Amino Acids Amino Acids Amino acids are building

Amino Acids
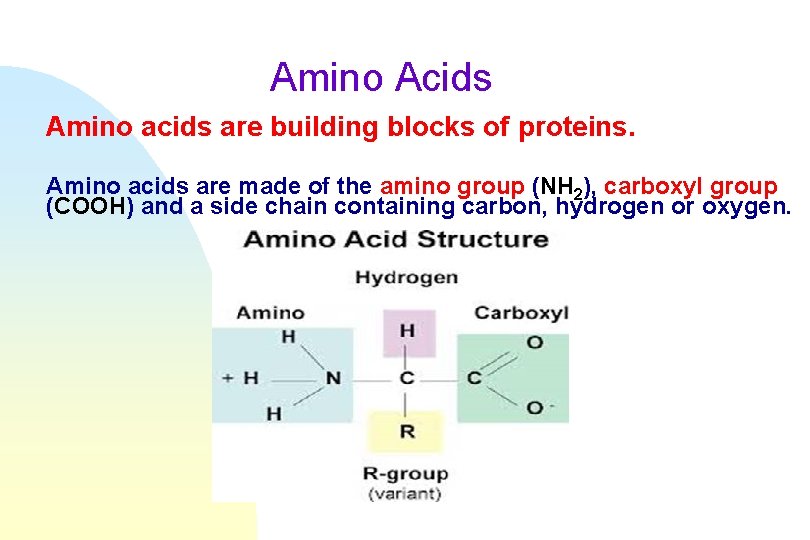
Amino Acids Amino acids are building blocks of proteins. Amino acids are made of the amino group (NH 2), carboxyl group (COOH) and a side chain containing carbon, hydrogen or oxygen.

Amino Acids, they all contain a carboxylic acid group and an amino group. The carbon adjacent to a carboxyl carbon is designated the α carbon. Since the naturally occurring amino acids have the amino group on the a carbon they are α amino acids.

Amino Acids Amino acids are the building blocks of protein in the body. There are twenty amino acids in the body. Some of these amino acids are naturally synthesized in the liver without need for supplementation through diet or other nutritional sources. Eleven of the twenty amino acids in the body cannot be made by the liver. If the amino acid must be consumed, it is called an essential amino acid.

Naturally occurring forms are called non-essential amino acids. Non-essential and essential amino acids have a different amino acid structure affecting the way they work in the body. • Non-essential amino acids, those synthesized in the body are - alanine - asparagine - aspartic acid - cysteine - glutamic acid - glutamine - glycine - serine - proline

Essential amino acids are histidine, isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. All of the 20 amino acids are necessary constituents of human protein. Adequate amounts of 11 of the 20 amino acids can be synthesized from carbohydrates and lipids in the body if а source of nitrogen is also available.

n n Classification of Amino Acids Amino acids are classified mainly into three groups depending on their reaction in solution as Neutral u Aromatic amino acids: Phenylalanine, Tyrosine, Tryptophan (Heterocyclic amino acids) u Aliphatic amino Acids: Glycine, Alanine, Valine, Leucine, Isoleucine, Serine/Threonine (-OH group containing amino acids) Cysteine, Methionine (Sulfur containing amino acids) Acidic amino acids u Aliphatic amino acids Aspartic acid, Asparagine, Glutamic acid, Glutamine Basic amino acids u Aliphatic amino acids Lysine, Arginine u Heterocyclic amino acid Histidine
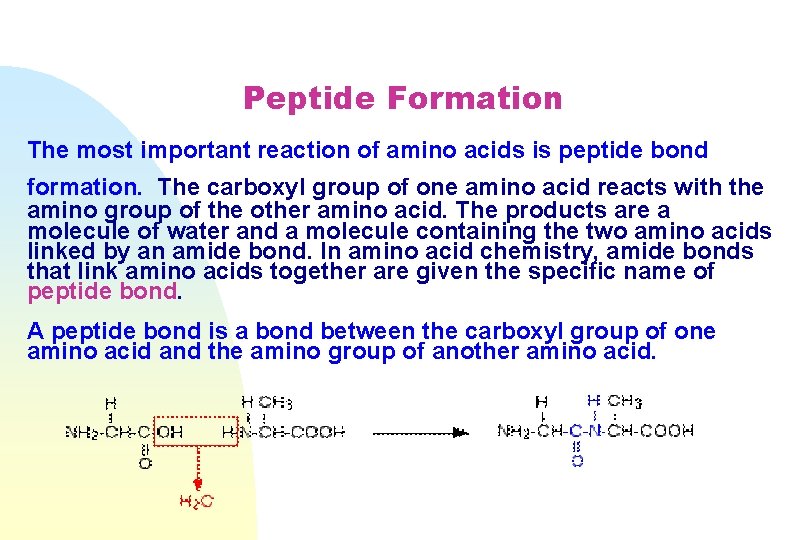
Peptide Formation The most important reaction of amino acids is peptide bond formation. The carboxyl group of one amino acid reacts with the amino group of the other amino acid. The products are а molecule of water and а molecule containing the two amino acids linked by an amide bond. In amino acid chemistry, amide bonds that link amino acids together are given the specific name of peptide bond. А peptide bond is а bond between the carboxyl group of one amino acid and the amino group of another amino acid.

Only L-α-Amino Acids Occur in Proteins n n Amino Acids contain both amino and carboxylic acid functional groups. In an α-amino acid, both are attached to the same carbon atom. The functional groups of amino acids dictate their chemical reactions (salt formation, esterification, and acylation)

Glucogenic and Ketogenic Amino Acids n n n If the carbon skeleton of amino acid can be converted into glucose in the body, such amino acids are called glucogenic amino acids. Similarly, if the carbon skeleton of amino acid is converted into ketone body (acetoacetic acid), such amino acids are called ketogenic amino acids. If one part of the carbon skeleton is converted into glucose and other part is converted into ketone body, such amino acids are termed both glucogenic and ketogenic amino acids.

Glucogenic and Ketogenic Amino Acids n n n Glucogenic amino acids: Glycine, alanine, serine, threonine, valine, cysteine, proline, methionine, asparagine, aspartic acid, glutamine, histidine, arginine. Ketogenic amino acids: Leucine Glucogenic and ketogenic amino acids: Lysine, Isoleucine, phenylalanine, tyrosine, tryptophan

Ninhydrin Reaction of Amino Acids n Ninhydrin reaction is used to detect and quantify the amount of amino acid. When amino acids are heated with ninhydrin, the free α–amino groups react and give a purple colored product.
Naturally Occuring Peptides n n Peptides are chains of amino acids. Biologically occurring peptides range in size from small molecules containing only two or three amino acids to macromolecules containing thousands of amino acids.
- Slides: 13