Amino Acids Peptides Protein Structure Andy Howard Biochemistry
Amino Acids, Peptides, Protein Structure Andy Howard Biochemistry Lectures, Spring 2019 24 January 2019
Let’s study amino acids, peptides, & proteins! n n n We’ll talk a bit more about amino acids themselves, and oligomers and polymers of amino acids We’ll discuss structure Then we’ll talk about methods of dealing with proteins 01/24/2019 Amino acids, Peptides, Proteins Page 2 of 67
Topics for today n n n The 20 amino acids n Helices and Sheets Main-chain chemistry n Protein structure Side-chain chemistry n Structural proteins Peptide bonds n Globular proteins Disulfides n Tertiary & Quaternary Primary & Secondary Structures 01/24/2019 Amino acids, Peptides, Proteins Page 3 of 67
Properties of the amino acids n n We presented the structures of the 20 ribosomally encoded amino acids on Tuesday. Today we’ll explore their properties, starting with their acid-base properties and moving on to other characteristics. 01/24/2019 Amino acids, Peptides, Proteins Page 4 of 67
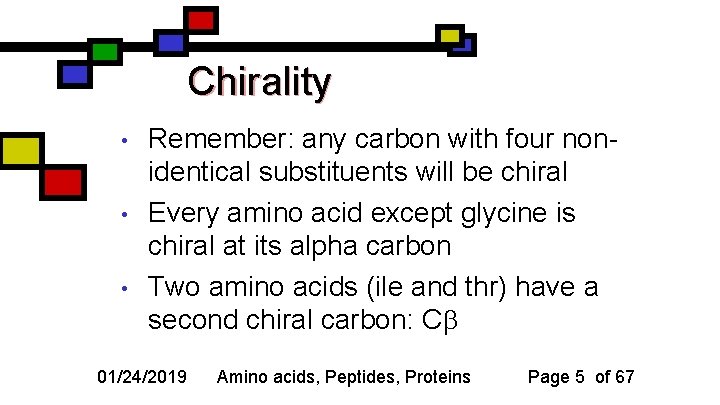
Chirality • • • Remember: any carbon with four nonidentical substituents will be chiral Every amino acid except glycine is chiral at its alpha carbon Two amino acids (ile and thr) have a second chiral carbon: C 01/24/2019 Amino acids, Peptides, Proteins Page 5 of 67
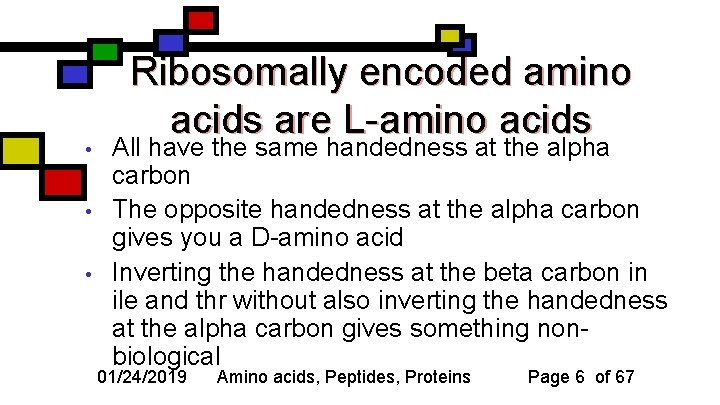
• • • Ribosomally encoded amino acids are L-amino acids All have the same handedness at the alpha carbon The opposite handedness at the alpha carbon gives you a D-amino acid Inverting the handedness at the beta carbon in ile and thr without also inverting the handedness at the alpha carbon gives something nonbiological 01/24/2019 Amino acids, Peptides, Proteins Page 6 of 67
n n n We do care about D-amino acids There are D-amino acids in many organisms Bacteria incorporate them into structures of their cell walls Makes those structures resistant to standard proteolytic enzymes, which only attack amino acids with L specificity 01/24/2019 Amino acids, Peptides, Proteins Page 7 of 67
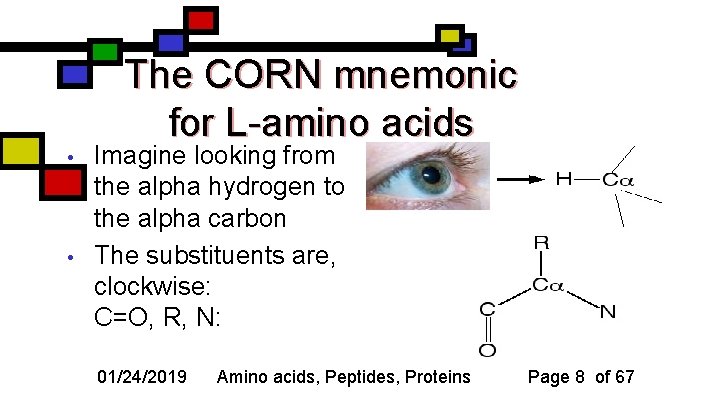
The CORN mnemonic for L-amino acids • • Imagine looking from the alpha hydrogen to the alpha carbon The substituents are, clockwise: C=O, R, N: 01/24/2019 Amino acids, Peptides, Proteins Page 8 of 67
Abbreviations for amino acids n 3 -letter and one-letter codes exist n n n All the 3 -letter codes are logical Most of the 1 -letter codes are too 6 unused letters, obviously n n U used for selenocysteine O used for pyrrolysine B, J, Z are used for ambiguous cases: B is asp/asn, J is ile/leu, Z is glu/gln X for “totally unknown” 01/24/2019 Amino acids, Peptides, Proteins Page 9 of 67
Nomenclature n n http: //www. chem. qmul. ac. uk/ iupac/Amino. Acid/A 2021. html Wikipedia article is pretty complete too 01/24/2019 Amino acids, Peptides, Proteins Page 10 of 67
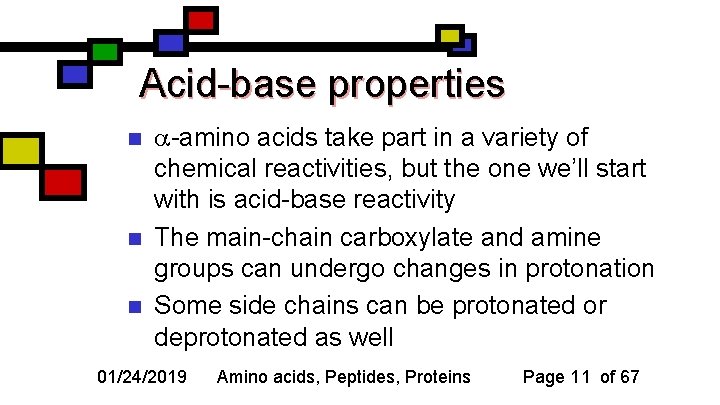
Acid-base properties n n n -amino acids take part in a variety of chemical reactivities, but the one we’ll start with is acid-base reactivity The main-chain carboxylate and amine groups can undergo changes in protonation Some side chains can be protonated or deprotonated as well 01/24/2019 Amino acids, Peptides, Proteins Page 11 of 67
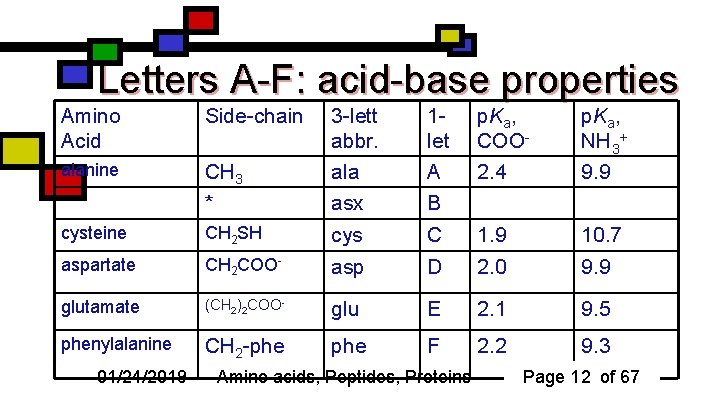
Letters A-F: acid-base properties Amino Acid Side-chain CH 3 * 3 -lett abbr. ala asx 1 let A B p. Ka, COO 2. 4 p. Ka, NH 3+ 9. 9 alanine cysteine CH 2 SH cys C 1. 9 10. 7 aspartate CH 2 COO- asp D 2. 0 9. 9 glutamate (CH 2)2 COO- glu E 2. 1 9. 5 phenylalanine CH 2 -phe F 2. 2 9. 3 01/24/2019 Amino acids, Peptides, Proteins Page 12 of 67
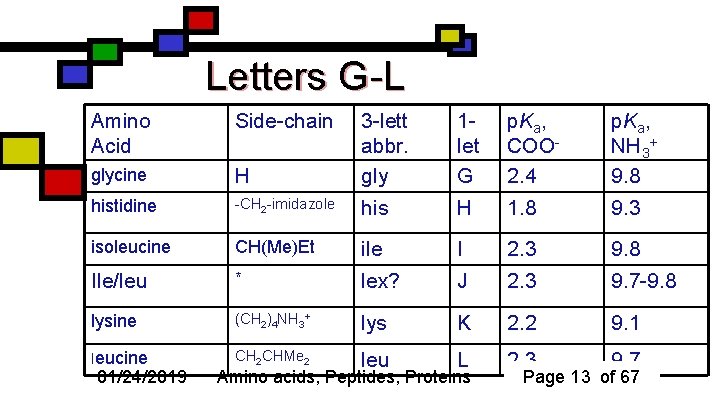
Letters G-L Amino Acid Side-chain H 3 -lett abbr. gly 1 let G p. Ka, COO 2. 4 p. Ka, NH 3+ 9. 8 glycine histidine -CH 2 -imidazole his H 1. 8 9. 3 isoleucine CH(Me)Et ile I 2. 3 9. 8 Ile/leu * lex? J 2. 3 9. 7 -9. 8 lysine (CH 2)4 NH 3+ lys K 2. 2 9. 1 leucine CH 2 CHMe 2 leu L 2. 3 9. 7 01/24/2019 Amino acids, Peptides, Proteins Page 13 of 67
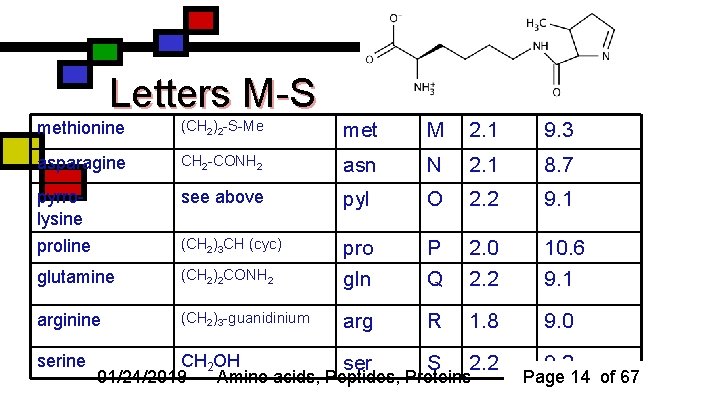
Letters M-S methionine (CH 2)2 -S-Me met M 2. 1 9. 3 asparagine CH 2 -CONH 2 asn N 2. 1 8. 7 pyrrolysine see above pyl O 2. 2 9. 1 proline (CH 2)3 CH (cyc) pro P 2. 0 10. 6 glutamine (CH 2)2 CONH 2 gln Q 2. 2 9. 1 arginine (CH 2)3 -guanidinium arg R 1. 8 9. 0 serine CH 2 OH ser S 2. 2 01/24/2019 Amino acids, Peptides, Proteins 9. 2 Page 14 of 67
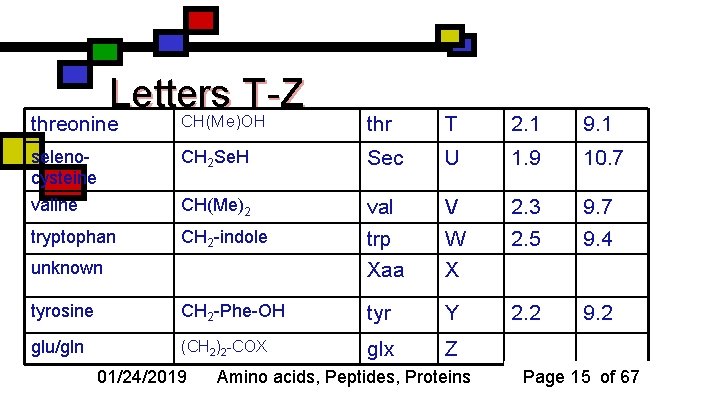
Letters T-Z CH(Me)OH threonine thr T 2. 1 9. 1 selenocysteine CH 2 Se. H Sec U 1. 9 10. 7 valine CH(Me)2 val V 2. 3 9. 7 tryptophan CH 2 -indole trp W 2. 5 9. 4 Xaa X 2. 2 9. 2 unknown tyrosine CH 2 -Phe-OH tyr Y glu/gln (CH 2)2 -COX glx Z 01/24/2019 Amino acids, Peptides, Proteins Page 15 of 67
Remembering the abbreviations n n A, C, G, H, I, L, M, P, S, T, V easy F: phenylalanine sounds like an F R: talk like a pirate D, E similar and they’re adjacent 01/24/2019 Amino acids, Peptides, Proteins Page 16 of 67
One-letter abbreviations, concluded n n n N: contains a nitrogen W: say tryptophan with a lisp Y: second letter is a Y Q: almost follows N, and gln is like asn You’re on your own for K, O, J, B, Z, U, X 01/24/2019 Amino acids, Peptides, Proteins Page 17 of 67
Do you need to memorize these structures? n Yes, for the 20 major ones n (not B, J, O, U, X, Z) The only other complex structures I’ll ask you to memorize are: n n DNA, RNA bases Ribose, glucose, deoxyribose, glyceraldehyde Cholesterol, stearate, palmitate, oleate A few others I won’t enumerate right now. 01/24/2019 Amino acids, Peptides, Proteins Page 18 of 67
How hard is it to memorize the structures? n n n Very easy: G, A, S, C, V Relatively easy: F, Y, D, E, N, Q Harder: I, K, L, M, P, T Hardest: H, R, W Again, I’m not asking you to memorize the oneletter codes, but they do make life a lot easier. 01/24/2019 Amino acids, Peptides, Proteins Page 19 of 67
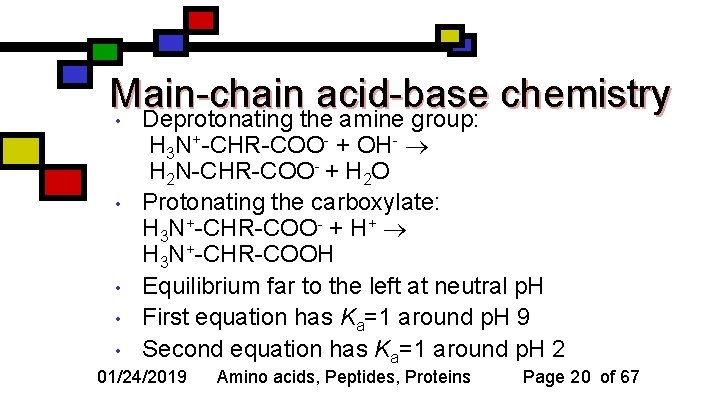
Main-chain acid-base chemistry • Deprotonating the amine group: • • • H 3 N+-CHR-COO- + OH- H 2 N-CHR-COO- + H 2 O Protonating the carboxylate: H 3 N+-CHR-COO- + H+ H 3 N+-CHR-COOH Equilibrium far to the left at neutral p. H First equation has Ka=1 around p. H 9 Second equation has Ka=1 around p. H 2 • 01/24/2019 Amino acids, Peptides, Proteins Page 20 of 67
Why does a main-chain p. Ka depend on the side chain? n n Opportunities for hydrogen bonding or other ionic interactions stabilize some charges more than others More variability in the amino terminus, i. e. the p. Ka of the carboxylate group doesn’t depend as much on R as the p. Ka of the amine group 01/24/2019 Amino acids, Peptides, Proteins Page 21 of 67
When do these p. Ka values apply? n n n The values given in the table are for the free amino acids The main-chain p. Ka values aren’t relevant for internal amino acids in proteins The side-chain p. Ka values vary a lot depending on molecular environment: a 9. 4 here doesn’t mean a 9. 4 in a protein! 01/24/2019 Amino acids, Peptides, Proteins Page 22 of 67
Relating p. Ka to percentage ionization • Derivable from Henderson-Hasselbalch • • • equation If p. H = p. Ka, half-ionized: [HA] = [A-] One unit below: n 91% at more positive charge state, n 9% at less + charge state One unit above: 9% / 91% 01/24/2019 Amino acids, Peptides, Proteins Page 23 of 67
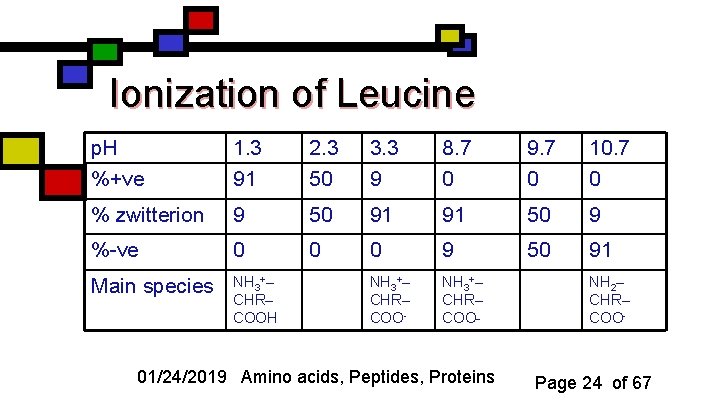
Ionization of Leucine p. H %+ve 1. 3 91 2. 3 50 3. 3 9 8. 7 0 9. 7 0 10. 7 0 % zwitterion 9 50 91 91 50 9 %-ve 0 0 0 9 50 91 Main species NH 3+– CHR– COOH NH 3+– CHR– COO- 01/24/2019 Amino acids, Peptides, Proteins NH 2– CHR– COO- Page 24 of 67
Side-chain reactivity • • • Not all the chemical reactivity of amino acids involves the main-chain amino and carboxyl groups Side chains can participate in reactions: n Acid-base reactions n Other reactions In proteins and peptides, side-chain reactivity is more important because main chain is locked up! 01/24/2019 Amino acids, Peptides, Proteins Page 25 of 67
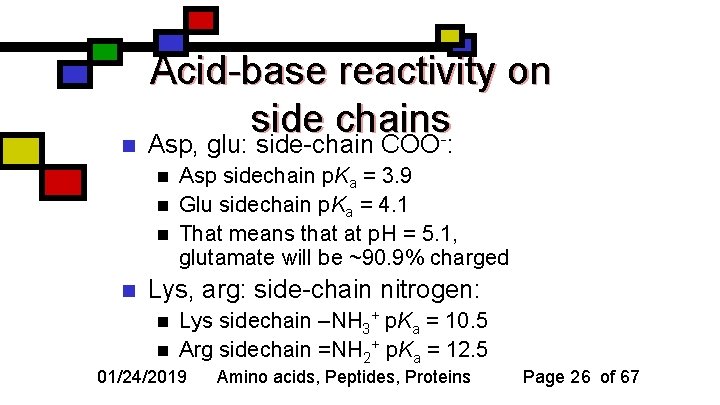
n Acid-base reactivity on side chains. Asp, glu: side-chain COO : n n Asp sidechain p. Ka = 3. 9 Glu sidechain p. Ka = 4. 1 That means that at p. H = 5. 1, glutamate will be ~90. 9% charged Lys, arg: side-chain nitrogen: n n Lys sidechain –NH 3+ p. Ka = 10. 5 Arg sidechain =NH 2+ p. Ka = 12. 5 01/24/2019 Amino acids, Peptides, Proteins Page 26 of 67
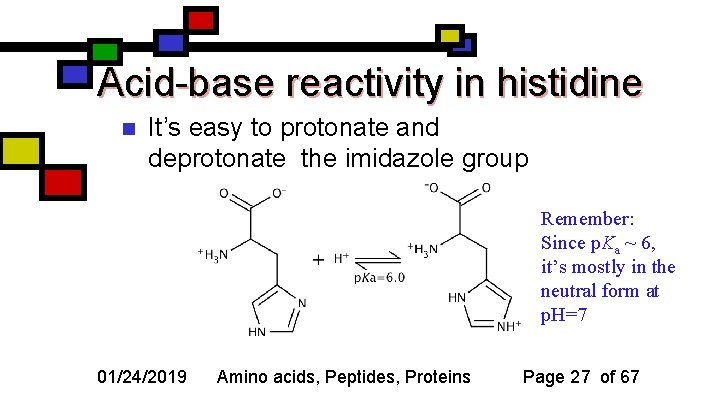
Acid-base reactivity in histidine n It’s easy to protonate and deprotonate the imidazole group Remember: Since p. Ka ~ 6, it’s mostly in the neutral form at p. H=7 01/24/2019 Amino acids, Peptides, Proteins Page 27 of 67
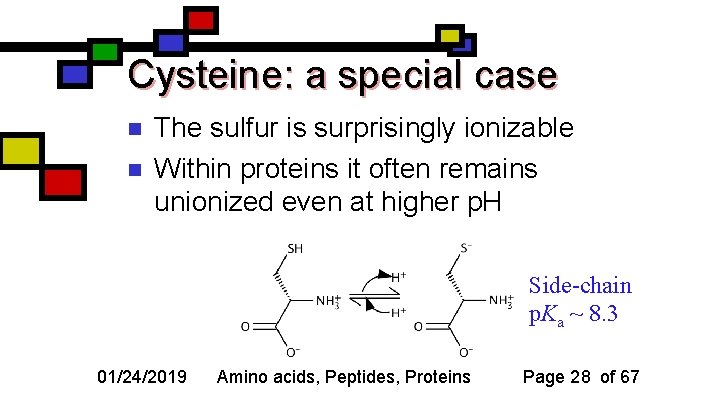
Cysteine: a special case n n The sulfur is surprisingly ionizable Within proteins it often remains unionized even at higher p. H Side-chain p. Ka ~ 8. 3 01/24/2019 Amino acids, Peptides, Proteins Page 28 of 67

Why isn’t that a big deal? n n It turns out many cysteines are involved in disulfide bonds (-S—S-), so the sulfhydryls aren’t available for ionization This is particularly true under oxidizing conditions 01/24/2019 Amino acids, Peptides, Proteins Page 29 of 67
Ionizing hydroxyls n n X–O–H X–O- + H+ Tyrosine is easy, ser and thr hard: n n n Tyr p. Ka = 10. 5 Ser, Thr p. Ka = ~13 Difference due to resonance stabilization of phenolate ion: 01/24/2019 Amino acids, Peptides, Proteins Page 30 of 67
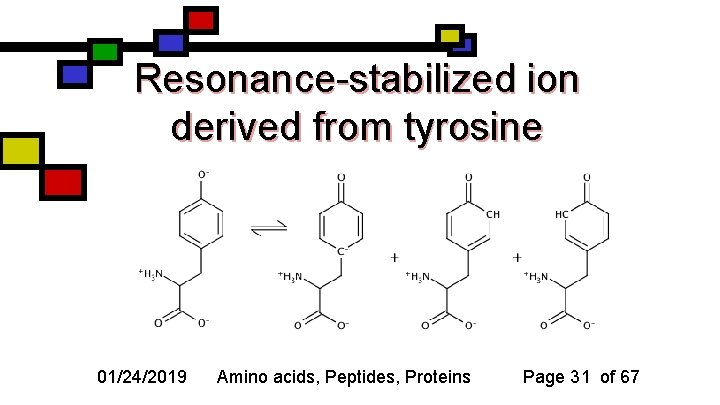
Resonance-stabilized ion derived from tyrosine 01/24/2019 Amino acids, Peptides, Proteins Page 31 of 67
Other side-chain reactions n n n Little activity in hydrophobic amino acids other than van der Waals Sulfurs (especially in cysteines) can be oxidized to sulfates, sulfites, … Nitrogens in his can covalently bond to various ligands Hydroxyls can form ethers, esters Salt bridges (e. g. lys - asp) 01/24/2019 Amino acids, Peptides, Proteins Page 32 of 67
Phosphorylation n n ATP donates terminal phosphate to side-chain hydroxyl of ser, thr, tyr Some phosphorylation of H, D, E too ATP + Ser-OH ADP + Ser-O-(P) Often involved in activating or inactivating enzymes Under careful control of enzymes called kinases (add (P)) and phosphatases (remove (P)) 01/24/2019 Amino acids, Peptides, Proteins Page 33 of 67

Angles, angles… n n By now you’re accustomed to thinking about bond angles, which involve three atoms that are covalently attached We also need to deal with torsion angles, which involve 4 covalently-attached atoms 01/24/2019 Amino acids, Peptides, Proteins Page 34 of 67
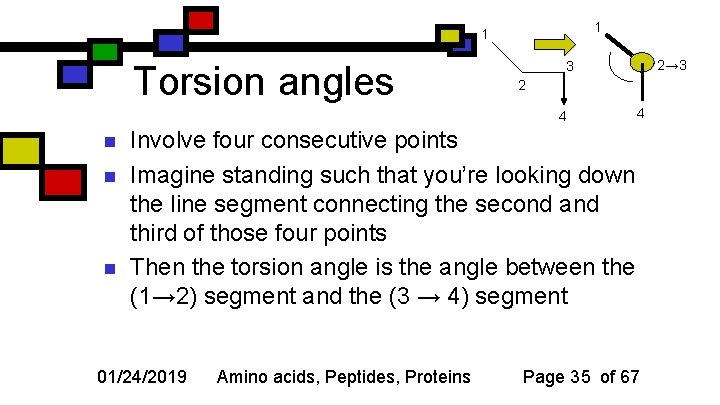
1 1 Torsion angles 2 4 n n n 2→ 3 3 4 Involve four consecutive points Imagine standing such that you’re looking down the line segment connecting the second and third of those four points Then the torsion angle is the angle between the (1→ 2) segment and the (3 → 4) segment 01/24/2019 Amino acids, Peptides, Proteins Page 35 of 67
Peptides & proteins n n n Peptides are oligomers of amino acids Proteins are polymers Dividing line is a little vague: ~ 50 -80 aa. All are created, both formally and in practice, by stepwise polymerization Water eliminated at each step 01/24/2019 Amino acids, Peptides, Proteins Page 36 of 67
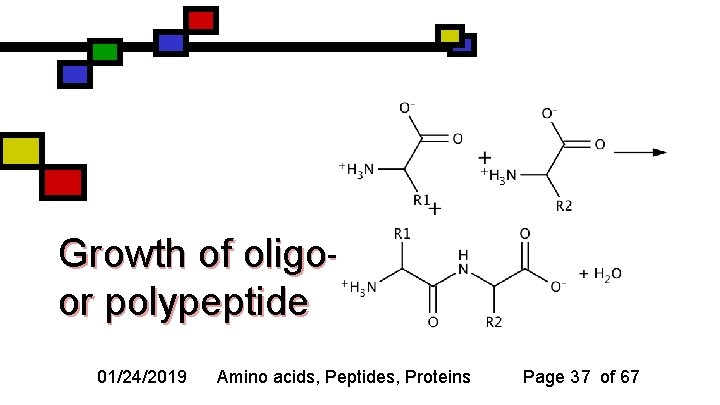
Growth of oligoor polypeptide 01/24/2019 Amino acids, Peptides, Proteins Page 37 of 67
The peptide bond n n The amide bond between two successive amino acids is known as a peptide bond The C-N bond between the first amino acid’s carbonyl carbon and the second amino acid’s amine nitrogen has some double bond character 01/24/2019 Amino acids, Peptides, Proteins Page 38 of 67
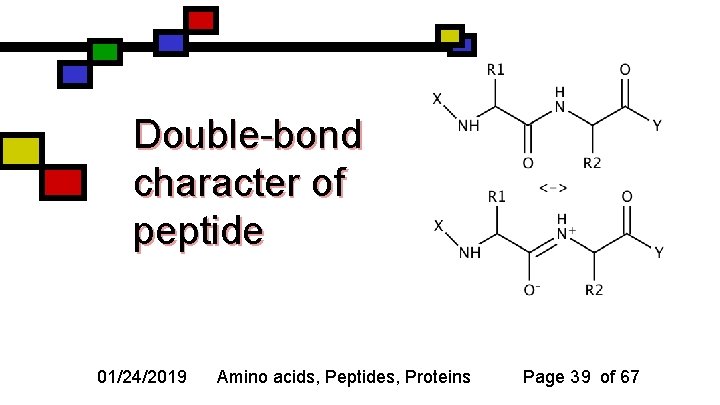
Double-bond character of peptide 01/24/2019 Amino acids, Peptides, Proteins Page 39 of 67
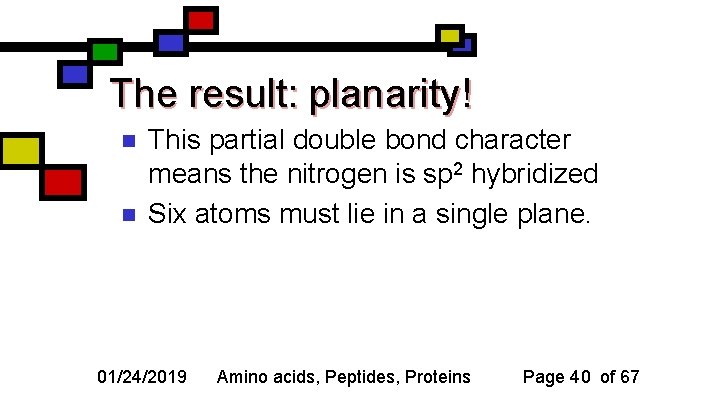
The result: planarity! n n This partial double bond character means the nitrogen is sp 2 hybridized Six atoms must lie in a single plane. 01/24/2019 Amino acids, Peptides, Proteins Page 40 of 67

n n n Which six atoms are constrained? First amino acid’s alpha carbon Carbonyl oxygen Second amino acid’s amide nitrogen Amide hydrogen Second amino acid’s alpha carbon 01/24/2019 Amino acids, Peptides, Proteins Page 41 of 67
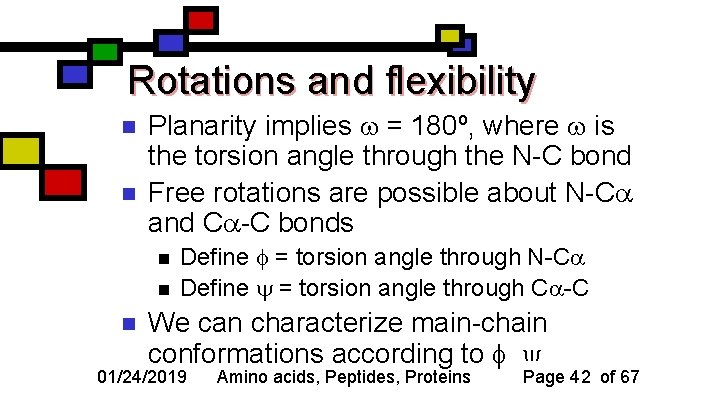
Rotations and flexibility n n Planarity implies = 180º, where is the torsion angle through the N-C bond Free rotations are possible about N-C and C -C bonds n n n Define = torsion angle through N-C Define = torsion angle through C -C We can characterize main-chain conformations according to , 01/24/2019 Amino acids, Peptides, Proteins Page 42 of 67
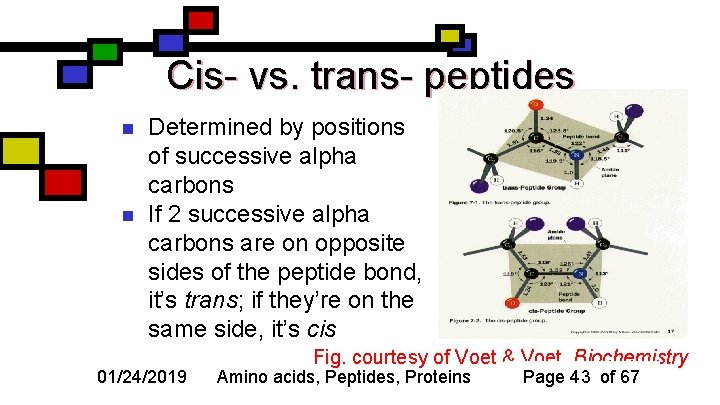
Cis- vs. trans- peptides n n Determined by positions of successive alpha carbons If 2 successive alpha carbons are on opposite sides of the peptide bond, it’s trans; if they’re on the same side, it’s cis 01/24/2019 Fig. courtesy of Voet & Voet, Biochemistry Amino acids, Peptides, Proteins Page 43 of 67
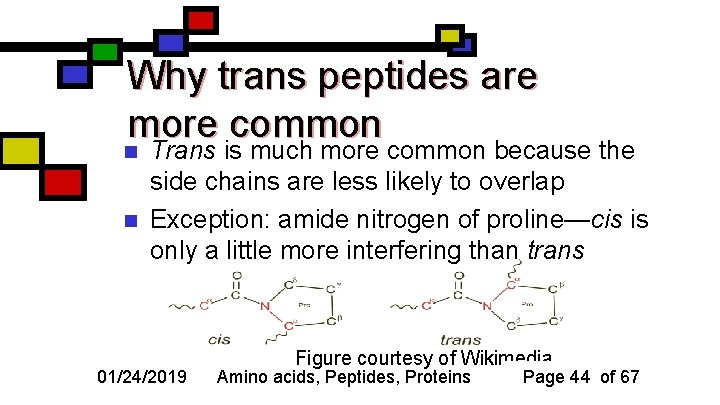
Why trans peptides are more common n n Trans is much more common because the side chains are less likely to overlap Exception: amide nitrogen of proline—cis is only a little more interfering than trans 01/24/2019 Figure courtesy of Wikimedia Amino acids, Peptides, Proteins Page 44 of 67
Ramachandran angles G. N. Ramachandran 01/24/2019 Amino acids, Peptides, Proteins Page 45 of 67
Preferred Values of and n n n Steric hindrance makes some values unlikely Specific values are characteristic of particular types of secondary structure Most structures with forbidden values of and turn out to be errors 01/24/2019 Amino acids, Peptides, Proteins Page 46 of 67
How far from 180º can vary? n n Remember partial double bond character of the C-N main-chain bond That imposes planarity In practice it rarely varies by more than a few degrees from 180º. Aromatic amino acids are the most likely to have non-planar peptides 01/24/2019 Amino acids, Peptides, Proteins Page 47 of 67
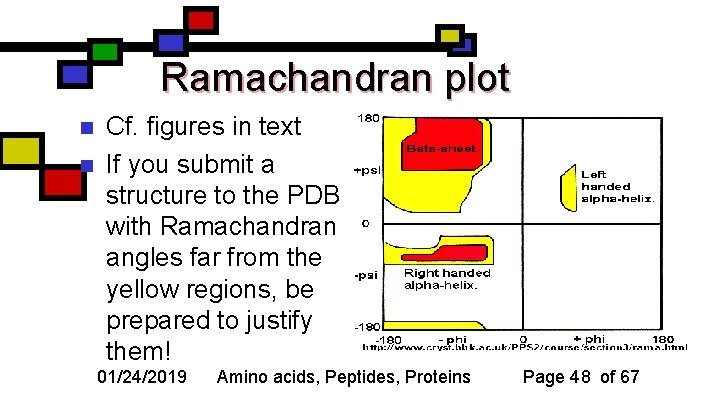
Ramachandran plot n n Cf. figures in text If you submit a structure to the PDB with Ramachandran angles far from the yellow regions, be prepared to justify them! 01/24/2019 Amino acids, Peptides, Proteins Page 48 of 67
How are oligo- and polypeptides synthesized? n n n Formation of the peptide linkages occurs in ribosome under careful enzymatic control Polymerization is endergonic and requires energy in the form of GTP (like ATP, only with guanosine): GTP + n-length-peptide + amino acid GDP + Pi + (n+1)-length peptide 01/24/2019 Amino acids, Peptides, Proteins Page 49 of 67
What happens at the ends? n n Usually there’s a free amino end a free carboxyl end: H 3 N+–CHR–CO–(peptide)n–NH–CHR–COOCyclic peptides do occur Cyclization doesn’t happen at the ribosome: it involves a separate, enzymatic step. 01/24/2019 Amino acids, Peptides, Proteins Page 50 of 67
Reactivity in peptides & proteins n n Main-chain acid-base reactivity unavailable except on the ends Side-chain reactivity available but with slightly modified p. Kas. Terminal main-chain p. Kavalues modified too Environment of protein side chain is often hydrophobic, unlike free amino acid side chain 01/24/2019 Amino acids, Peptides, Proteins Page 51 of 67
i. Clicker question 1 1. What’s the net charge on ELVIS at p. H 7? n n n 01/24/2019 (a) 0 (b) +1 (c) -1 (d) +2 (e) -2 Amino acids, Peptides, Proteins Page 52 of 67
i. Clicker question 2 n 2. Leucine is one of the more common amino acids in proteins. In a typical protein, I would expect the leucine content to be about: n n (a) 53%; (b) 7%; (c) 5%; (d) 3% (e) We do not have enough information to know. 01/24/2019 Amino acids, Peptides, Proteins Page 53 of 67
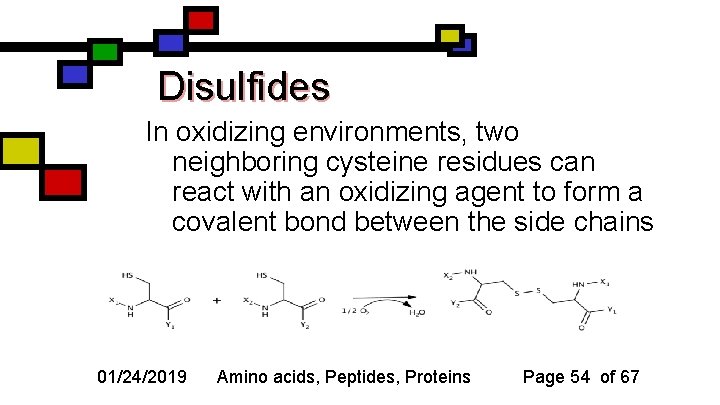
Disulfides In oxidizing environments, two neighboring cysteine residues can react with an oxidizing agent to form a covalent bond between the side chains 01/24/2019 Amino acids, Peptides, Proteins Page 54 of 67
What could this do? n n Can bring portions of a protein that are distant in amino acid sequence into close proximity with one another This can influence protein stability 01/24/2019 Amino acids, Peptides, Proteins Page 55 of 67
n n n Proteins have definable structures! This isn’t intuitively obvious Many biomolecules are much more flexible in terms of the number of conformations they actually take on in the real world Why are protein structures definable? n n They’re big enough to have an interior Hydrophobic in, hydrophilic out imposes order 01/24/2019 Amino acids, Peptides, Proteins Page 56 of 67
Levels of Protein Structure (CF&M § 4. 1 -4. 5) n We conventionally describe proteins at four levels of structure, from most local to most global— primary, secondary, tertiary, and quaternary 01/24/2019 Amino acids, Peptides, Proteins Page 57 of 67
The four levels of structure n n Primary: linear sequence of peptide units Secondary: main-chain H-bonds that define short-range order in structure Tertiary: three-dimensional fold of a single polypeptide Quaternary: Folds of multiple polypeptide chains to form a complete oligomeric unit 01/24/2019 Amino acids, Peptides, Proteins Page 58 of 67
Not all proteins have all four levels of structure n n n Monomeric proteins don’t have quaternary structure Tertiary structure: subsumed into 2 ndry structure for many structural proteins (keratin, silk fibroin, …) Some proteins (usually small ones) have no definite secondary or tertiary structure; they flop around! 01/24/2019 Amino acids, Peptides, Proteins Page 59 of 67
n n What does the primary structure look like? -ala-glu-val-thr-asp-pro-gly- … Can be determined by amino acid sequencing of the protein Can also be determined by sequencing the gene and then using the codon information to define the protein sequence Amino acid analysis means percentages; that’s less informative than the sequence 01/24/2019 Amino acids, Peptides, Proteins Page 60 of 67
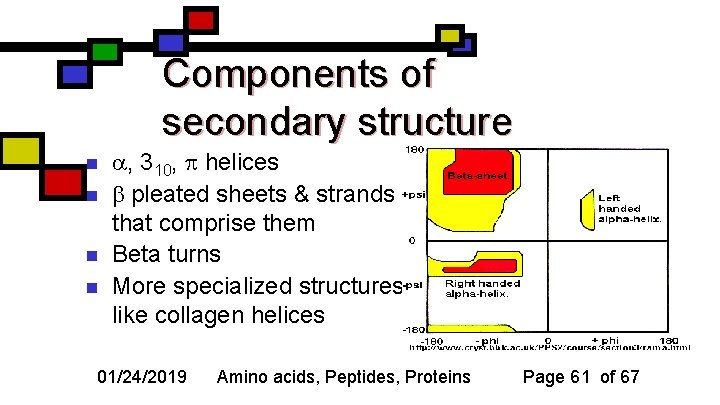
Components of secondary structure n n , 310, helices pleated sheets & strands that comprise them Beta turns More specialized structures like collagen helices 01/24/2019 Amino acids, Peptides, Proteins Page 61 of 67
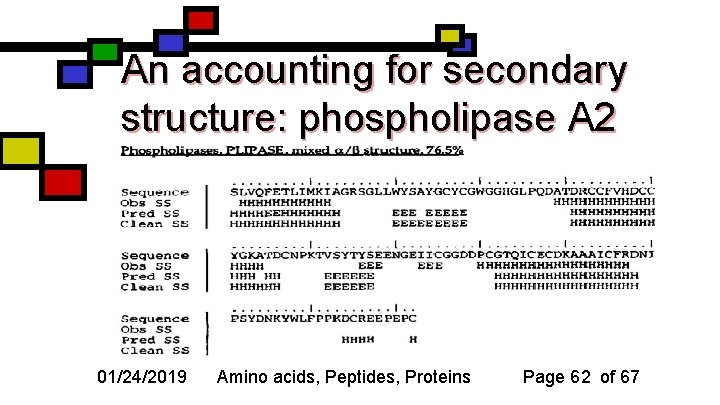
An accounting for secondary structure: phospholipase A 2 01/24/2019 Amino acids, Peptides, Proteins Page 62 of 67
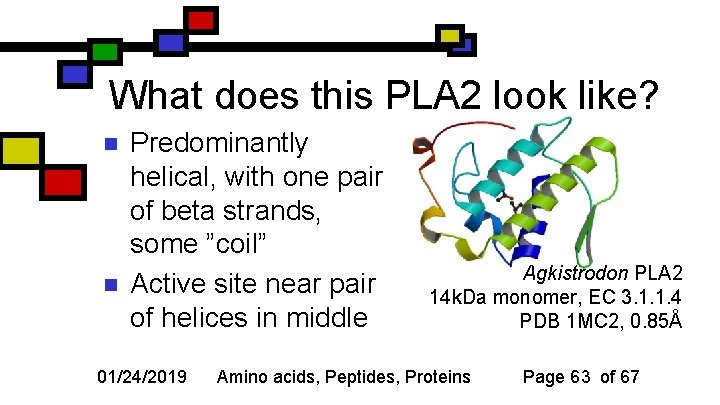
What does this PLA 2 look like? n n Predominantly helical, with one pair of beta strands, some ”coil” Active site near pair of helices in middle 01/24/2019 Agkistrodon PLA 2 14 k. Da monomer, EC 3. 1. 1. 4 PDB 1 MC 2, 0. 85Å Amino acids, Peptides, Proteins Page 63 of 67
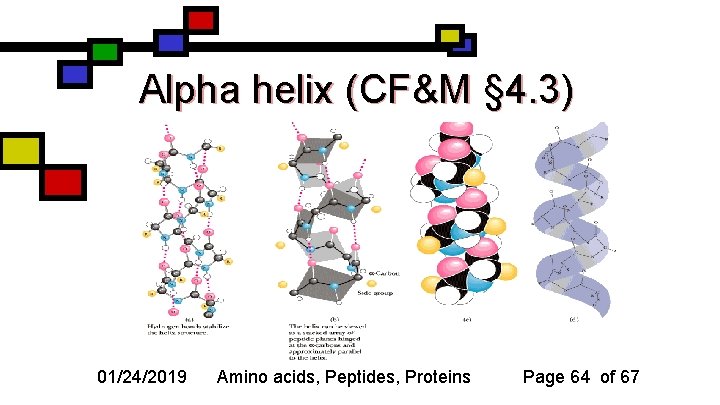
Alpha helix (CF&M § 4. 3) 01/24/2019 Amino acids, Peptides, Proteins Page 64 of 67
Characteristics of helices n n Hydrogen bonding from amino nitrogen to carbonyl oxygen in the residue 4 earlier in the chain 3. 6 residues per turn Amino acid side chains face outward ~ 10 residues long in globular proteins 01/24/2019 Amino acids, Peptides, Proteins Page 65 of 67

What would disrupt this? n n Not much: the side chains don’t bump into one another Proline residue will disrupt it: n n n Main-chain N can’t H-bond The ring forces a kink Glycines sometimes disrupt because they tend to be flexible 01/24/2019 Amino acids, Peptides, Proteins Page 66 of 67
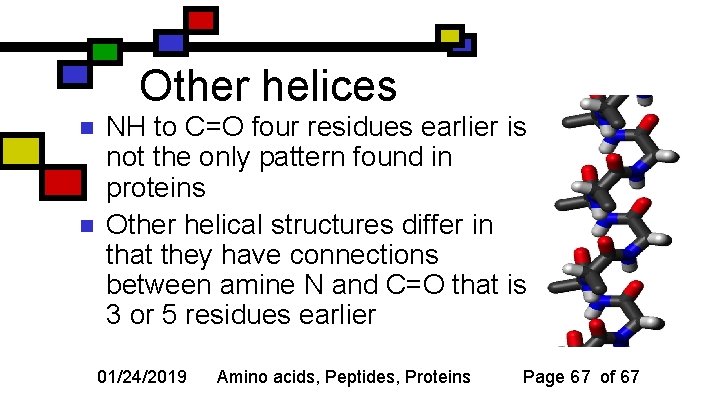
Other helices n n NH to C=O four residues earlier is not the only pattern found in proteins Other helical structures differ in that they have connections between amine N and C=O that is 3 or 5 residues earlier 01/24/2019 Amino acids, Peptides, Proteins Page 67 of 67
- Slides: 67