Amino acids Building blocks of proteins Amino acids

Amino acids: Building blocks of proteins Amino acids constitute the basic monomeric units of proteins, joined together by peptide bonds. The twenty standard amino acids can be arranged in several ways giving rise to numerous proteins having different structures and properties. Harini Chandra Affiliations
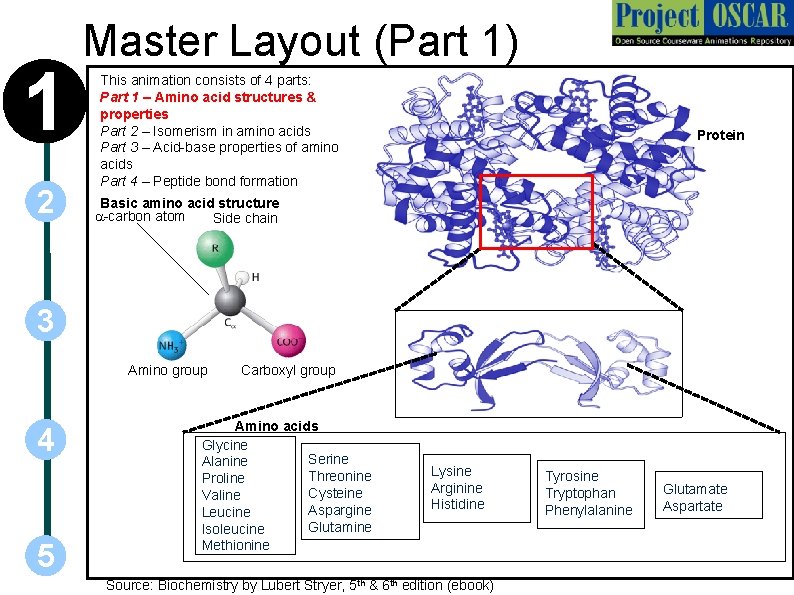
1 2 Master Layout (Part 1) This animation consists of 4 parts: Part 1 – Amino acid structures & properties Part 2 – Isomerism in amino acids Part 3 – Acid-base properties of amino acids Part 4 – Peptide bond formation Protein Basic amino acid structure a-carbon atom Side chain 3 Amino group 4 5 Carboxyl group Amino acids Glycine Serine Alanine Threonine Proline Cysteine Valine Aspargine Leucine Glutamine Isoleucine Methionine Lysine Arginine Histidine Source: Biochemistry by Lubert Stryer, 5 th & 6 th edition (ebook) Tyrosine Tryptophan Phenylalanine Glutamate Aspartate
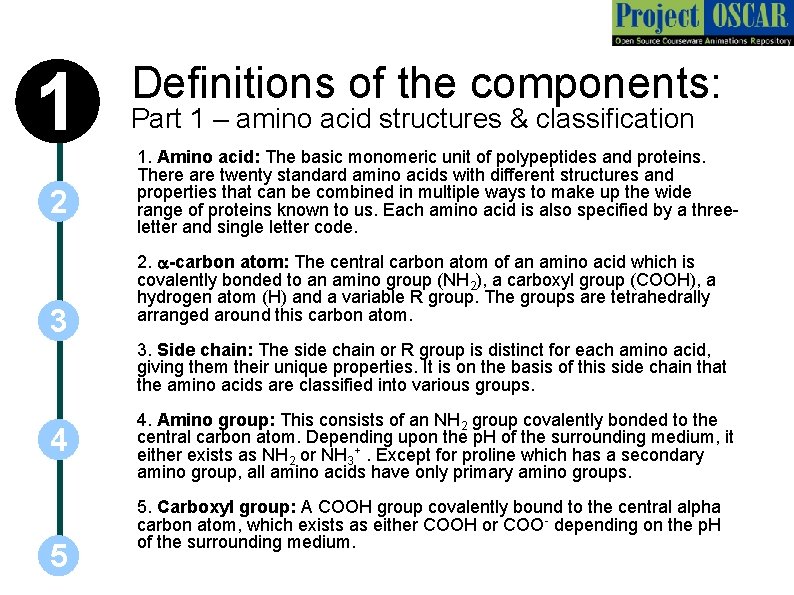
1 2 3 Definitions of the components: Part 1 – amino acid structures & classification 1. Amino acid: The basic monomeric unit of polypeptides and proteins. There are twenty standard amino acids with different structures and properties that can be combined in multiple ways to make up the wide range of proteins known to us. Each amino acid is also specified by a threeletter and single letter code. 2. a-carbon atom: The central carbon atom of an amino acid which is covalently bonded to an amino group (NH 2), a carboxyl group (COOH), a hydrogen atom (H) and a variable R group. The groups are tetrahedrally arranged around this carbon atom. 3. Side chain: The side chain or R group is distinct for each amino acid, giving them their unique properties. It is on the basis of this side chain that the amino acids are classified into various groups. 4 5 4. Amino group: This consists of an NH 2 group covalently bonded to the central carbon atom. Depending upon the p. H of the surrounding medium, it either exists as NH 2 or NH 3+. Except for proline which has a secondary amino group, all amino acids have only primary amino groups. 5. Carboxyl group: A COOH group covalently bound to the central alpha carbon atom, which exists as either COOH or COO- depending on the p. H of the surrounding medium.
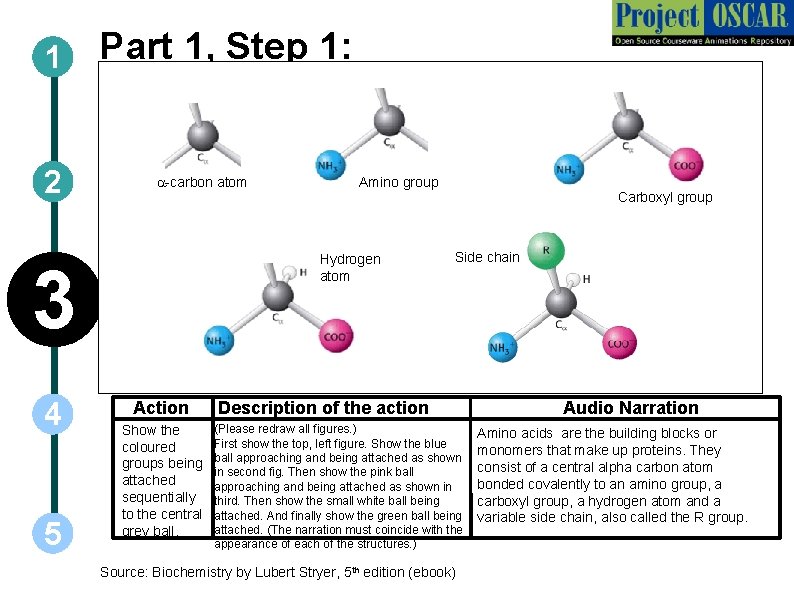
1 2 Part 1, Step 1: a-carbon atom Hydrogen atom 3 4 5 Amino group Action Show the coloured groups being attached sequentially to the central grey ball. Carboxyl group Side chain Description of the action (Please redraw all figures. ) First show the top, left figure. Show the blue ball approaching and being attached as shown in second fig. Then show the pink ball approaching and being attached as shown in third. Then show the small white ball being attached. And finally show the green ball being attached. (The narration must coincide with the appearance of each of the structures. ) Source: Biochemistry by Lubert Stryer, 5 th edition (ebook) Audio Narration Amino acids are the building blocks or monomers that make up proteins. They consist of a central alpha carbon atom bonded covalently to an amino group, a carboxyl group, a hydrogen atom and a variable side chain, also called the R group.
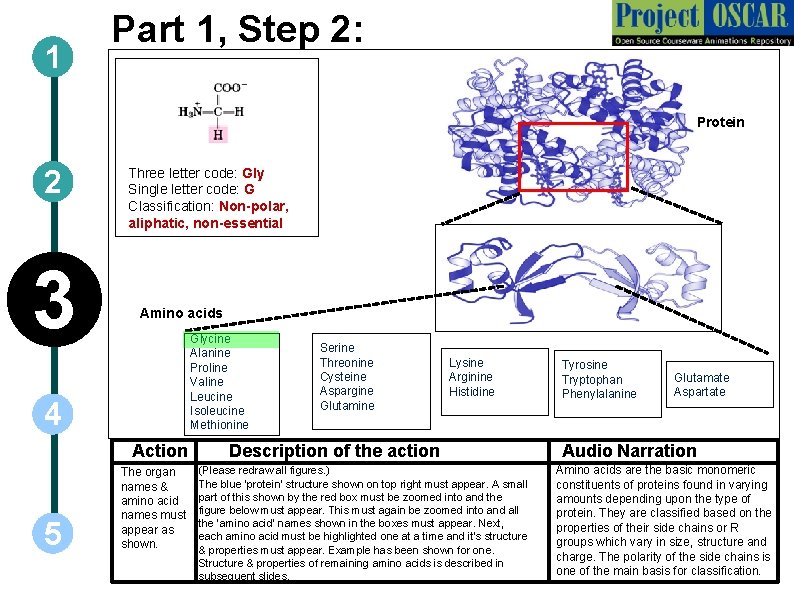
1 Part 1, Step 2: Protein 2 3 Three letter code: Gly Single letter code: G Classification: Non-polar, aliphatic, non-essential Amino acids Glycine Alanine Proline Valine Leucine Isoleucine Methionine 4 Action 5 The organ names & amino acid names must appear as shown. Serine Threonine Cysteine Aspargine Glutamine Lysine Arginine Histidine Description of the action (Please redraw all figures. ) The blue ‘protein’ structure shown on top right must appear. A small part of this shown by the red box must be zoomed into and the figure below must appear. This must again be zoomed into and all the ‘amino acid’ names shown in the boxes must appear. Next, each amino acid must be highlighted one at a time and it’s structure & properties must appear. Example has been shown for one. Structure & properties of remaining amino acids is described in subsequent slides. Tyrosine Tryptophan Phenylalanine Glutamate Aspartate Audio Narration Amino acids are the basic monomeric constituents of proteins found in varying amounts depending upon the type of protein. They are classified based on the properties of their side chains or R groups which vary in size, structure and charge. The polarity of the side chains is one of the main basis for classification.
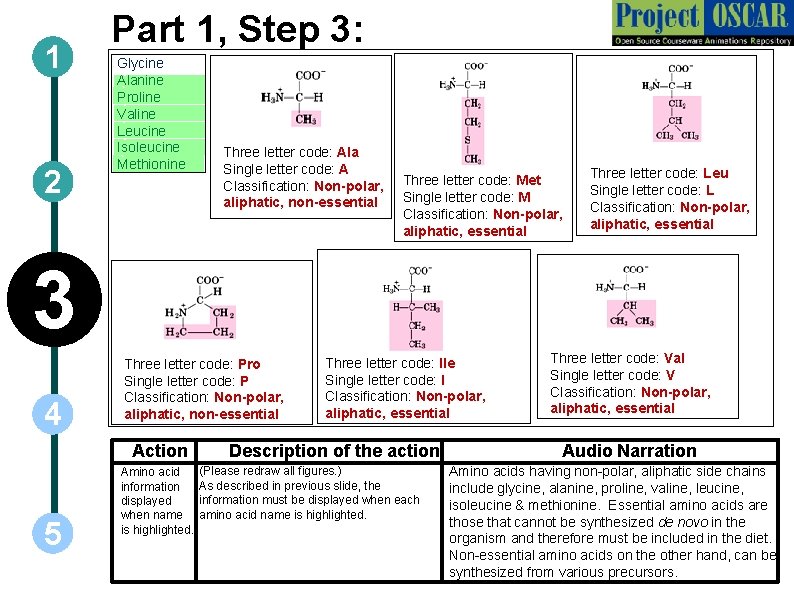
1 2 Part 1, Step 3: Glycine Alanine ` ` Proline ` Valine ` Leucine ` Isoleucine ` Methionine Three letter code: Ala Single letter code: A Classification: Non-polar, aliphatic, non-essential Three letter code: Met Single letter code: M Classification: Non-polar, aliphatic, essential Three letter code: Leu Single letter code: L Classification: Non-polar, aliphatic, essential 3 4 Three letter code: Pro Single letter code: P Classification: Non-polar, aliphatic, non-essential Action 5 Amino acid information displayed when name is highlighted. Three letter code: Ile Single letter code: I Classification: Non-polar, aliphatic, essential Description of the action (Please redraw all figures. ) As described in previous slide, the information must be displayed when each amino acid name is highlighted. Three letter code: Val Single letter code: V Classification: Non-polar, aliphatic, essential Audio Narration Amino acids having non-polar, aliphatic side chains include glycine, alanine, proline, valine, leucine, isoleucine & methionine. Essential amino acids are those that cannot be synthesized de novo in the organism and therefore must be included in the diet. Non-essential amino acids on the other hand, can be synthesized from various precursors.
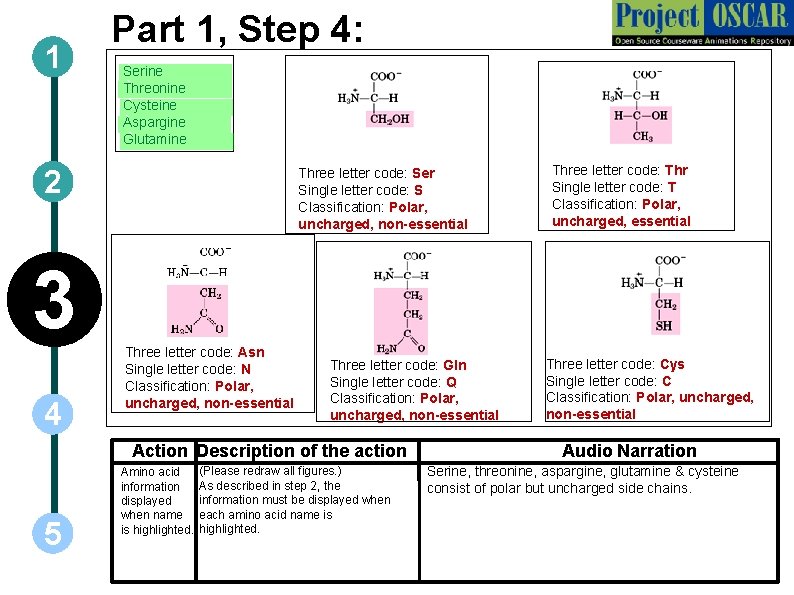
1 Part 1, Step 4: ` Serine Threonine ` Cysteine ` Aspargine ` Glutamine ` 2 Three letter code: Ser Single letter code: S Classification: Polar, uncharged, non-essential ` 3 4 Three letter code: Asn Single letter code: N Classification: Polar, uncharged, non-essential Three letter code: Gln Single letter code: Q Classification: Polar, uncharged, non-essential Action Description of the action 5 Amino acid information displayed when name is highlighted. (Please redraw all figures. ) As described in step 2, the information must be displayed when each amino acid name is highlighted. Three letter code: Thr Single letter code: T Classification: Polar, uncharged, essential Three letter code: Cys Single letter code: C Classification: Polar, uncharged, non-essential Audio Narration Serine, threonine, aspargine, glutamine & cysteine consist of polar but uncharged side chains.
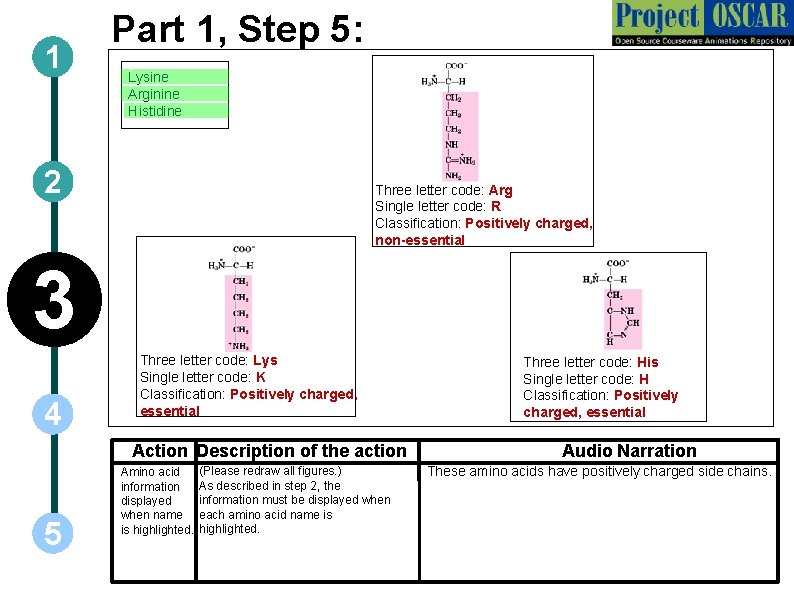
1 Part 1, Step 5: ` Lysine ` Arginine ` Histidine 2 Three letter code: Arg Single letter code: R Classification: Positively charged, non-essential 3 4 Three letter code: Lys Single letter code: K Classification: Positively charged, essential Action Description of the action 5 Amino acid information displayed when name is highlighted. (Please redraw all figures. ) As described in step 2, the information must be displayed when each amino acid name is highlighted. Three letter code: His Single letter code: H Classification: Positively charged, essential Audio Narration These amino acids have positively charged side chains.
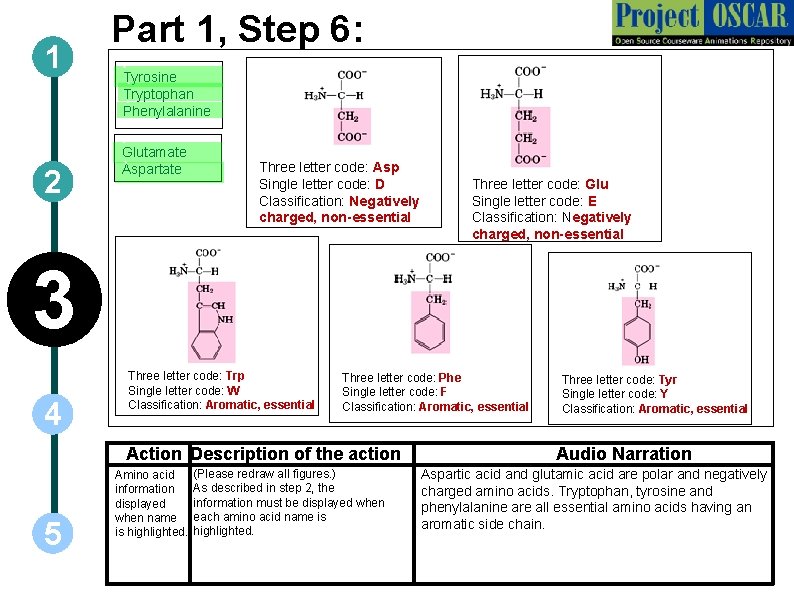
1 2 Part 1, Step 6: `Tyrosine ` Tryptophan ` Phenylalanine `Glutamate Aspartate ` Three letter code: Asp Single letter code: D Classification: Negatively charged, non-essential Three letter code: Glu Single letter code: E Classification: Negatively charged, non-essential 3 4 Three letter code: Trp Single letter code: W Classification: Aromatic, essential Three letter code: Phe Single letter code: F Classification: Aromatic, essential Action Description of the action 5 Amino acid information displayed when name is highlighted. (Please redraw all figures. ) As described in step 2, the information must be displayed when each amino acid name is highlighted. Three letter code: Tyr Single letter code: Y Classification: Aromatic, essential Audio Narration Aspartic acid and glutamic acid are polar and negatively charged amino acids. Tryptophan, tyrosine and phenylalanine are all essential amino acids having an aromatic side chain.
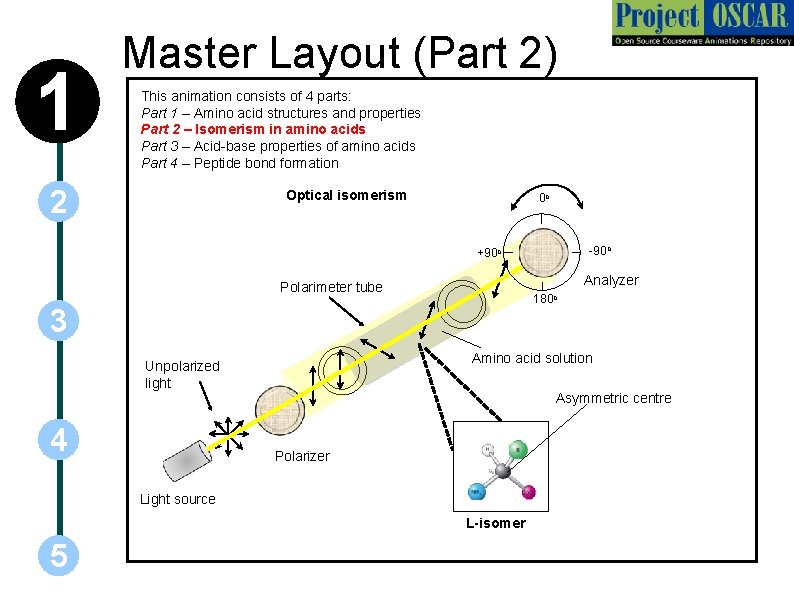
1 Master Layout (Part 2) This animation consists of 4 parts: Part 1 – Amino acid structures and properties Part 2 – Isomerism in amino acids Part 3 – Acid-base properties of amino acids Part 4 – Peptide bond formation 2 Optical isomerism 0 o -90 o +90 o Analyzer Polarimeter tube 180 o 3 Amino acid solution Unpolarized light 4 Asymmetric centre Polarizer Light source L-isomer 5

1 2 3 4 Definitions of the components: Part 2 – isomerism in amino acids 1. Optical isomerism: Chiral molecules interact with plane polarized light such that they rotate the plane of polarization either in the clockwise or counter-clockwise direction. Depending on which direction the molecule rotates the plane of polarization, they are designated as (+) or dextrorotatory (d) and (-) or laevorotatory (l). This nomenclature is not the same as the D and L designations, which refer to the absolute configurations specified on the basis of their relationship with D and L glyceraldehyde. Majority of the amino acids found in proteins are of the l configuration. 2. Asymmetric centre: Except for glycine, the a-carbon atom of all other amino acids have four different groups attached to it in a tetrahedral arrangement. This carbon atom is known as an asymmetric or chiral centre and gives rise to the phenomenon of optical isomerism. There are two amino acids, threonine and isoleucine, that have two chiral centres. 3. Enantiomers: Molecules with a chiral centre have a non-superimposable mirror image and the two forms of this molecule are known as enantiomers. They are designated as D and L or R (rectus) and S (sinister) depending on the arrangement of groups around the asymmetric carbon atom. R and S nomenclature is based on priority of atomic numbers of atoms directly attached to the central asymmetric centre. 4. Light source: A source of light that gives out unpolarized light i. e. light in which the electric vector is oriented in random, unpredictable ways. 5. Unpolarized light: Light in which the orientation of the electric field vectors is random and uncorrelated is said to be unpolarized. 5 6. Polarizer: A polarizer is a device that is capable of converting unpolarized light into plane polarized light such that the electromagnetic waves are oriented in only a single plane.
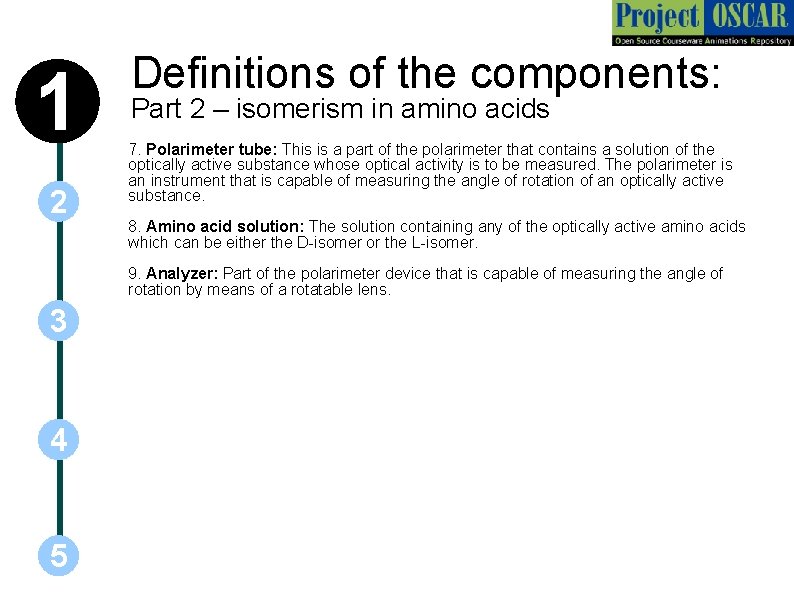
1 2 Definitions of the components: Part 2 – isomerism in amino acids 7. Polarimeter tube: This is a part of the polarimeter that contains a solution of the optically active substance whose optical activity is to be measured. The polarimeter is an instrument that is capable of measuring the angle of rotation of an optically active substance. 8. Amino acid solution: The solution containing any of the optically active amino acids which can be either the D-isomer or the L-isomer. 9. Analyzer: Part of the polarimeter device that is capable of measuring the angle of rotation by means of a rotatable lens. 3 4 5
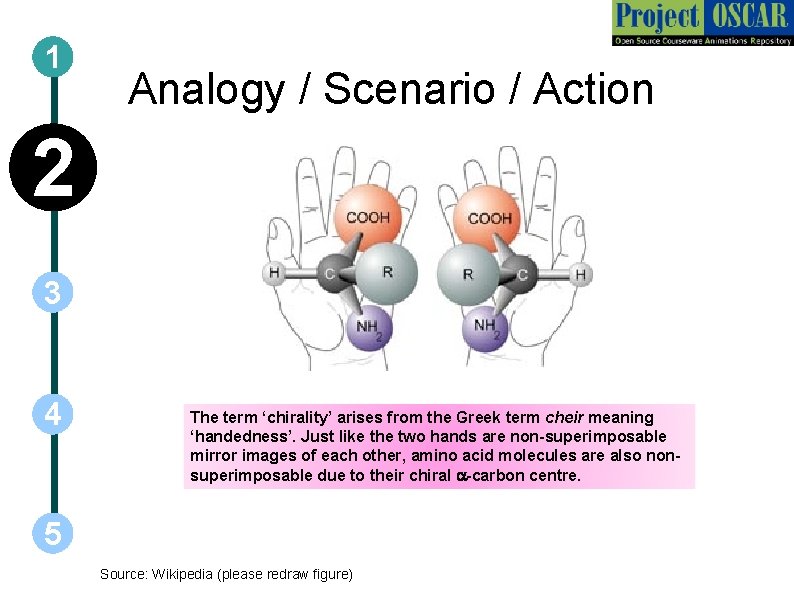
1 Analogy / Scenario / Action 2 3 4 The term ‘chirality’ arises from the Greek term cheir meaning ‘handedness’. Just like the two hands are non-superimposable mirror images of each other, amino acid molecules are also nonsuperimposable due to their chiral a-carbon centre. 5 Source: Wikipedia (please redraw figure)
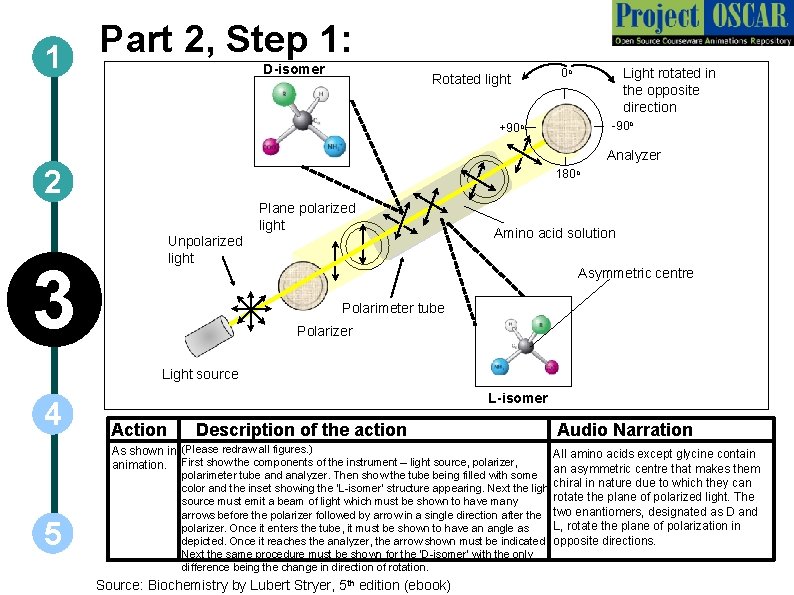
Part 2, Step 1: 1 D-isomer Rotated light Light rotated in the opposite direction 0 o -90 o +90 o Analyzer 2 180 o Plane polarized light Unpolarized light 3 Amino acid solution Asymmetric centre Polarimeter tube Polarizer Light source 4 L-isomer Action Description of the action As shown in (Please redraw all figures. ) animation. First show the components of the instrument – light source, polarizer, 5 Audio Narration All amino acids except glycine contain an asymmetric centre that makes them polarimeter tube and analyzer. Then show the tube being filled with some color and the inset showing the ‘L-isomer’ structure appearing. Next the light chiral in nature due to which they can rotate the plane of polarized light. The source must emit a beam of light which must be shown to have many arrows before the polarizer followed by arrow in a single direction after the two enantiomers, designated as D and L, rotate the plane of polarization in polarizer. Once it enters the tube, it must be shown to have an angle as depicted. Once it reaches the analyzer, the arrow shown must be indicated. opposite directions. Next the same procedure must be shown for the ‘D-isomer’ with the only difference being the change in direction of rotation. Source: Biochemistry by Lubert Stryer, 5 th edition (ebook)
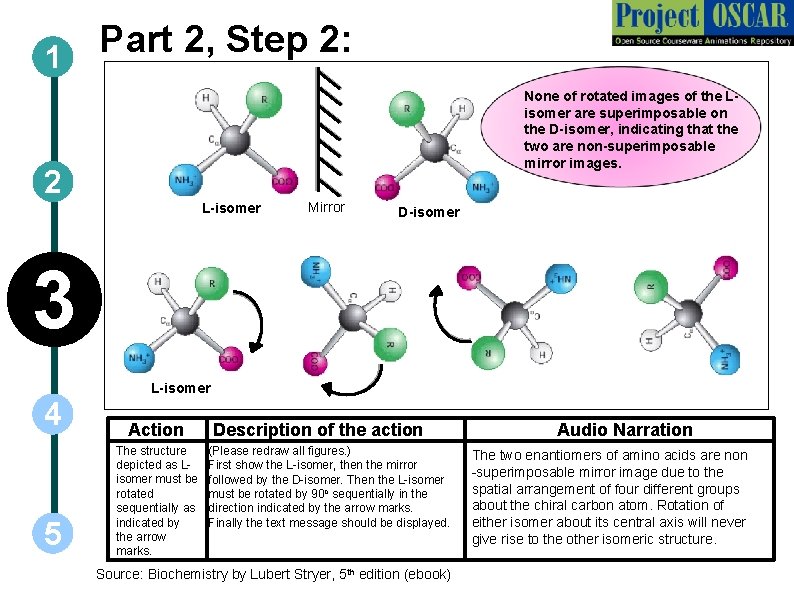
Part 2, Step 2: 1 None of rotated images of the Lisomer are superimposable on the D-isomer, indicating that the two are non-superimposable mirror images. 2 L-isomer Mirror D-isomer 3 L-isomer 4 5 Action The structure depicted as Lisomer must be rotated sequentially as indicated by the arrow marks. Description of the action (Please redraw all figures. ) First show the L-isomer, then the mirror followed by the D-isomer. Then the L-isomer must be rotated by 90 o sequentially in the direction indicated by the arrow marks. Finally the text message should be displayed. Source: Biochemistry by Lubert Stryer, 5 th edition (ebook) Audio Narration The two enantiomers of amino acids are non -superimposable mirror image due to the spatial arrangement of four different groups about the chiral carbon atom. Rotation of either isomer about its central axis will never give rise to the other isomeric structure.
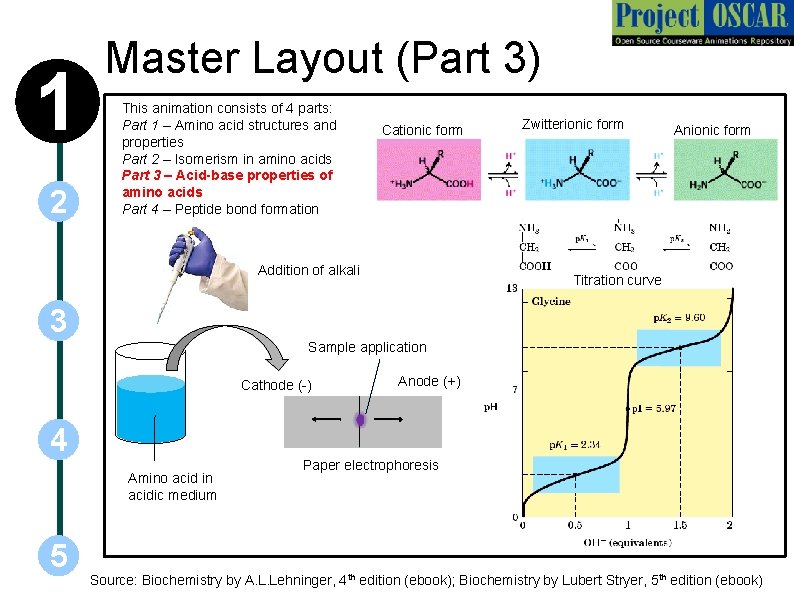
1 2 Master Layout (Part 3) This animation consists of 4 parts: Part 1 – Amino acid structures and properties Part 2 – Isomerism in amino acids Part 3 – Acid-base properties of amino acids Part 4 – Peptide bond formation Cationic form Addition of alkali Zwitterionic form Anionic form Titration curve 3 Sample application Cathode (-) 4 Amino acid in acidic medium 5 Anode (+) Paper electrophoresis Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook); Biochemistry by Lubert Stryer, 5 th edition (ebook)

1 Definitions of the components: Part 3 – acid base properties of amino acids 1. Cationic form: All amino acids exist in the completely protonated form in acidic medium, known as the cationic form. Both amino and carboxyl groups are protonated here. 2 2. Zwitterionic form: The state in which the amino acid has no net charge is known as the zwitterion. It is neutral due to the presence of NH 3+ and COO- groups. 3. Anionic form: In a highly alkaline medium, all amino acids exist in their anionic form due to the presence of COO- group. 3 4 5 4. Amino acid in acidic medium: To obtain the titration curve of an amino acid, it is first taken in a highly acidic medium such that it exists entirely in the cationic form. 5. Addition of alkali: 0. 1 N Na. OH solution is added in drops to the acidic amino acid solution. This progressively converts the cationic form into zwitterion and finally into the anionic form. 6. Paper electrophoresis: One of the easiest and quickest methods to detect whether the molecule of interest is present in its positive, negative or neutral state. The sample is applied at the centre of a moistened strip of filter paper and current passed through it. Depending upon the net charge of the molecule, it will either migrate towards the cathode or anode or remain stationary at the point of application. Amino acid solutions although colourless can be detected after electrophoresis by means of the ninhydrin reagent which gives it a violet colour. 7. Cathode and anode: These are oppositely charged electrodes. The cathode is negatively charged while the anode is positively charged.

1 2 Definitions of the components: Part 3 – acid base properties of amino acids 8. Sample application: The sample to be analyzed is applied to the centre of the electrophoresis strip after which current is passed through it. 9. Titration curve: The number of equivalents of alkali being consumed during the process of addition of alkali to the amino acid solution is plotted against p. H of the solution in the flask to yield a unique titration curve for each amino acid. The titration curve depicted corresponds to that of glycine. 3 4 5 10. p. K: Negative log of the p. H at which the catonic and neutral forms inter-convert (p. K 1) and neutral and anionic forms inter-convert (p. K 2).
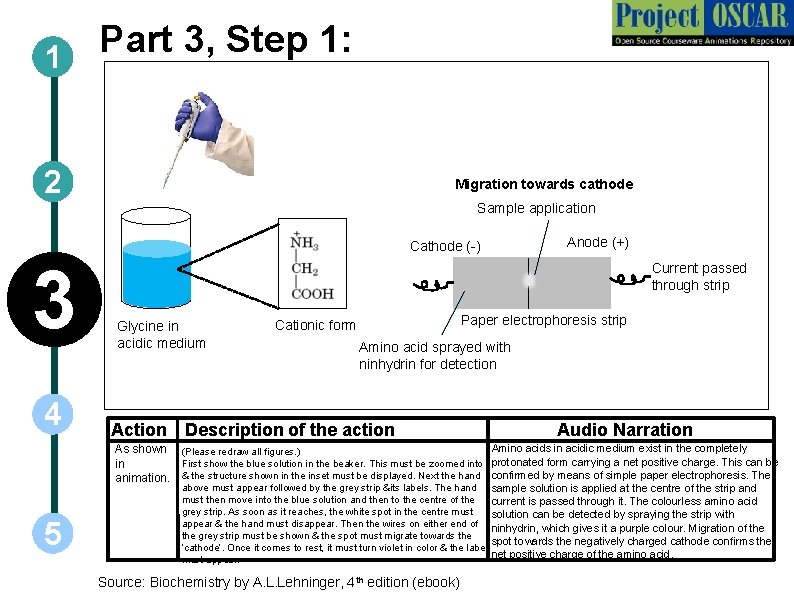
Part 3, Step 1: 1 2 Migration towards cathode Sample application Anode (+) Cathode (-) 3 4 Current passed through strip Glycine in acidic medium Paper electrophoresis strip Cationic form Amino acid sprayed with ninhydrin for detection Action Description of the action Audio Narration Amino acids in acidic medium exist in the completely As shown (Please redraw all figures. ) First show the blue solution in the beaker. This must be zoomed into protonated form carrying a net positive charge. This can be in animation. & the structure shown in the inset must be displayed. Next the hand confirmed by means of simple paper electrophoresis. The 5 above must appear followed by the grey strip &its labels. The hand must then move into the blue solution and then to the centre of the grey strip. As soon as it reaches, the white spot in the centre must appear & the hand must disappear. Then the wires on either end of the grey strip must be shown & the spot must migrate towards the ‘cathode’. Once it comes to rest, it must turn violet in color & the label must appear. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) sample solution is applied at the centre of the strip and current is passed through it. The colourless amino acid solution can be detected by spraying the strip with ninhydrin, which gives it a purple colour. Migration of the spot towards the negatively charged cathode confirms the net positive charge of the amino acid.
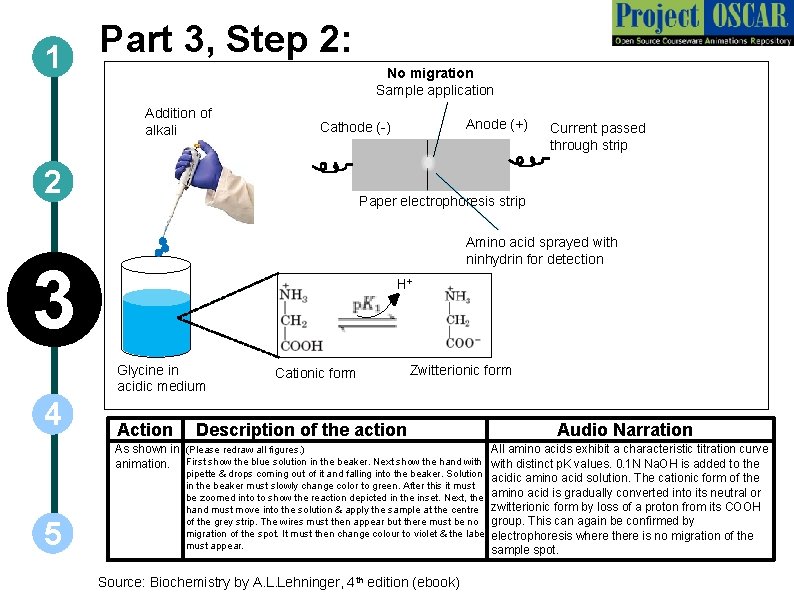
Part 3, Step 2: 1 Addition of alkali Current passed through strip Paper electrophoresis strip Amino acid sprayed with ninhydrin for detection 3 H+ Glycine in acidic medium 5 Anode (+) Cathode (-) 2 4 No migration Sample application Action Cationic form Zwitterionic form Description of the action Audio Narration As shown in (Please redraw all figures. ) All amino acids exhibit a characteristic titration curve animation. First show the blue solution in the beaker. Next show the hand with distinct p. K values. 0. 1 N Na. OH is added to the pipette & drops coming out of it and falling into the beaker. Solution acidic amino acid solution. The cationic form of the in the beaker must slowly change color to green. After this it must be zoomed into to show the reaction depicted in the inset. Next, the amino acid is gradually converted into its neutral or hand must move into the solution & apply the sample at the centre zwitterionic form by loss of a proton from its COOH of the grey strip. The wires must then appear but there must be no group. This can again be confirmed by migration of the spot. It must then change colour to violet & the label electrophoresis where there is no migration of the must appear. sample spot. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook)
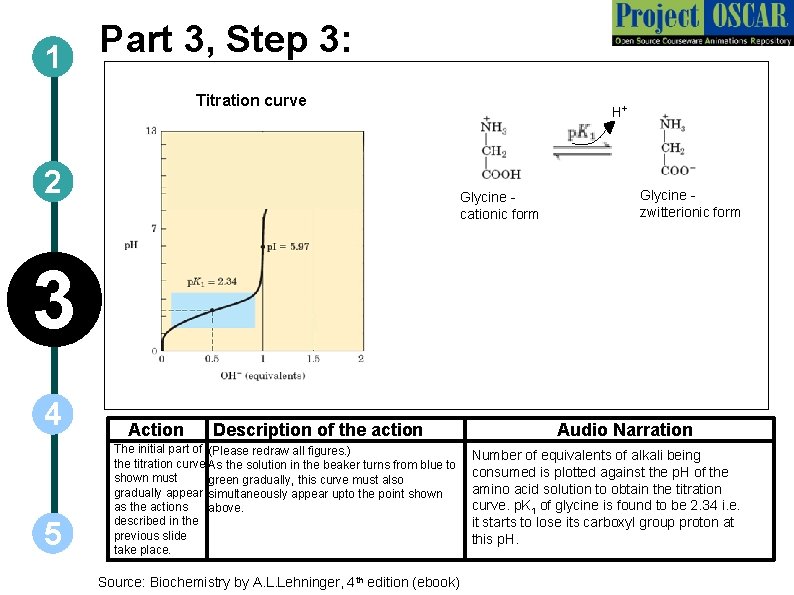
Part 3, Step 3: 1 Titration curve 2 H+ Glycine cationic form Glycine zwitterionic form 3 4 5 Action Description of the action The initial part of (Please redraw all figures. ) the titration curve As the solution in the beaker turns from blue to shown must green gradually, this curve must also gradually appear simultaneously appear upto the point shown as the actions above. described in the previous slide take place. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Audio Narration Number of equivalents of alkali being consumed is plotted against the p. H of the amino acid solution to obtain the titration curve. p. K 1 of glycine is found to be 2. 34 i. e. it starts to lose its carboxyl group proton at this p. H.
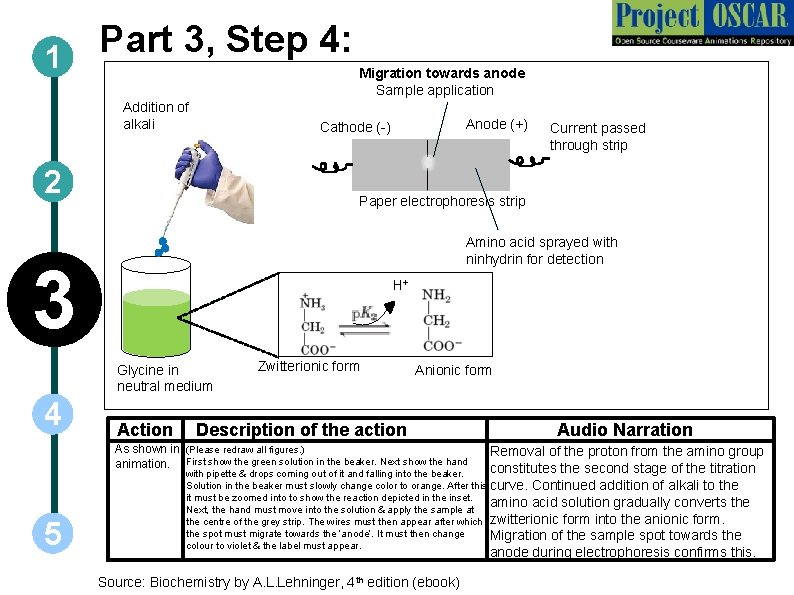
Part 3, Step 4: 1 Addition of alkali Migration towards anode Sample application 2 Amino acid sprayed with ninhydrin for detection H+ Glycine in neutral medium Action Zwitterionic form Anionic form Description of the action As shown in (Please redraw all figures. ) animation. First show the green solution in the beaker. Next show the hand 5 Current passed through strip Paper electrophoresis strip 3 4 Anode (+) Cathode (-) Audio Narration Removal of the proton from the amino group constitutes the second stage of the titration with pipette & drops coming out of it and falling into the beaker. Solution in the beaker must slowly change color to orange. After this curve. Continued addition of alkali to the it must be zoomed into to show the reaction depicted in the inset. amino acid solution gradually converts the Next, the hand must move into the solution & apply the sample at the centre of the grey strip. The wires must then appear after which zwitterionic form into the anionic form. the spot must migrate towards the ‘anode’. It must then change Migration of the sample spot towards the colour to violet & the label must appear. anode during electrophoresis confirms this. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook)
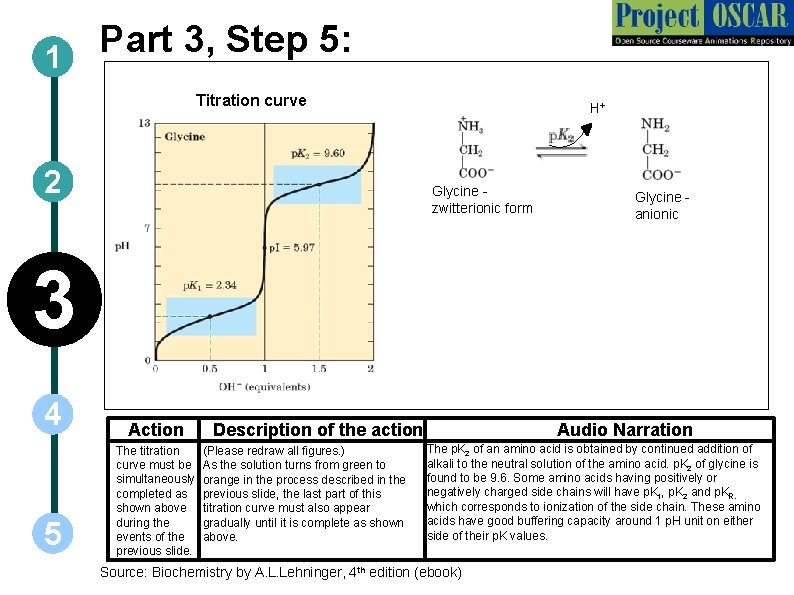
Part 3, Step 5: 1 Titration curve 2 H+ Glycine zwitterionic form Glycine anionic 3 4 5 Action The titration curve must be simultaneously completed as shown above during the events of the previous slide. Description of the action (Please redraw all figures. ) As the solution turns from green to orange in the process described in the previous slide, the last part of this titration curve must also appear gradually until it is complete as shown above. Audio Narration The p. K 2 of an amino acid is obtained by continued addition of alkali to the neutral solution of the amino acid. p. K 2 of glycine is found to be 9. 6. Some amino acids having positively or negatively charged side chains will have p. K 1, p. K 2 and p. KR, which corresponds to ionization of the side chain. These amino acids have good buffering capacity around 1 p. H unit on either side of their p. K values. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook)
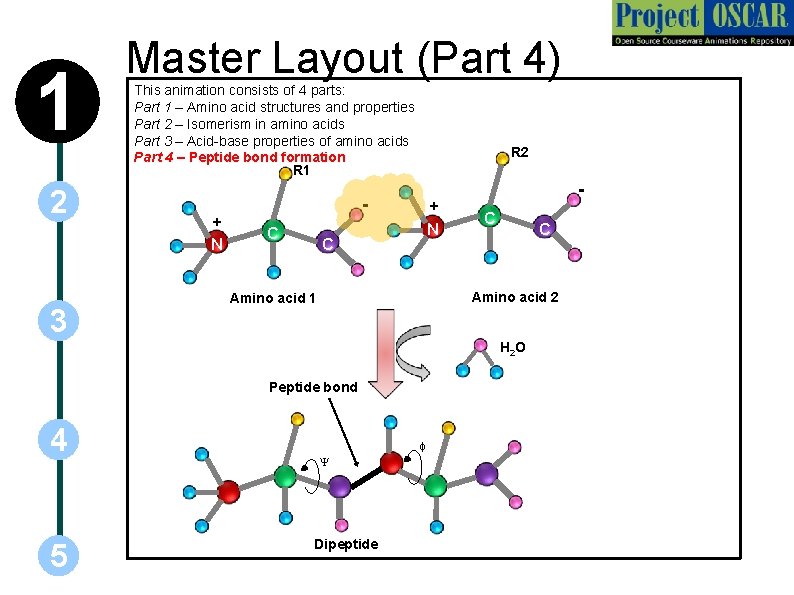
1 2 Master Layout (Part 4) This animation consists of 4 parts: Part 1 – Amino acid structures and properties Part 2 – Isomerism in amino acids Part 3 – Acid-base properties of amino acids Part 4 – Peptide bond formation R 1 + N 3 R 2 C + N C C C Amino acid 2 Amino acid 1 H 2 O Peptide bond 4 5 f Y Dipeptide
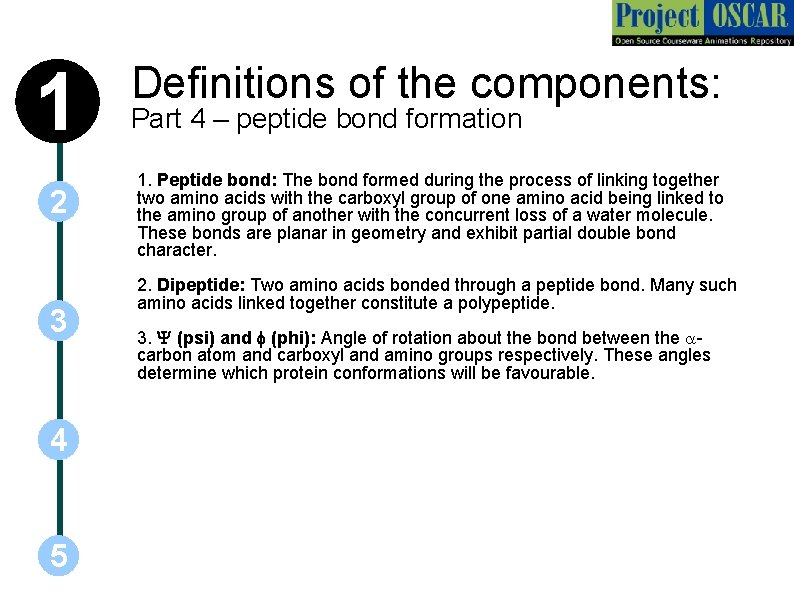
1 2 3 4 5 Definitions of the components: Part 4 – peptide bond formation 1. Peptide bond: The bond formed during the process of linking together two amino acids with the carboxyl group of one amino acid being linked to the amino group of another with the concurrent loss of a water molecule. These bonds are planar in geometry and exhibit partial double bond character. 2. Dipeptide: Two amino acids bonded through a peptide bond. Many such amino acids linked together constitute a polypeptide. 3. Y (psi) and f (phi): Angle of rotation about the bond between the acarbon atom and carboxyl and amino groups respectively. These angles determine which protein conformations will be favourable.
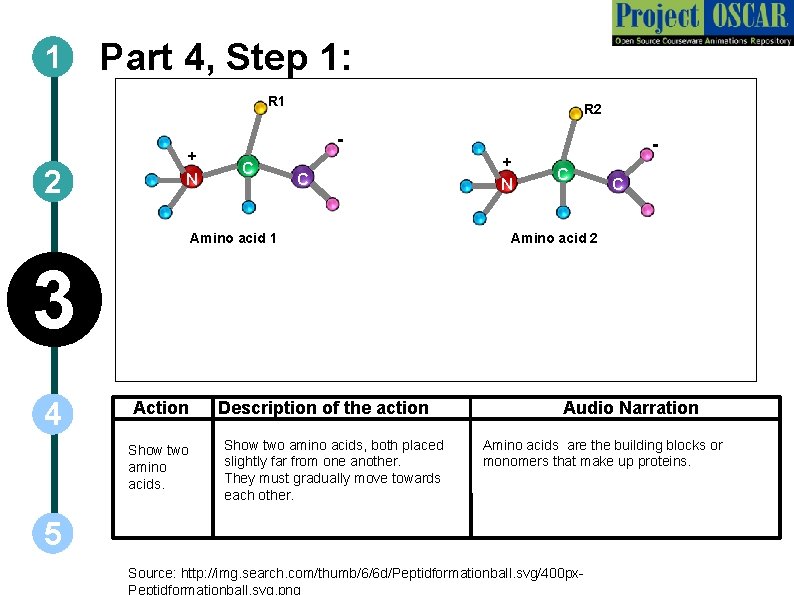
1 Part 4, Step 1: R 1 2 + N R 2 C + C Amino acid 1 N C C Amino acid 2 3 4 Action Show two amino acids. Description of the action Show two amino acids, both placed slightly far from one another. They must gradually move towards each other. Audio Narration Amino acids are the building blocks or monomers that make up proteins. 5 Source: http: //img. search. com/thumb/6/6 d/Peptidformationball. svg/400 px. Peptidformationball. svg. png
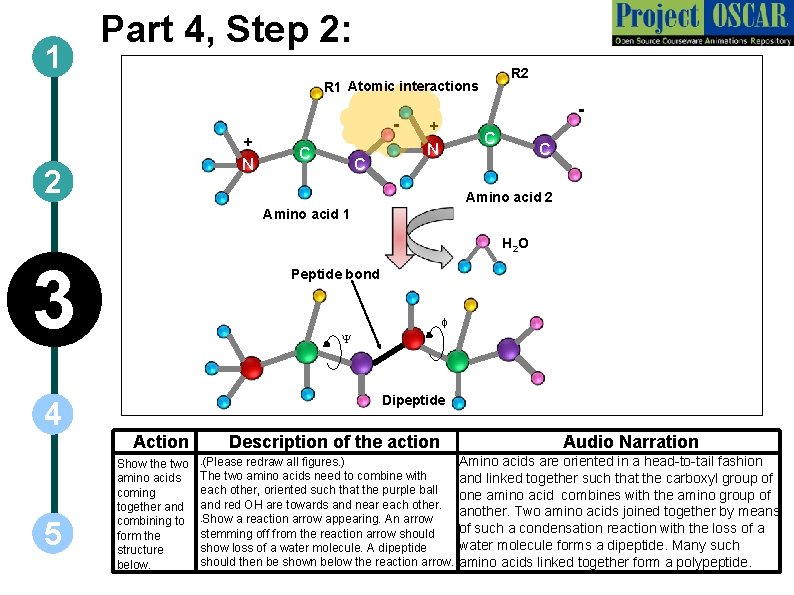
1 Part 4, Step 2: R 2 R 1 Atomic interactions + N 2 C C - + C N C Amino acid 2 Amino acid 1 H 2 O 3 4 5 Peptide bond f Y Dipeptide Action Show the two amino acids coming together and combining to form the structure below. Description of the action (Please redraw all figures. ) The two amino acids need to combine with each other, oriented such that the purple ball and red OH are towards and near each other. • Show a reaction arrow appearing. An arrow stemming off from the reaction arrow should show loss of a water molecule. A dipeptide should then be shown below the reaction arrow. • Audio Narration Amino acids are oriented in a head-to-tail fashion and linked together such that the carboxyl group of one amino acid combines with the amino group of another. Two amino acids joined together by means of such a condensation reaction with the loss of a water molecule forms a dipeptide. Many such amino acids linked together form a polypeptide.
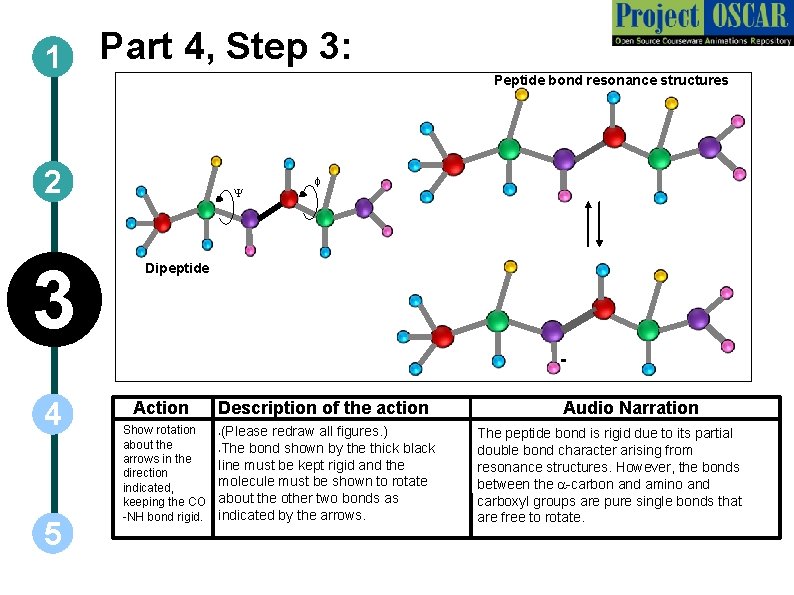
1 Part 4, Step 3: Peptide bond resonance structures 2 3 Y f Dipeptide - 4 5 Action Show rotation about the arrows in the direction indicated, keeping the CO -NH bond rigid. Description of the action (Please redraw all figures. ) • The bond shown by the thick black line must be kept rigid and the molecule must be shown to rotate about the other two bonds as indicated by the arrows. • Audio Narration The peptide bond is rigid due to its partial double bond character arising from resonance structures. However, the bonds between the a-carbon and amino and carboxyl groups are pure single bonds that are free to rotate.
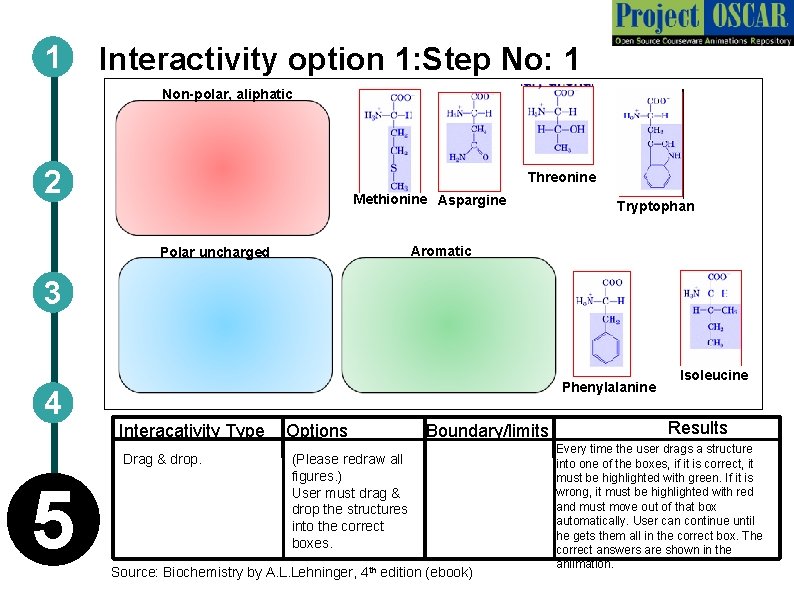
1 Interactivity option 1: Step No: 1 Non-polar, aliphatic 2 Threonine Methionine Aspargine Tryptophan Aromatic Polar uncharged 3 Phenylalanine 4 Interacativity Type Drag & drop. 5 Options Boundary/limits (Please redraw all figures. ) User must drag & drop the structures into the correct boxes. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Isoleucine Results Every time the user drags a structure into one of the boxes, if it is correct, it must be highlighted with green. If it is wrong, it must be highlighted with red and must move out of that box automatically. User can continue until he gets them all in the correct box. The correct answers are shown in the aniimation.
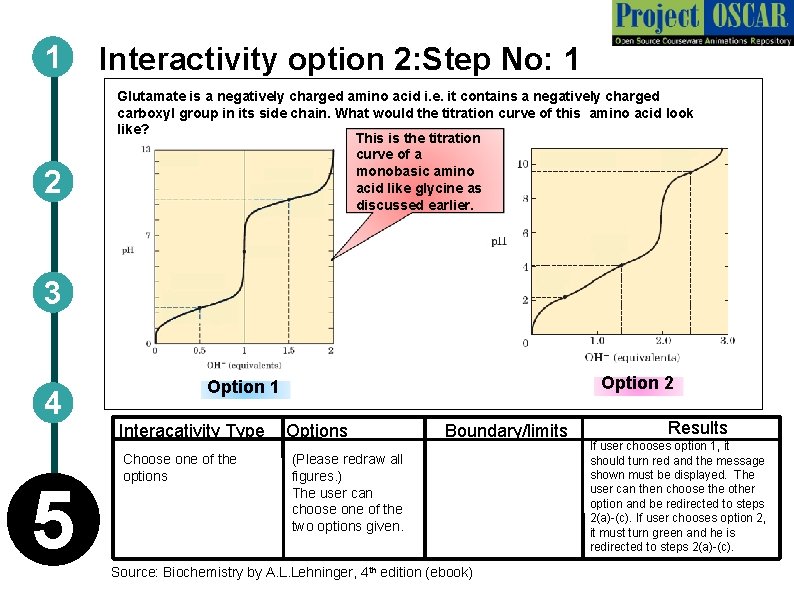
1 2 Interactivity option 2: Step No: 1 Glutamate is a negatively charged amino acid i. e. it contains a negatively charged carboxyl group in its side chain. What would the titration curve of this amino acid look like? This is the titration curve of a monobasic amino acid like glycine as discussed earlier. 3 4 Interacativity Type 5 Option 2 Option 1 Choose one of the options Options Boundary/limits (Please redraw all figures. ) The user can choose one of the two options given. Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Results If user chooses option 1, it should turn red and the message shown must be displayed. The user can then choose the other option and be redirected to steps 2(a)-(c). If user chooses option 2, it must turn green and he is redirected to steps 2(a)-(c).
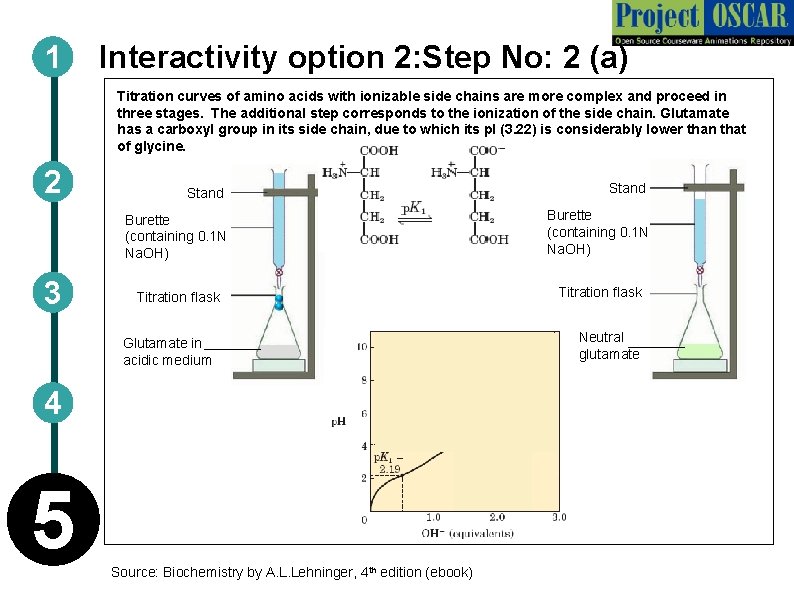
1 Interactivity option 2: Step No: 2 (a) Titration curves of amino acids with ionizable side chains are more complex and proceed in three stages. The additional step corresponds to the ionization of the side chain. Glutamate has a carboxyl group in its side chain, due to which its p. I (3. 22) is considerably lower than that of glycine. 2 3 Stand Burette (containing 0. 1 N Na. OH) Titration flask Glutamate in acidic medium 4 5 Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Neutral glutamate
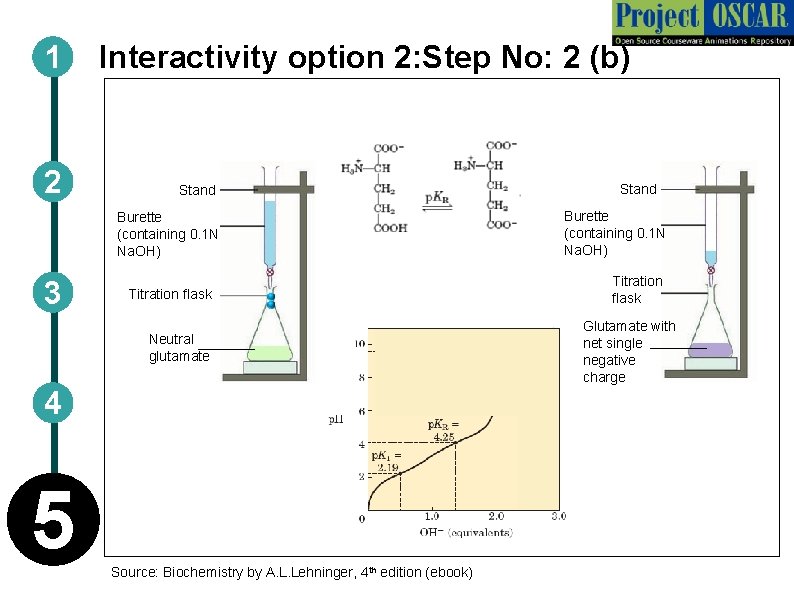
1 2 Interactivity option 2: Step No: 2 (b) Stand Burette (containing 0. 1 N Na. OH) 3 Titration flask Neutral glutamate 4 5 Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Stand Burette (containing 0. 1 N Na. OH) Titration flask Glutamate with net single negative charge
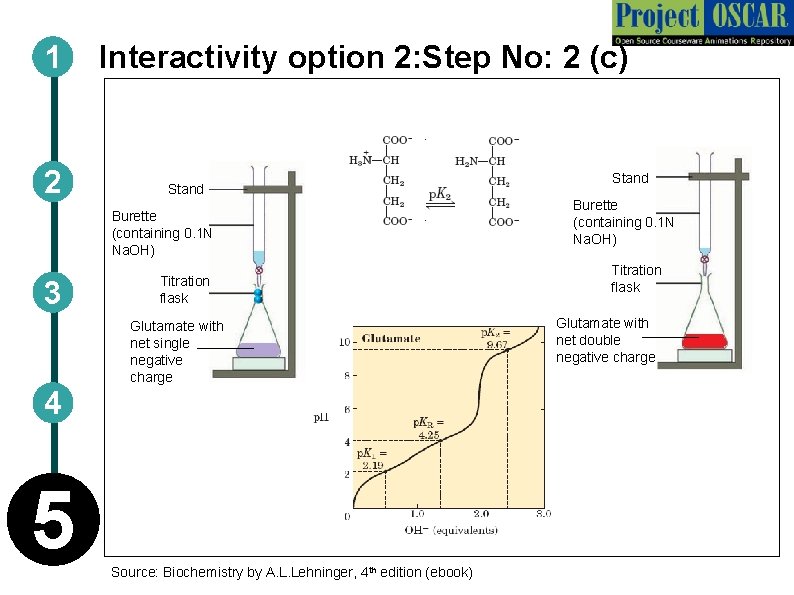
1 2 Interactivity option 2: Step No: 2 (c) Stand Burette (containing 0. 1 N Na. OH) 3 Titration flask Glutamate with net single negative charge 4 5 Source: Biochemistry by A. L. Lehninger, 4 th edition (ebook) Stand Burette (containing 0. 1 N Na. OH) Titration flask Glutamate with net double negative charge

1 Questionnaire 1. Which of the following amino acids has two asymmetric centres? Answers: a) Glycine b) Histidine c) Isoleucine d) Cysteine 2 2. Which amino acid has significant buffering capacity at near neutral p. Hs in intracellular and extracellular fluids of animals? Answers: a) Glutamic acid b) Histidine 3 c) Valine d) Methionine 3. Which of the following amino acids does not have an uncharged side chain? Answers: a) Aspartic acid b) Serine c) Threonine d) Glutamine 4 4. How many peptide bonds would be present in an octapeptide molecule? Answers: a) 8 b) 9 c) 6 d) 7 5. Out of the 20 standard amino acids, how many of them will have complex three-stage titration curves? Answers: a) 2 b) 3 c) 8 d) 5 5

Links for further reading Books: Biochemistry by Stryer et al. , 5 th edition Biochemistry by A. L. Lehninger et al. , 3 rd edition Biochemistry by Voet & Voet, 3 rd edition
- Slides: 35