Amicus Therapeutics Galafold migalastat Jiri Hermanek GM CEE




- Slides: 6

Amicus Therapeutics & Galafold (migalastat) Jiri Hermanek, GM CEE

Product Pipeline Multiple technology platforms PRODUCT/PLATFORM Fabry DISCOVERY PRECLINICAL PHASE 1 PHASE 2 Monotherapy - Personalized Medicine (Galafold) Migalastat Pharmacological Chaperone Monotherapy & Combination w/ ERT Co-Administration with ERT Next-Generation ERT Pompe Next-Generation ERT + Chaperone 2 PHASE 3 Regulatory

Fabry Disease: Pathophysiology Mutations in the GLA gene Reduced lysosomal alpha-galactosidase A (α-Gal A) activity Accumulation of globotriaosylceramide (GL-3) and Lyso-Gb 3 Multisystemic disease: Cardiovascular disease, renal failure, stroke, gastrointestinal symptoms, pain, hearing loss Premature death 3

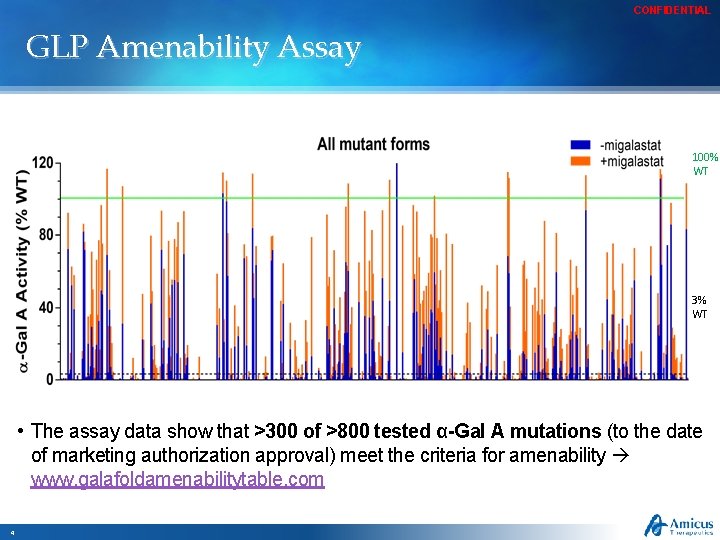
CONFIDENTIAL GLP Amenability Assay 100% WT 3% WT • The assay data show that >300 of >800 tested α-Gal A mutations (to the date of marketing authorization approval) meet the criteria for amenability www. galafoldamenabilitytable. com 4

Galafold (migalastat) Personalized medicine for Fabry patients § Novel personalized treatment concept for Fabry disease: only patients with amenable mutations can be treated with Galafold (migalastat) § First orally administered small molecule (every other day dosing): – Comparable clinical efficacy to ERT – No risk of immunogenicity (small molecule) – No infusion-associated reactions 5

Galafold (migalastat) Personalized medicine for Fabry patients § EU Marketing Authorization Approval granted May 30 th 2016 § Reimbursed already in multiple EU countries (Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Norway, Sweden, UK) >250 patients on therapy § Hungary: – Pricing & reimbursement application submitted to NEAK on December 22 nd 2016 recommendation for reimbursement in “individual patient request” category final stage of negotiating reimbursement agreement with NEAK available from January 2018 (? ) 6