Amino acids and proteins Amino acid Carboxyl Amine

Amino acids and proteins
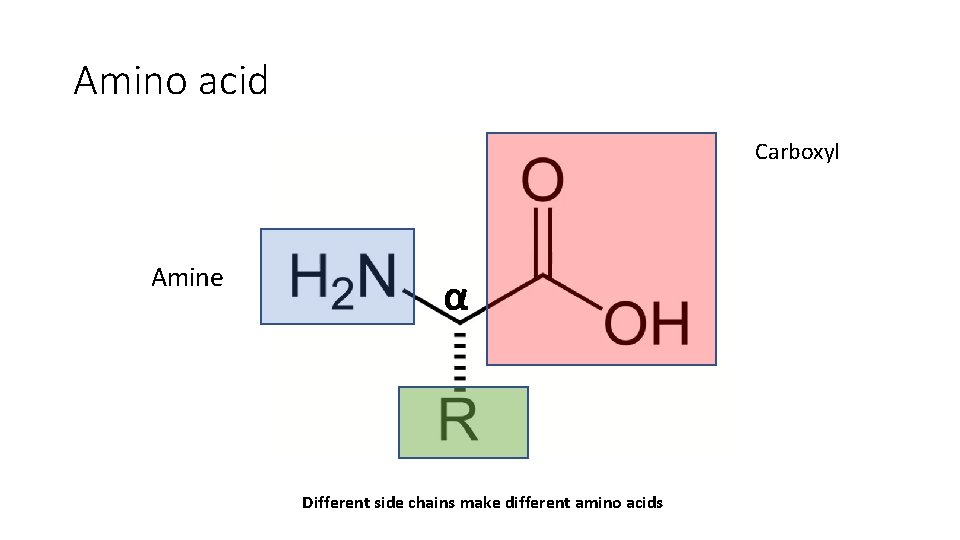
Amino acid Carboxyl Amine α Different side chains make different amino acids
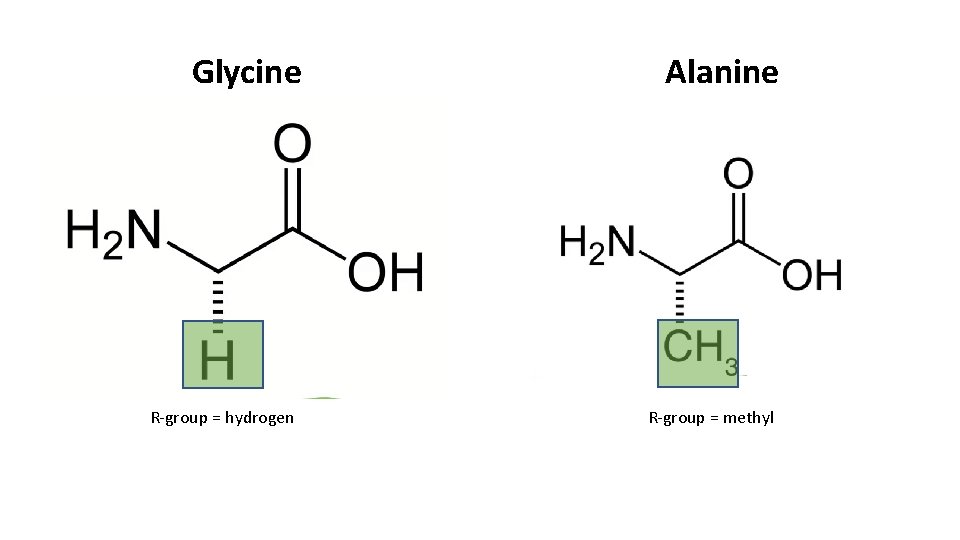
Glycine R-group = hydrogen Alanine R-group = methyl
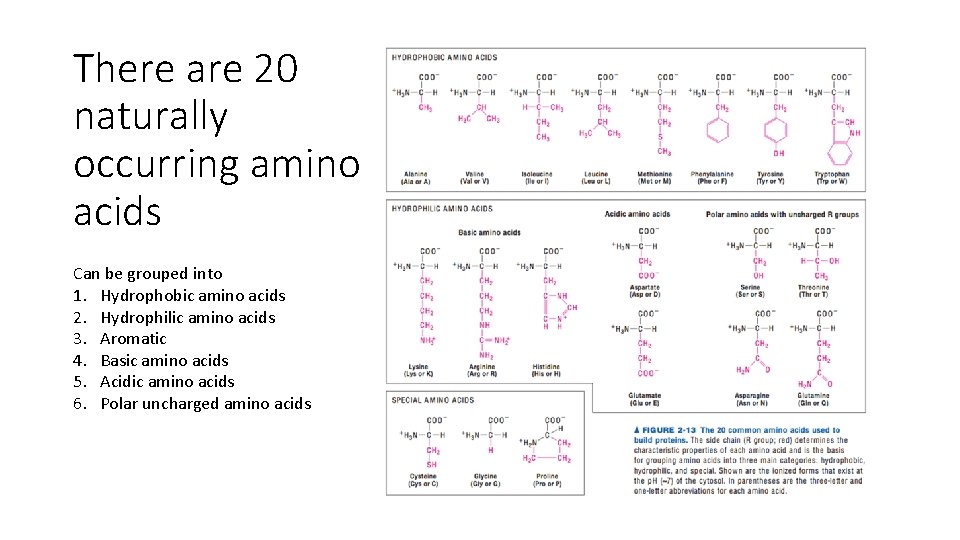
There are 20 naturally occurring amino acids Can be grouped into 1. Hydrophobic amino acids 2. Hydrophilic amino acids 3. Aromatic 4. Basic amino acids 5. Acidic amino acids 6. Polar uncharged amino acids

DO YOU KNOW AMINO ACID 21 AND 22? • Selenocysteine (Sec) and pyrrolysine (Pyl) are rare amino acids • Sec ---- Archaea, bacteria and Eukaryotes and requires the essential micronutrient Selenium (Se) for synthesis and function • Pyl ----- methanogenic archaea
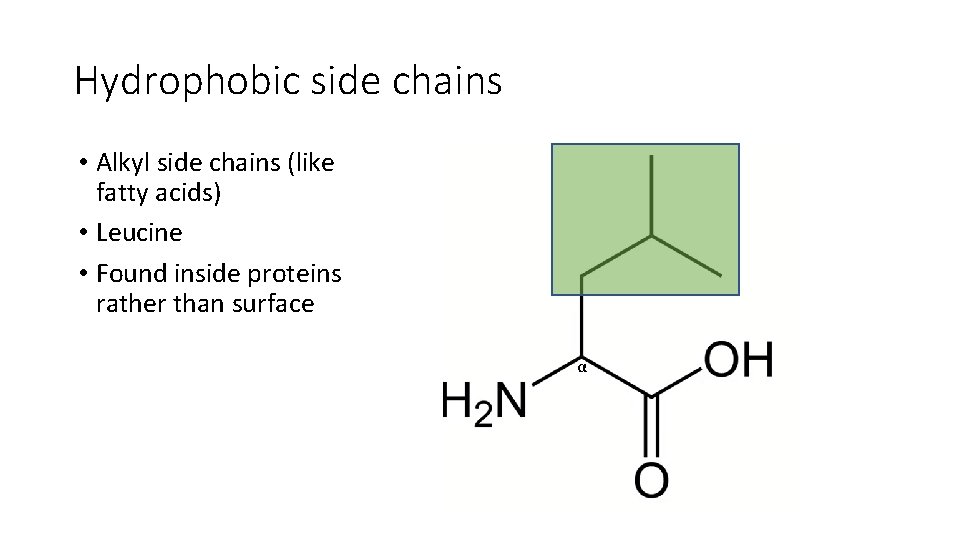
Hydrophobic side chains • Alkyl side chains (like fatty acids) • Leucine • Found inside proteins rather than surface α
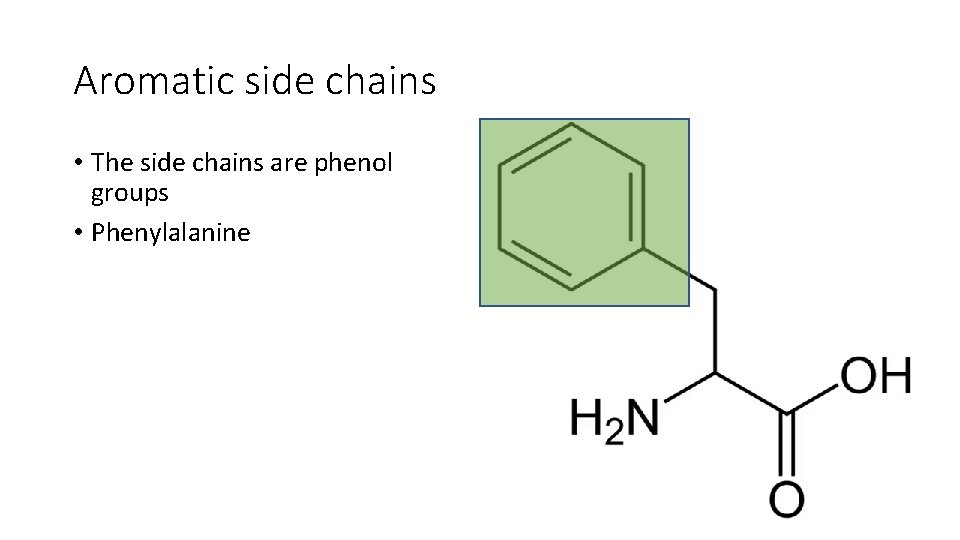
Aromatic side chains • The side chains are phenol groups • Phenylalanine
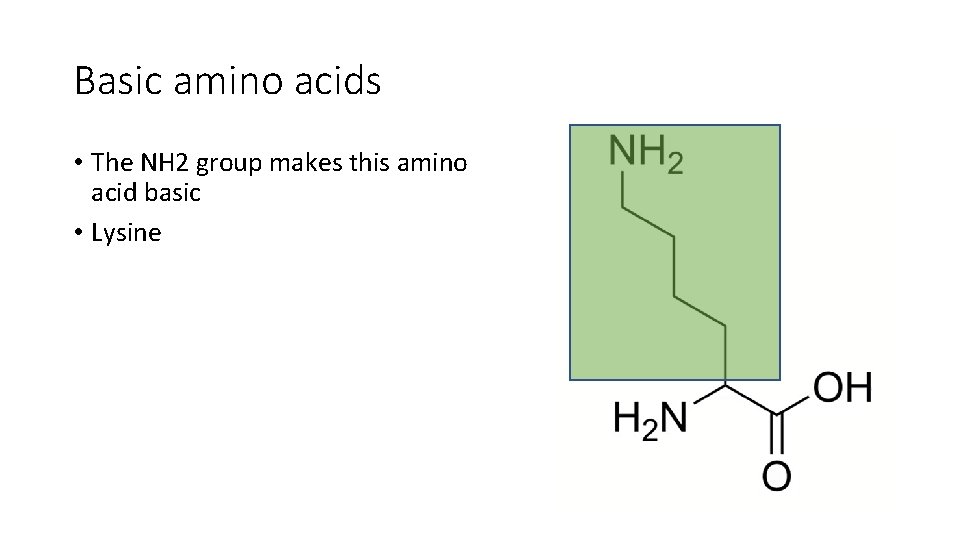
Basic amino acids • The NH 2 group makes this amino acid basic • Lysine
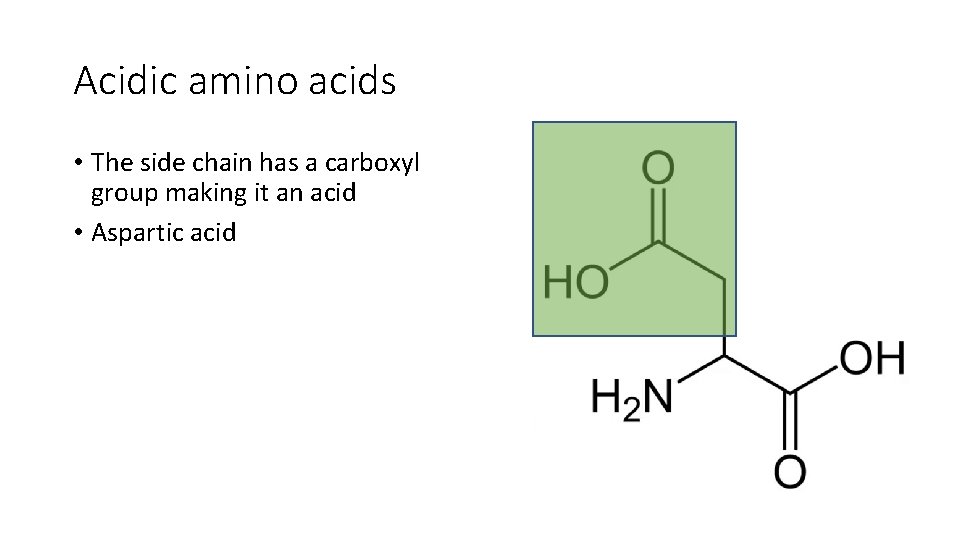
Acidic amino acids • The side chain has a carboxyl group making it an acid • Aspartic acid
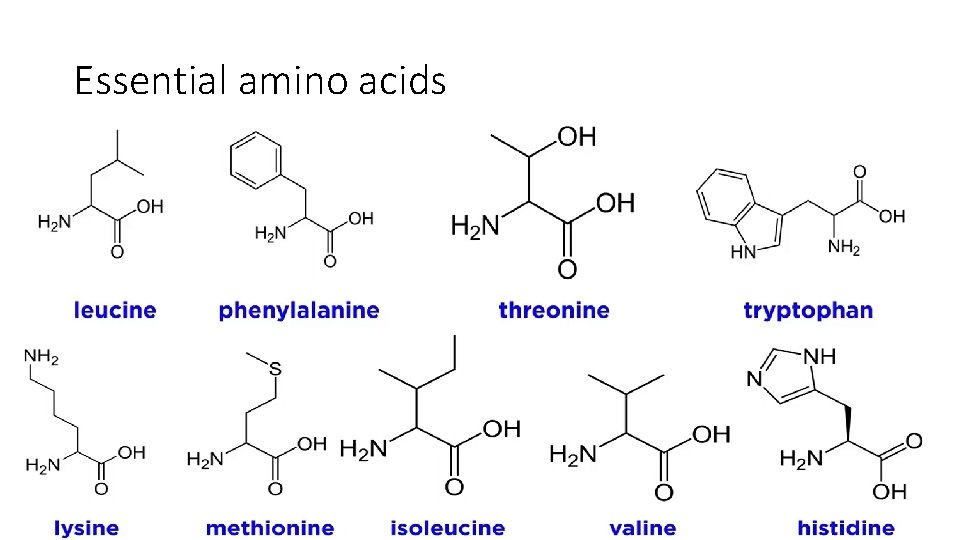
Essential amino acids
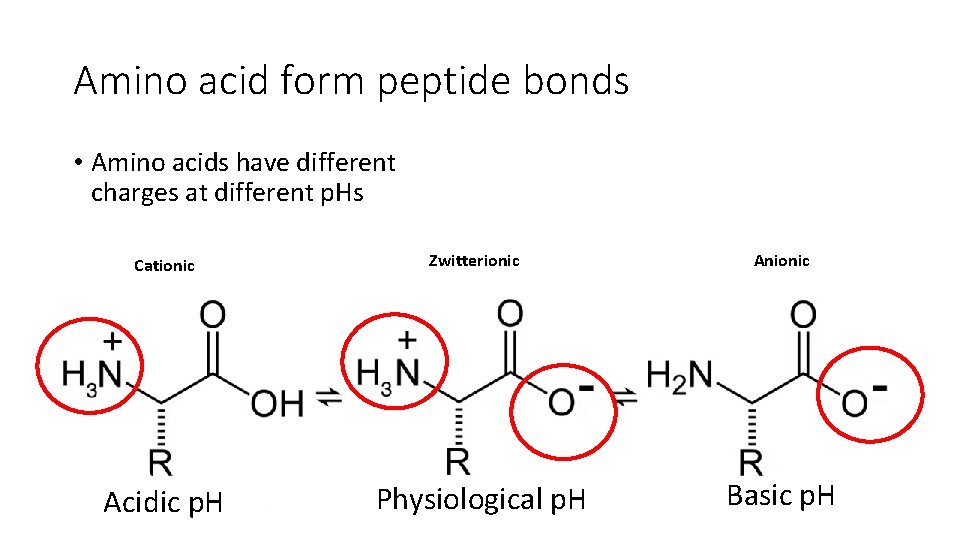
Amino acid form peptide bonds • Amino acids have different charges at different p. Hs Cationic Acidic p. H Zwitterionic Physiological p. H Anionic Basic p. H
Proteins are polymers of amino acids • Proteins are also called polypeptides • Made up of amino acid units. • Most diverse type of biomolecule • Different combinations of amino acids give different properties and shapes to the protein
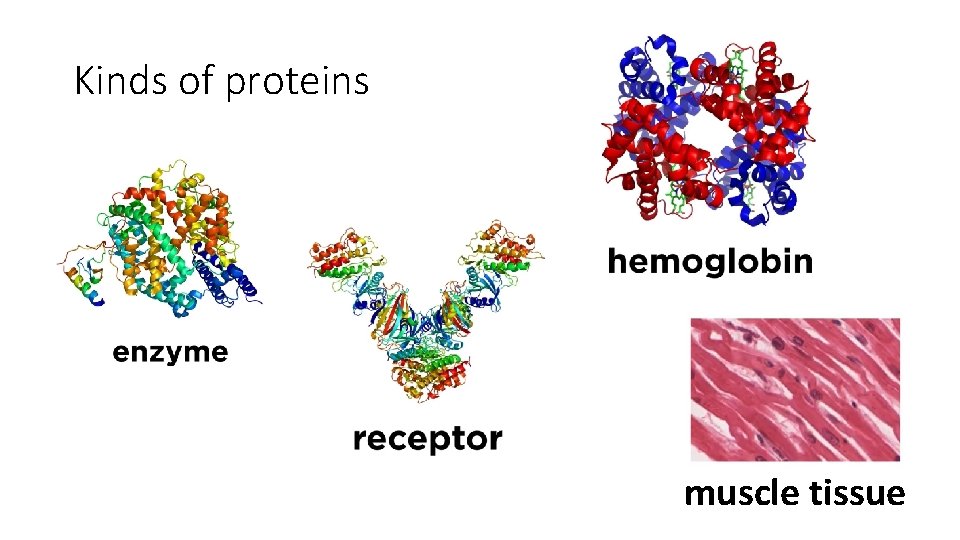
Kinds of proteins muscle tissue
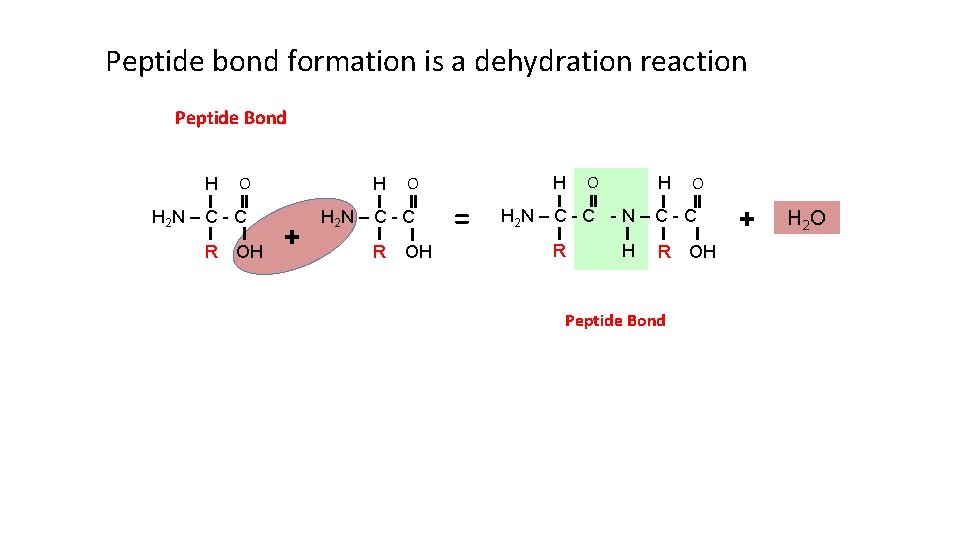
Peptide bond formation is a dehydration reaction Peptide Bond H O H 2 N – C - C R OH H + O H 2 N – C - C R OH H = O H 2 N – C - C R H R Peptide Bond OH + H 2 O
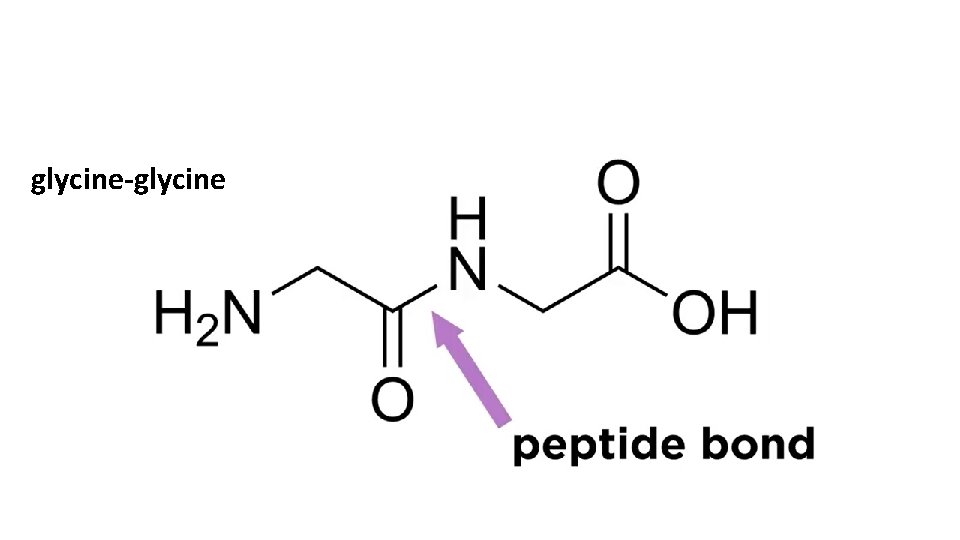
glycine-glycine
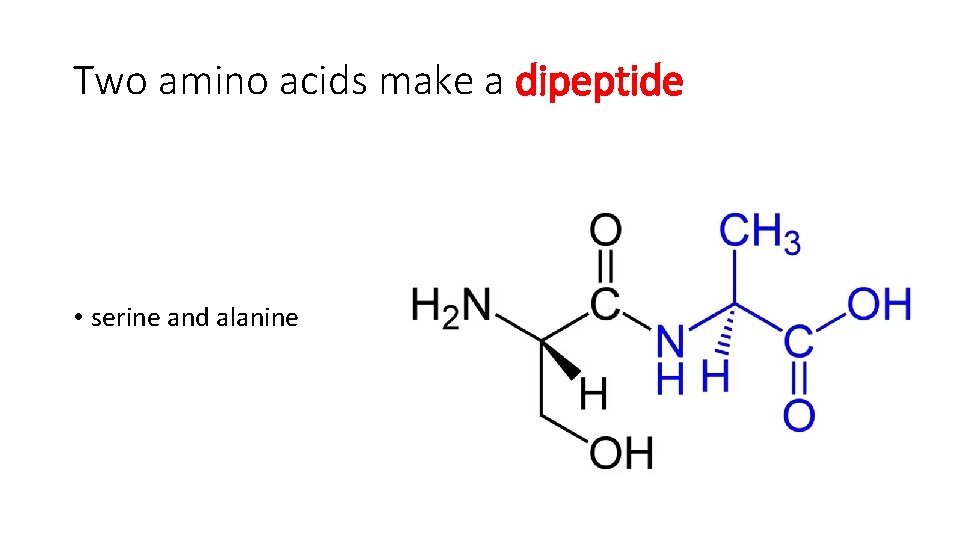
Two amino acids make a dipeptide • serine and alanine
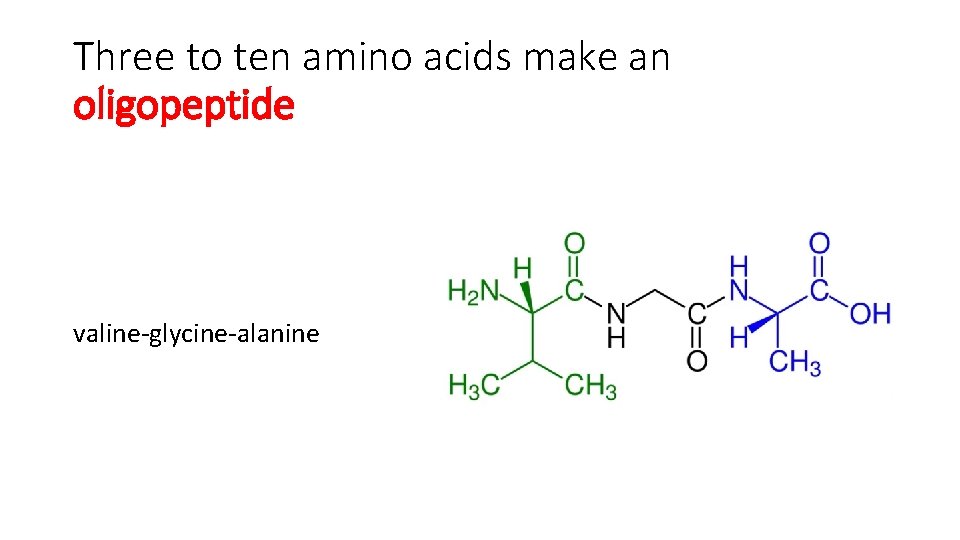
Three to ten amino acids make an oligopeptide valine-glycine-alanine
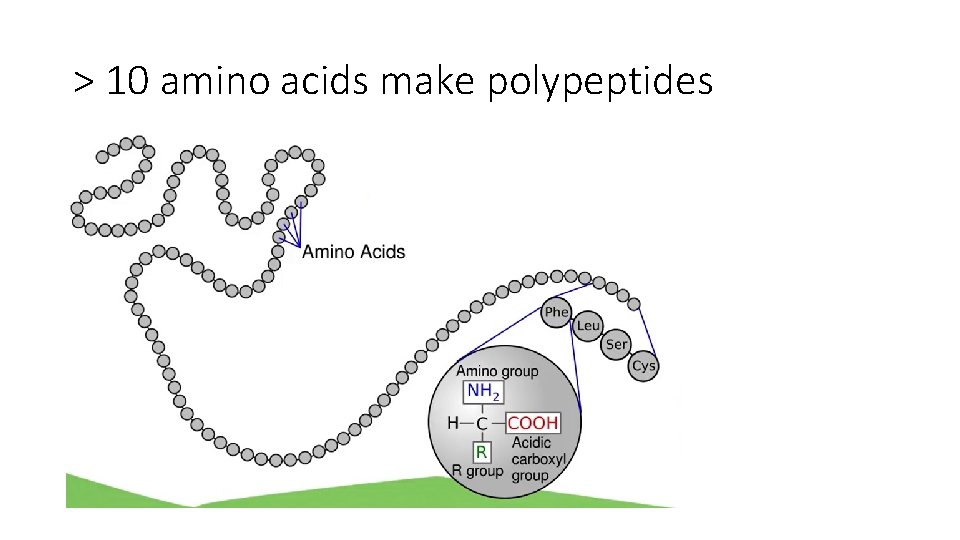
> 10 amino acids make polypeptides
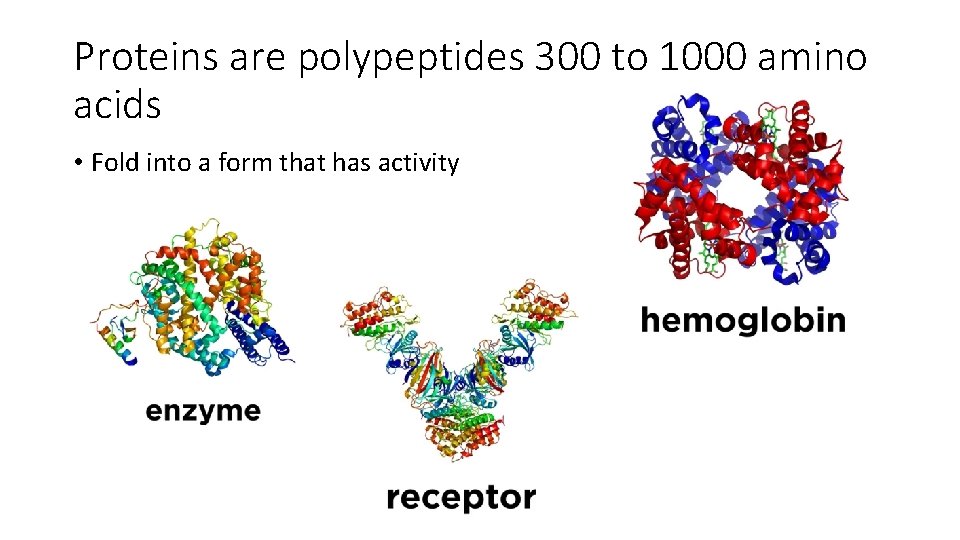
Proteins are polypeptides 300 to 1000 amino acids • Fold into a form that has activity

Proteins have N and C termini N-terminus C-terminus
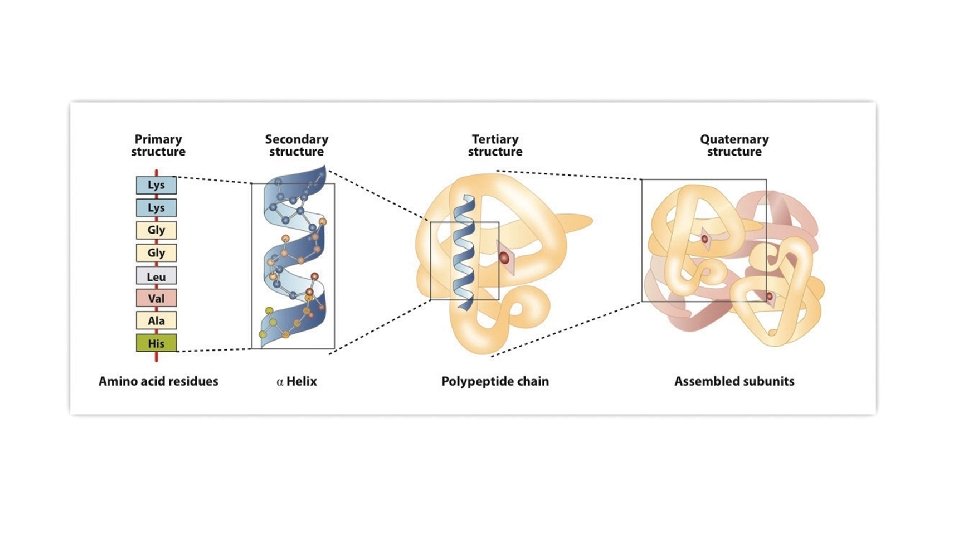
Primary Secondary Tertiary Quaternary
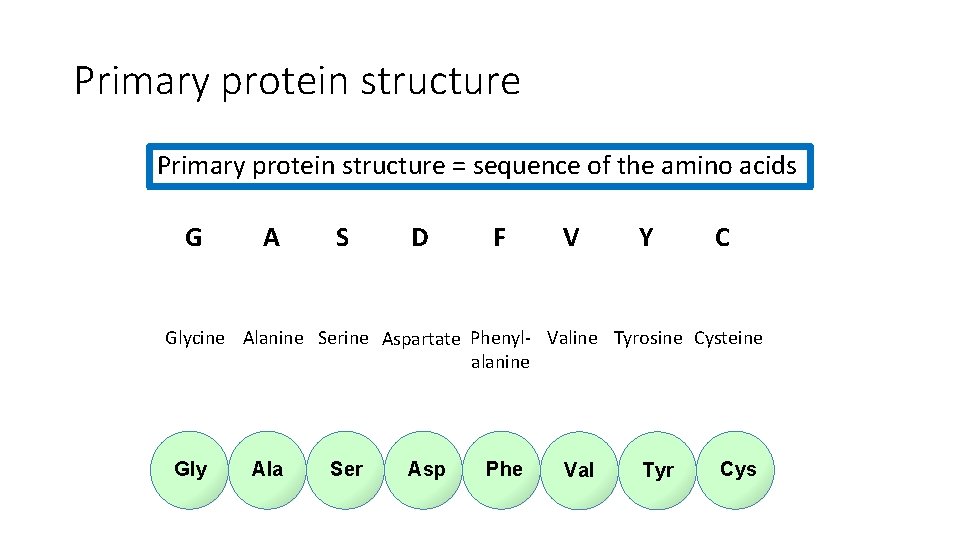
Primary protein structure = sequence of the amino acids G A S D F V Y C Glycine Alanine Serine Aspartate Phenyl- Valine Tyrosine Cysteine alanine Gly Ala Ser Asp Phe Val Tyr Cys
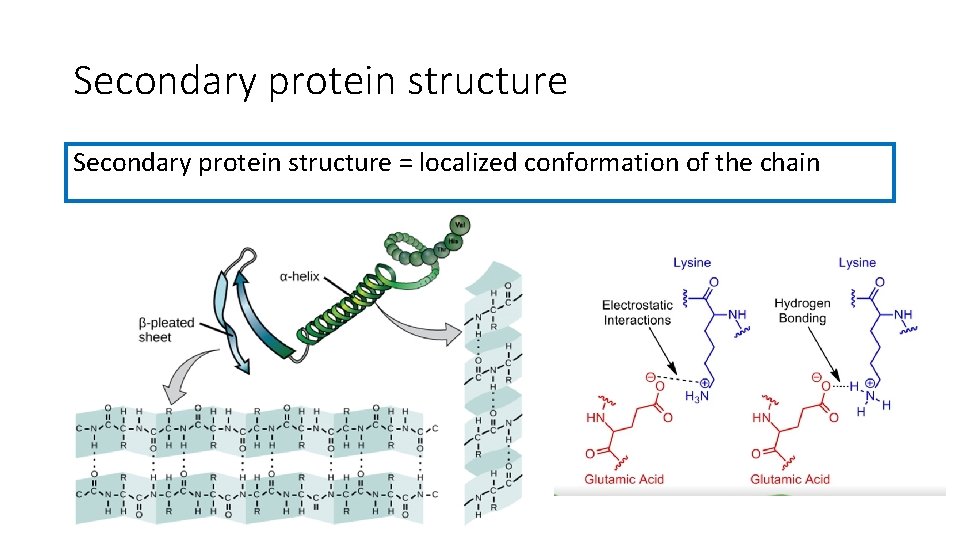
Secondary protein structure = localized conformation of the chain
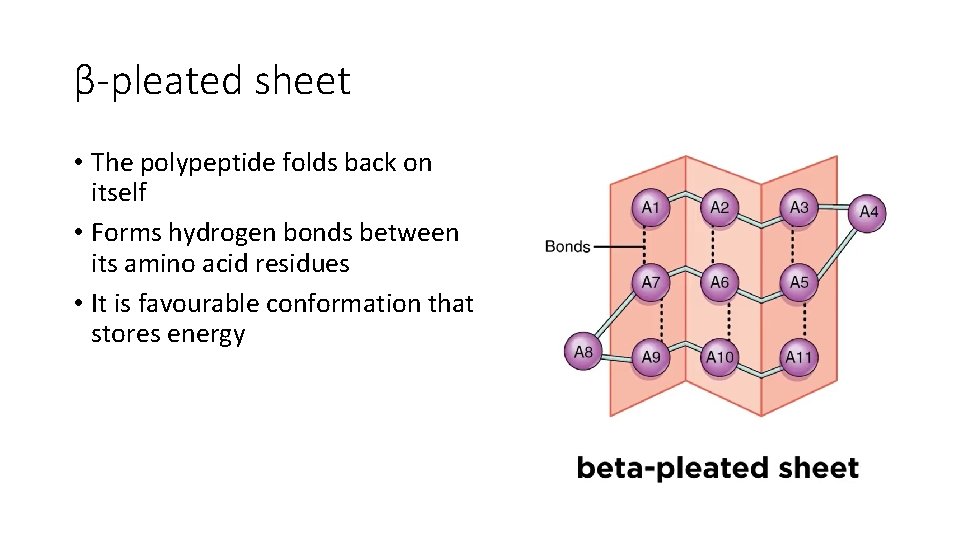
β-pleated sheet • The polypeptide folds back on itself • Forms hydrogen bonds between its amino acid residues • It is favourable conformation that stores energy
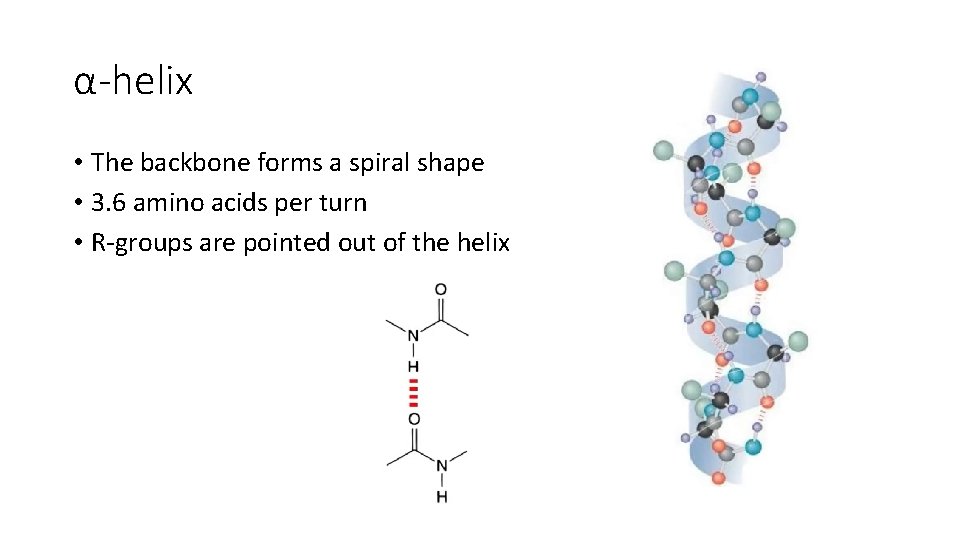
α-helix • The backbone forms a spiral shape • 3. 6 amino acids per turn • R-groups are pointed out of the helix
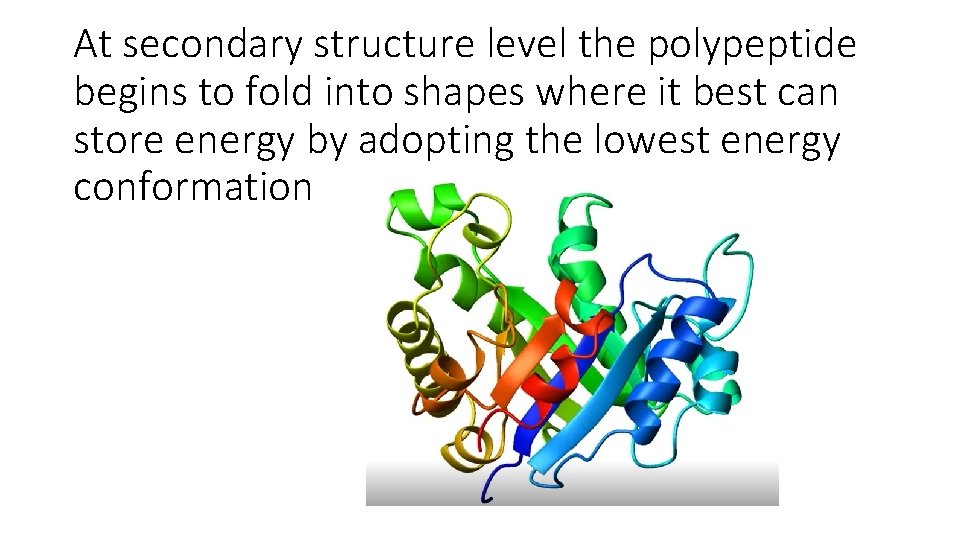
At secondary structure level the polypeptide begins to fold into shapes where it best can store energy by adopting the lowest energy conformation
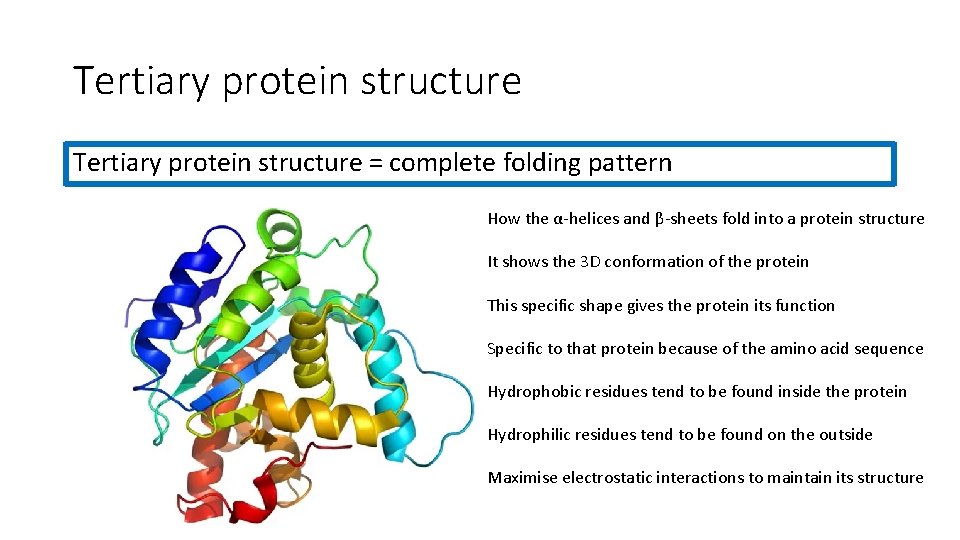
Tertiary protein structure = complete folding pattern How the α-helices and β-sheets fold into a protein structure It shows the 3 D conformation of the protein This specific shape gives the protein its function Specific to that protein because of the amino acid sequence Hydrophobic residues tend to be found inside the protein Hydrophilic residues tend to be found on the outside Maximise electrostatic interactions to maintain its structure
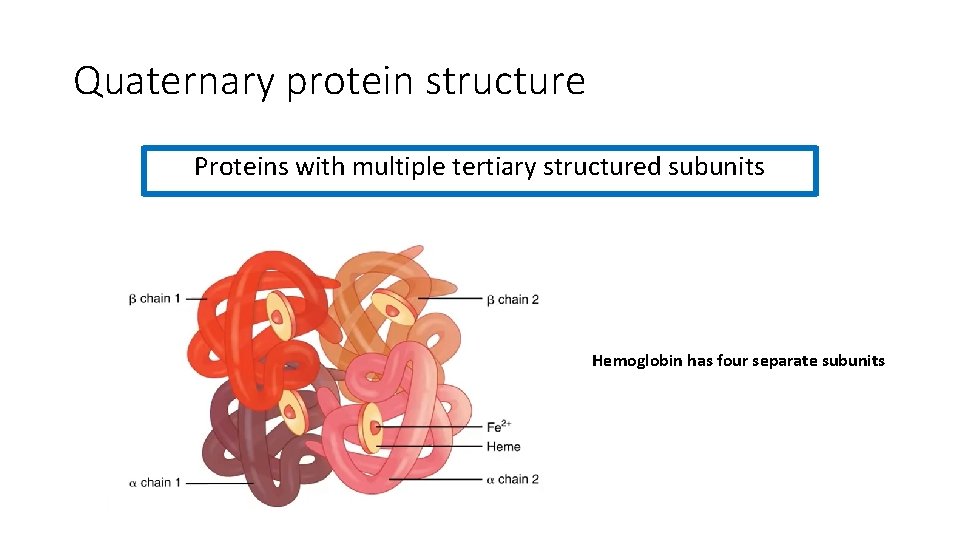
Quaternary protein structure Proteins with multiple tertiary structured subunits Hemoglobin has four separate subunits
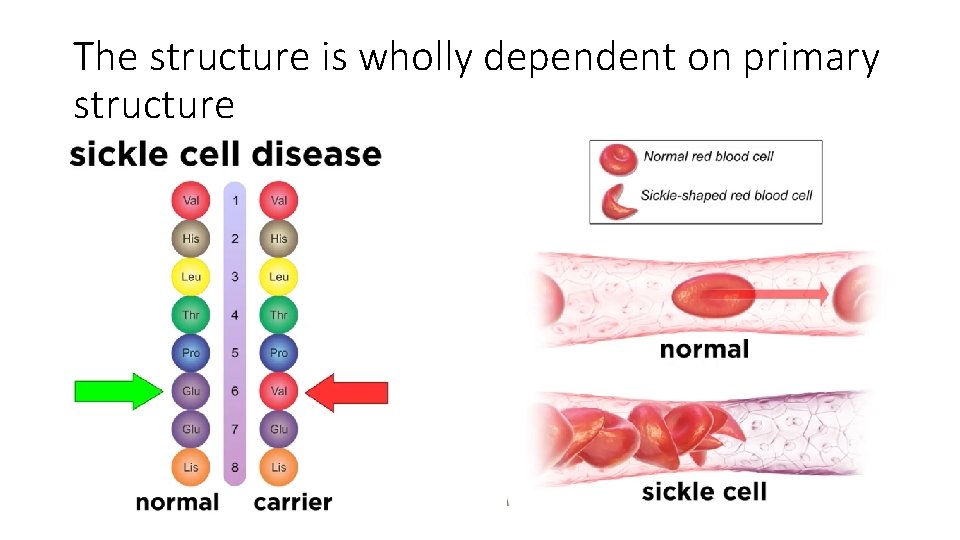
The structure is wholly dependent on primary structure
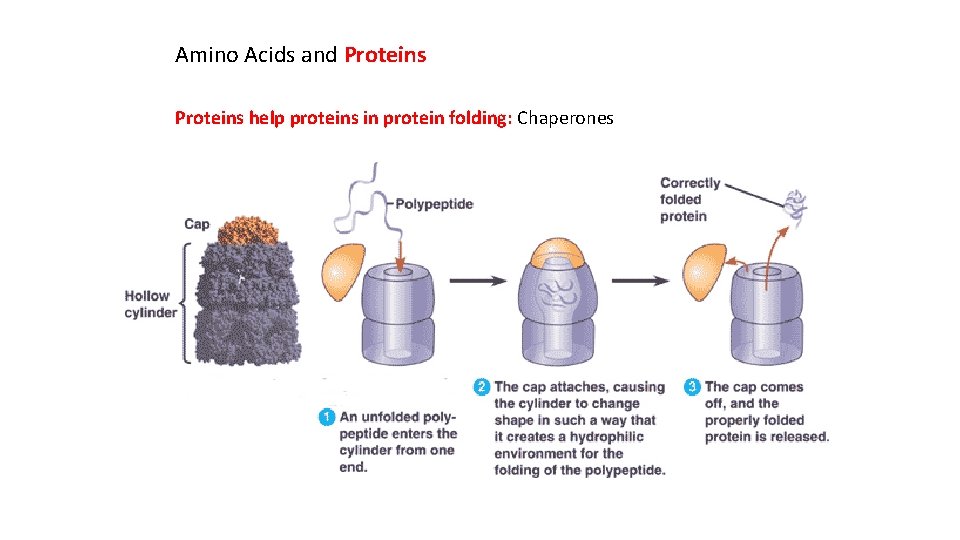
Amino Acids and Proteins help proteins in protein folding: Chaperones
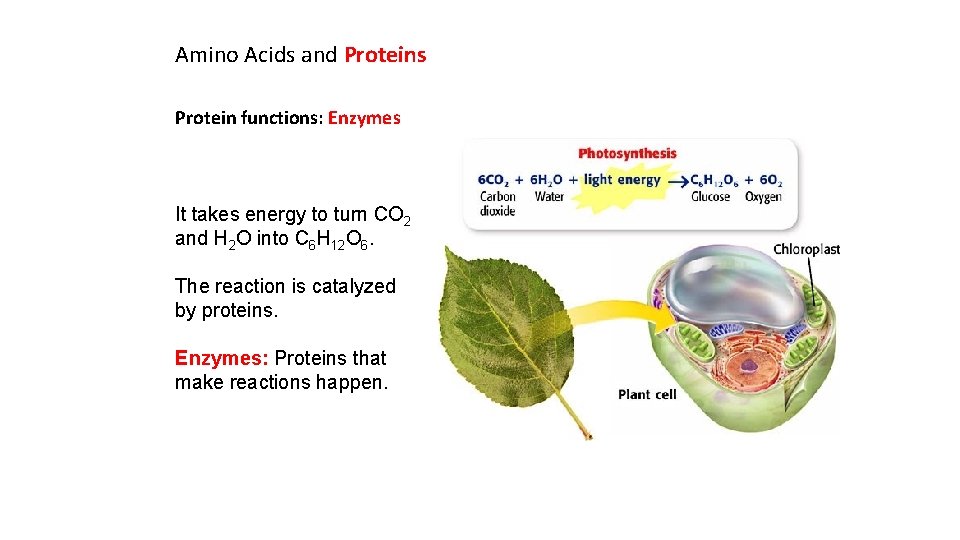
Amino Acids and Proteins Protein functions: Enzymes It takes energy to turn CO 2 and H 2 O into C 6 H 12 O 6. The reaction is catalyzed by proteins. Enzymes: Proteins that make reactions happen.
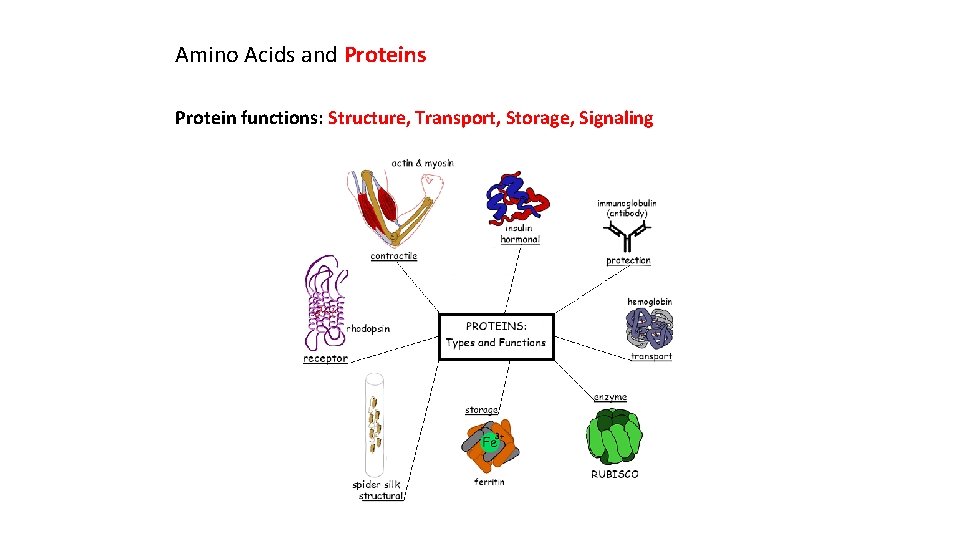
Amino Acids and Proteins Protein functions: Structure, Transport, Storage, Signaling
- Slides: 34