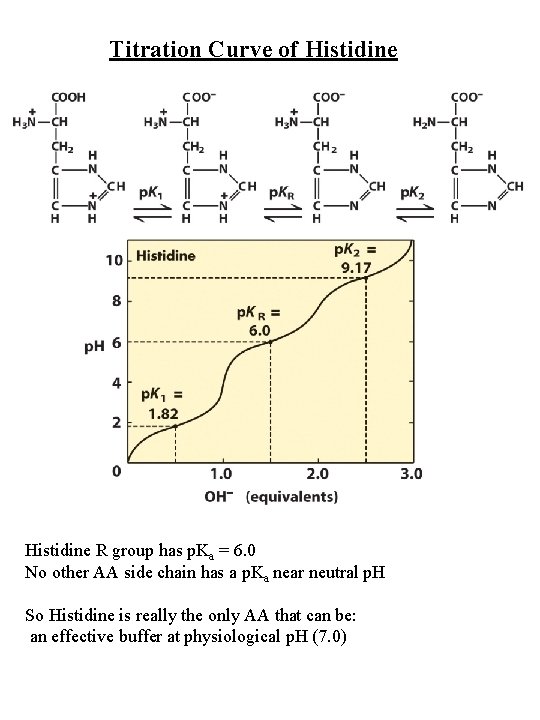
Titration Curve of Histidine R group has p. Ka = 6. 0 No other AA side chain has a p. Ka near neutral p. H So Histidine is really the only AA that can be: an effective buffer at physiological p. H (7. 0)

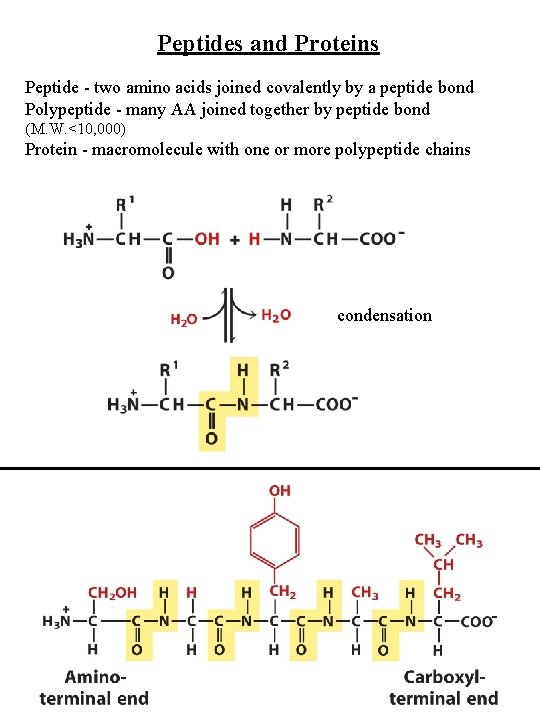
Peptides and Proteins Peptide - two amino acids joined covalently by a peptide bond Polypeptide - many AA joined together by peptide bond (M. W. <10, 000) Protein - macromolecule with one or more polypeptide chains condensation

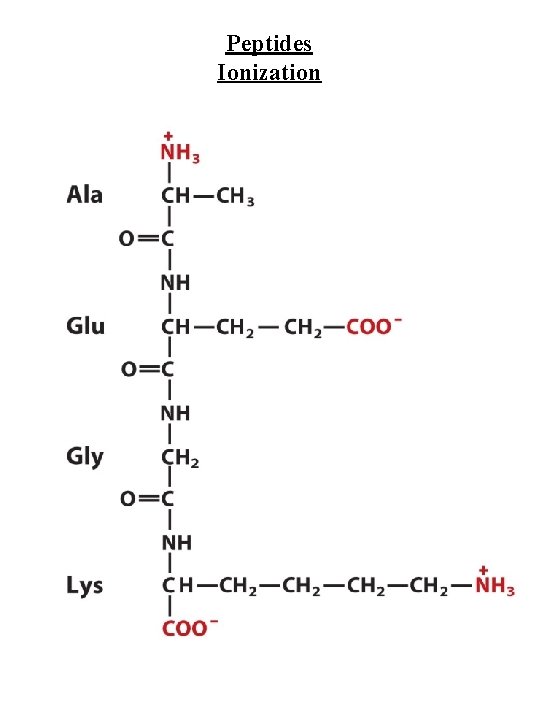
Peptides Ionization

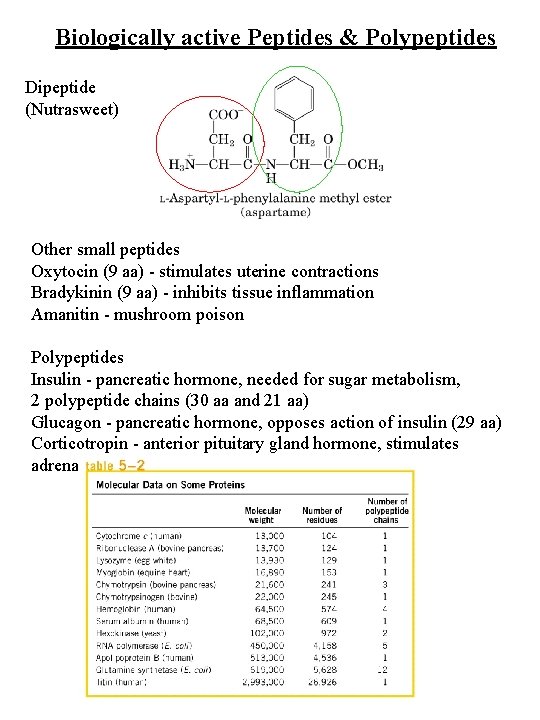
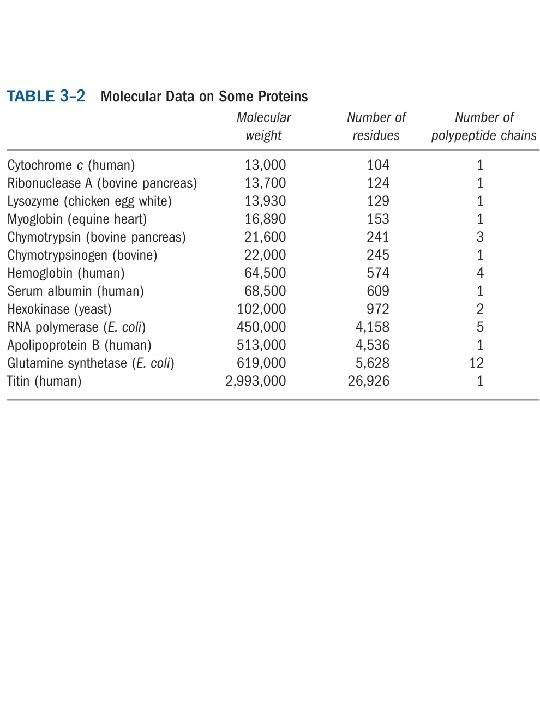
Biologically active Peptides & Polypeptides Dipeptide (Nutrasweet) Other small peptides Oxytocin (9 aa) - stimulates uterine contractions Bradykinin (9 aa) - inhibits tissue inflammation Amanitin - mushroom poison Polypeptides Insulin - pancreatic hormone, needed for sugar metabolism, 2 polypeptide chains (30 aa and 21 aa) Glucagon - pancreatic hormone, opposes action of insulin (29 aa) Corticotropin - anterior pituitary gland hormone, stimulates adrenal cortex (39 aa)


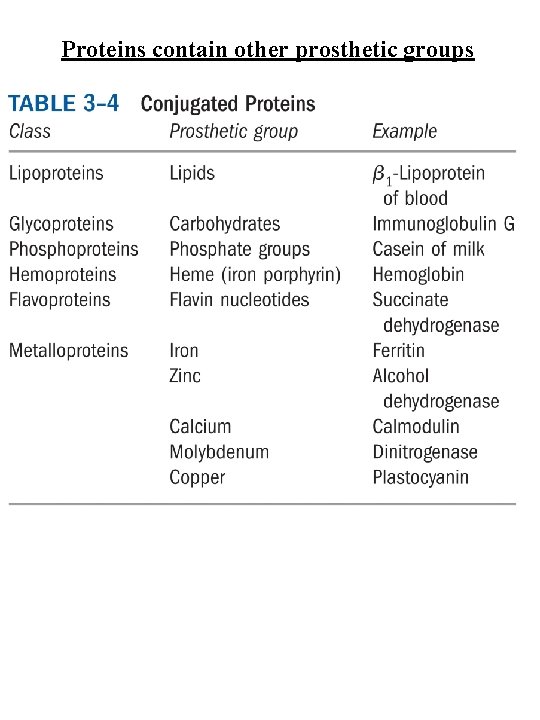
Proteins contain other prosthetic groups

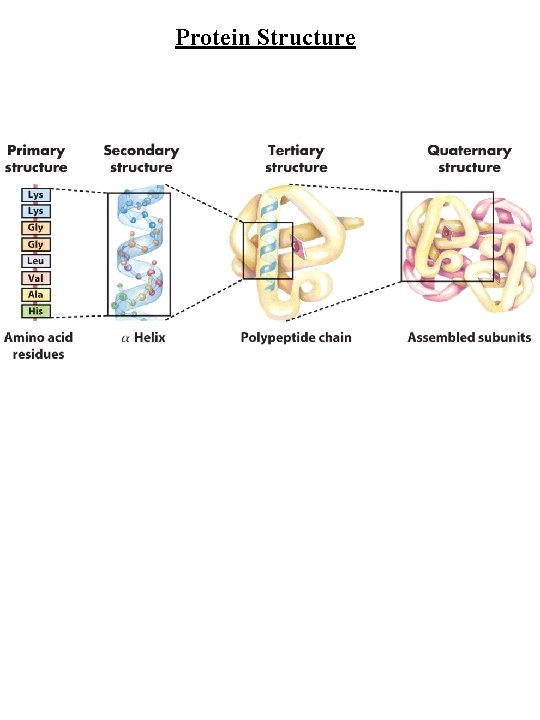
Protein Structure