Amines Chapter 20 Outline 1 Structure nomenclature and



- Slides: 30

Amines Chapter 20

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

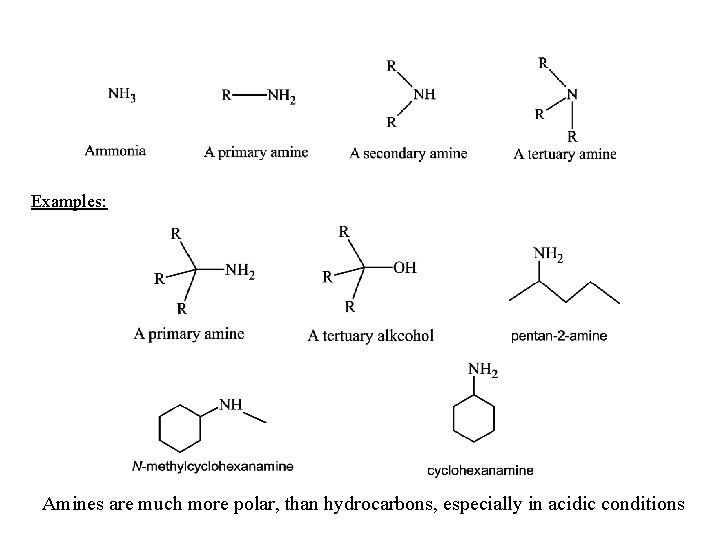
Examples: Amines are much more polar, than hydrocarbons, especially in acidic conditions

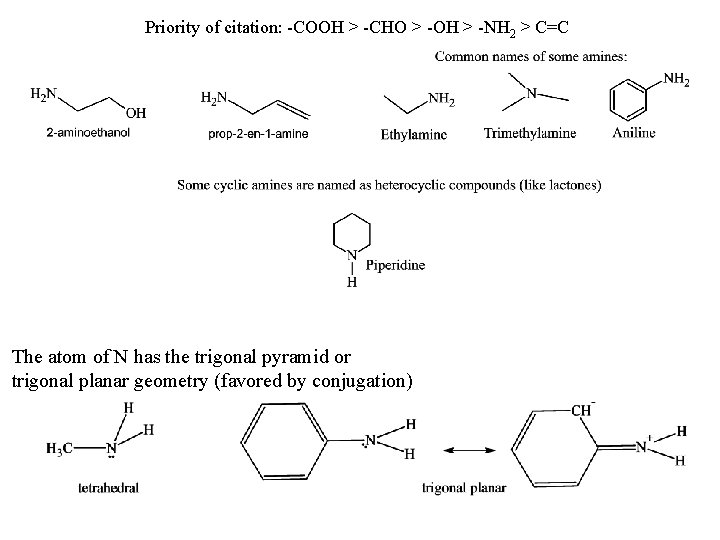
Priority of citation: -COOH > -CHO > -OH > -NH 2 > C=C The atom of N has the trigonal pyramid or trigonal planar geometry (favored by conjugation)

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

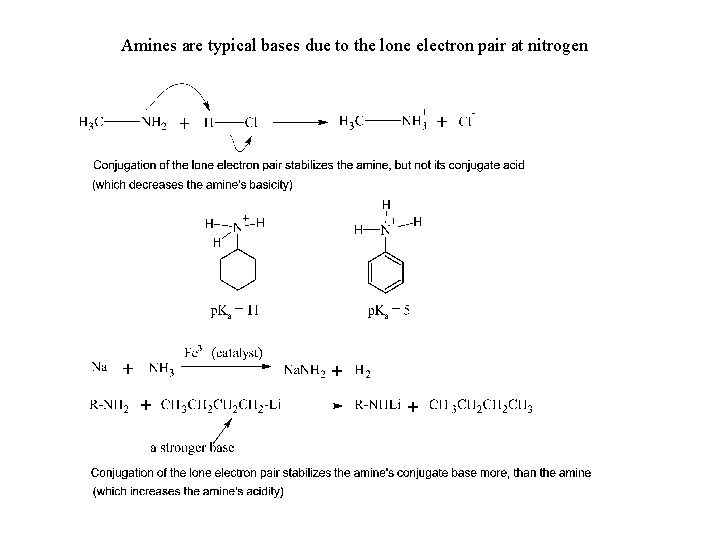
Amines are typical bases due to the lone electron pair at nitrogen

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

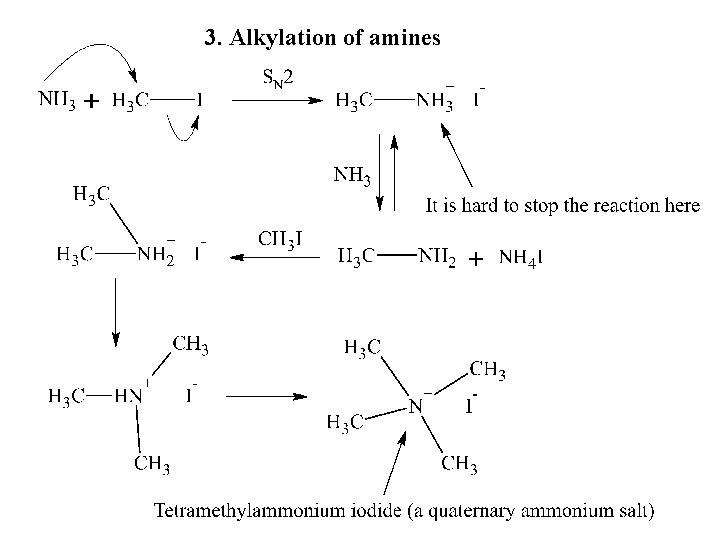
3. Alkylation of amines

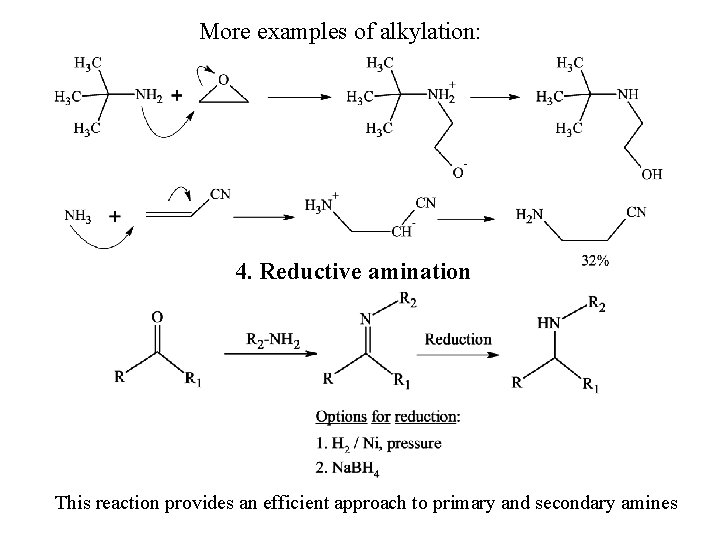
More examples of alkylation: 4. Reductive amination This reaction provides an efficient approach to primary and secondary amines

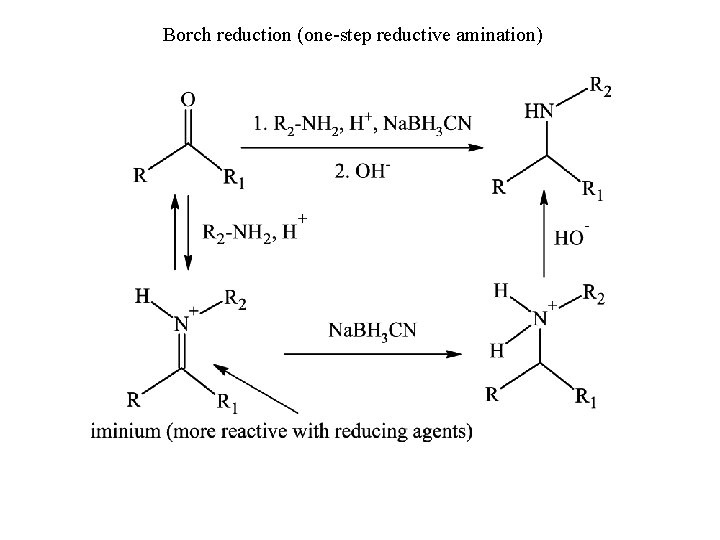
Borch reduction (one-step reductive amination)

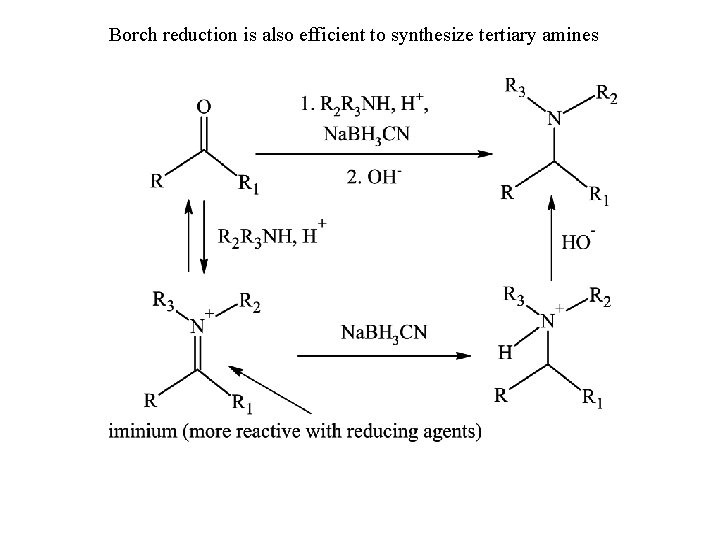
Borch reduction is also efficient to synthesize tertiary amines

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

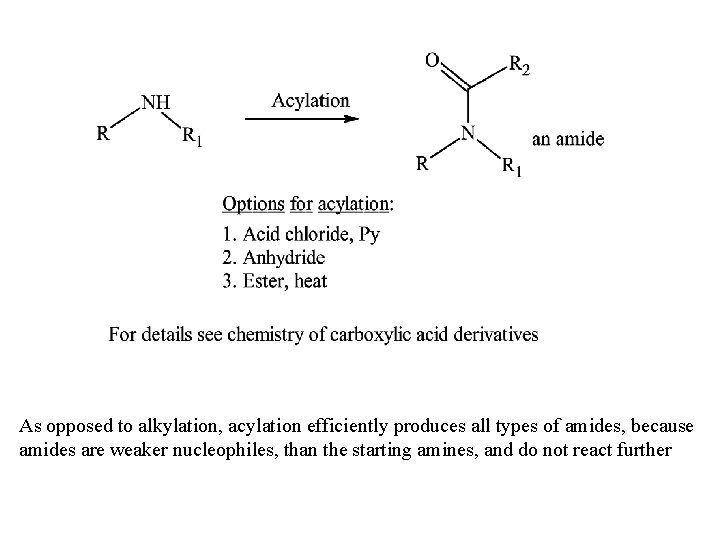
As opposed to alkylation, acylation efficiently produces all types of amides, because amides are weaker nucleophiles, than the starting amines, and do not react further

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

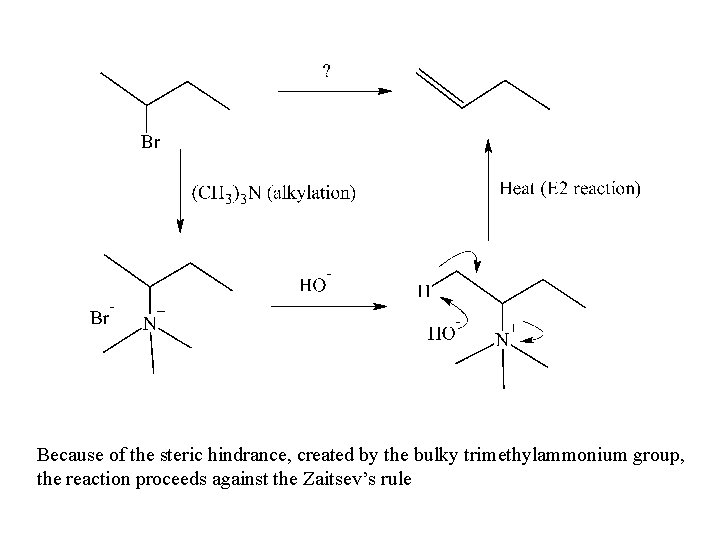
Because of the steric hindrance, created by the bulky trimethylammonium group, the reaction proceeds against the Zaitsev’s rule

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines


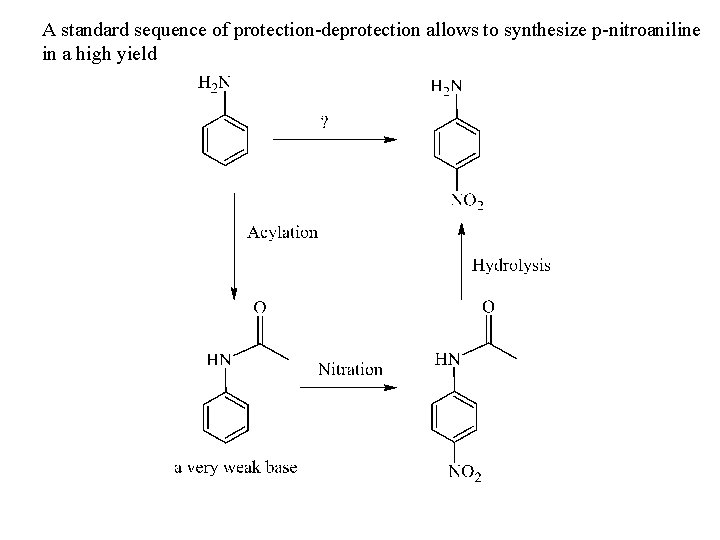
A standard sequence of protection-deprotection allows to synthesize p-nitroaniline in a high yield

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines


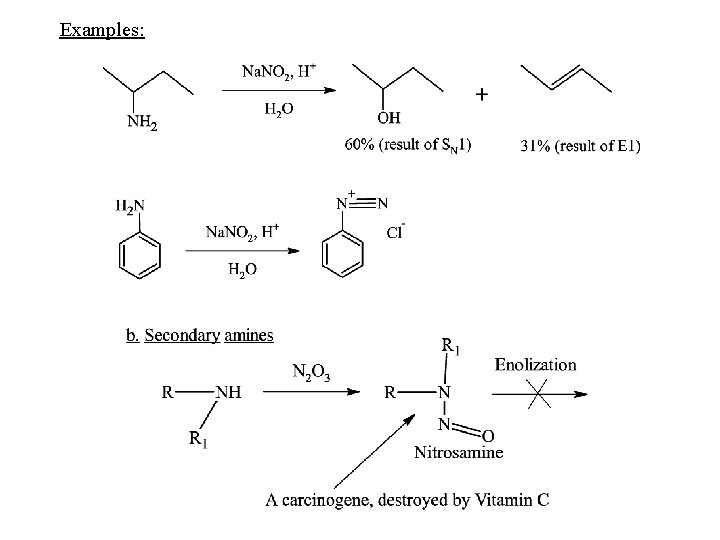
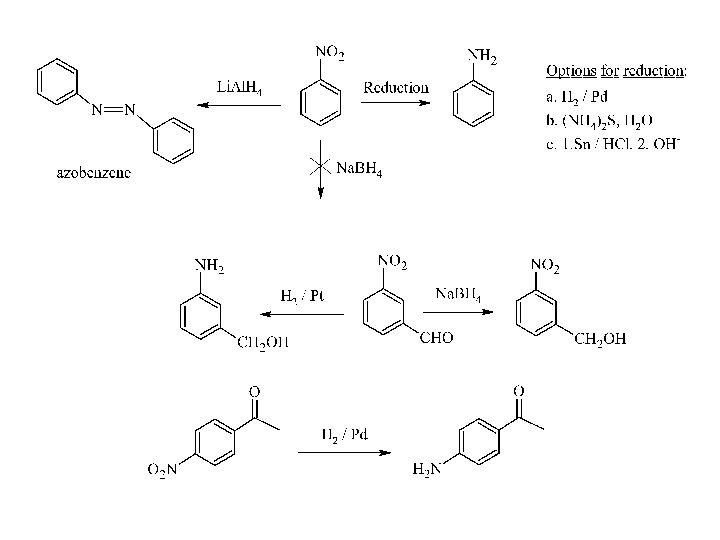
Examples:

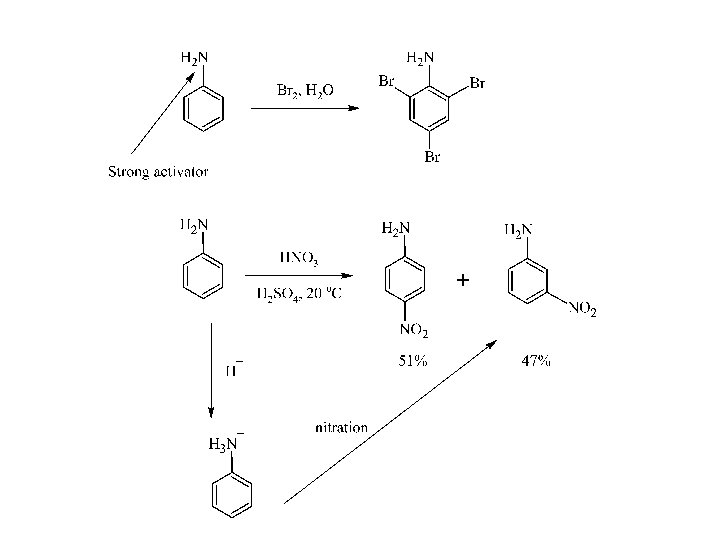
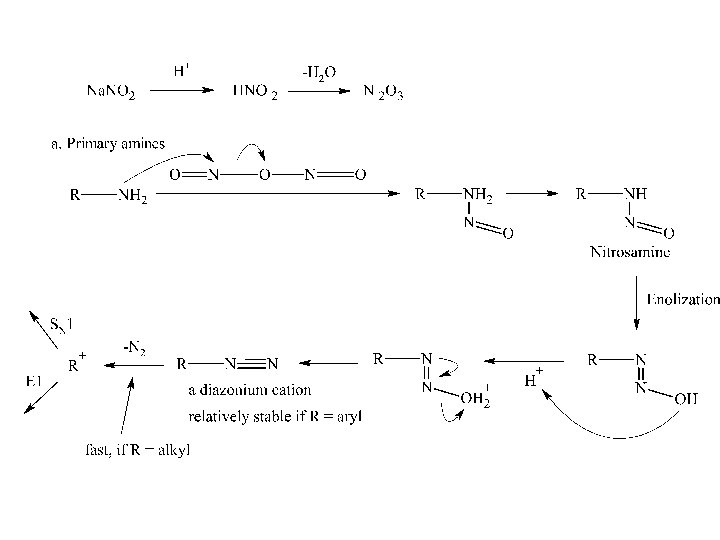
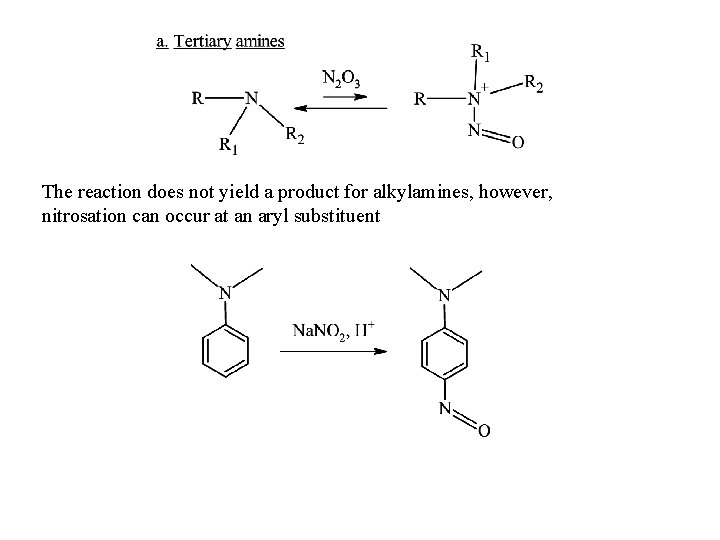
The reaction does not yield a product for alkylamines, however, nitrosation can occur at an aryl substituent

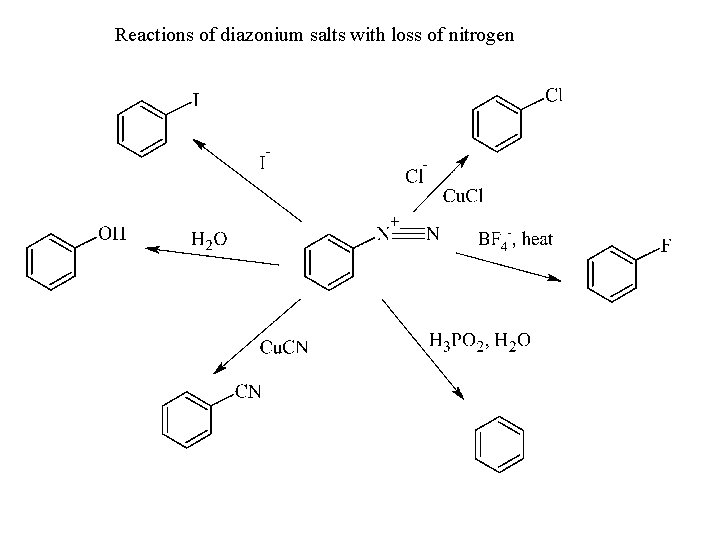
Reactions of diazonium salts with loss of nitrogen

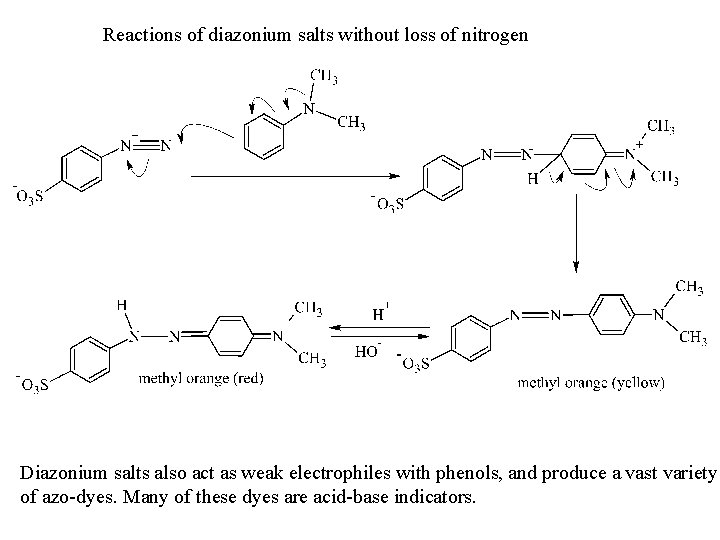
Reactions of diazonium salts without loss of nitrogen Diazonium salts also act as weak electrophiles with phenols, and produce a vast variety of azo-dyes. Many of these dyes are acid-base indicators.

Outline 1. Structure, nomenclature and physical properties of amines 2. Acidity and basicity of amines 3. Alkylation of amines 4. Reductive amination 5. Acylation of amines 6. Hoffmann elimination 7. Electrophilic reactions of aniline 8. Reaction of amines with nitrous acid. Diazonium salts 9. Synthesis of amines

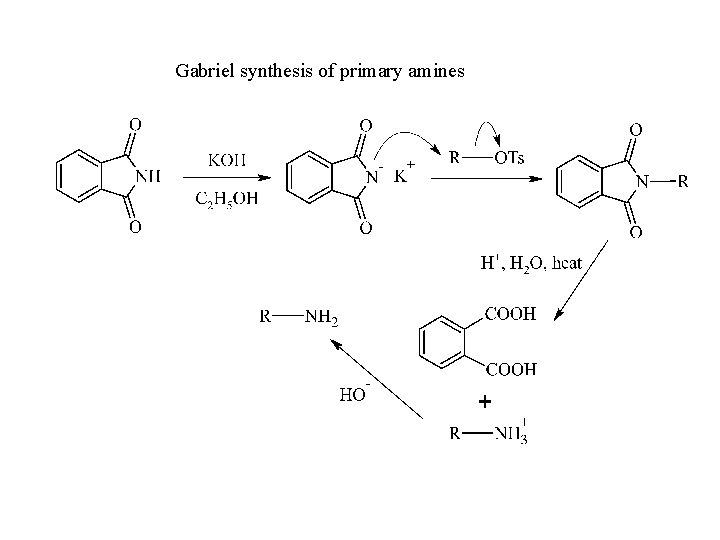
Gabriel synthesis of primary amines


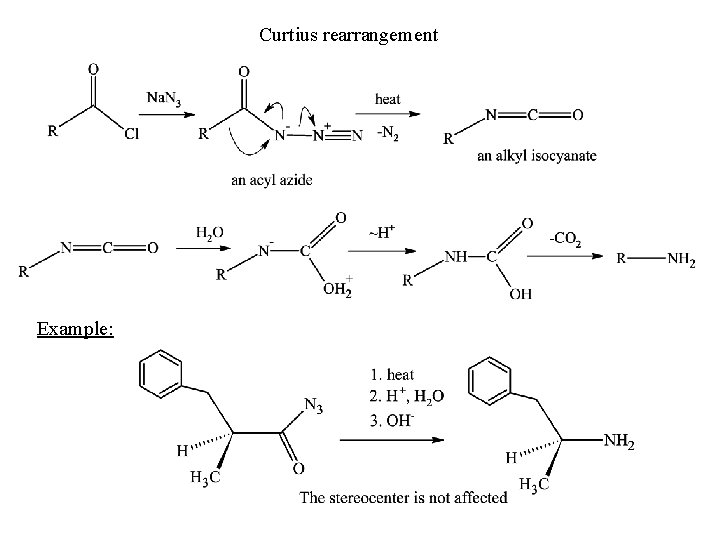
Curtius rearrangement Example:

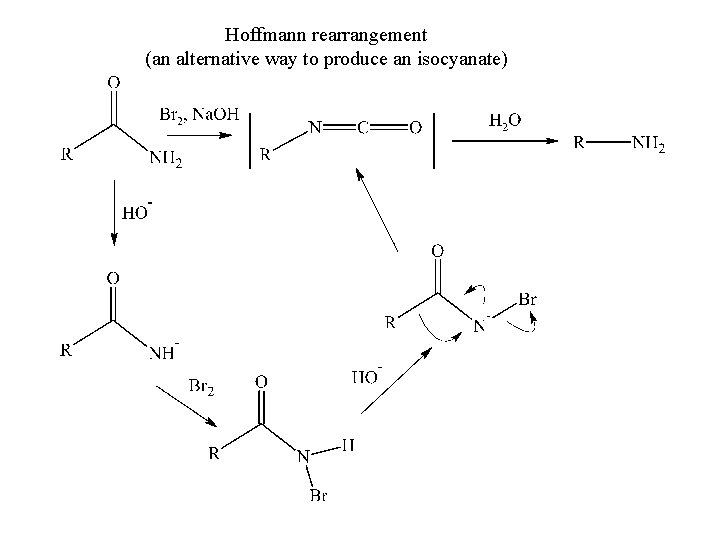
Hoffmann rearrangement (an alternative way to produce an isocyanate)