American Statistical Association 2004 FDAIndustry Statistics Workshop Washington










- Slides: 37

American Statistical Association 2004 FDA/Industry Statistics Workshop Washington, DC September 23, 2004 Beyond the CDISC SDTM V 3. 1 Model: Statistical & Programming Considerations William J. Qubeck, IV MS, MBA Electronic Submissions Data Group Leader Global Clinical Data Services, Pfizer Inc. 1

Agenda § Model Overview of CDISC SDTM V 3. 1 § Programming, Statistical and Submission Considerations § Cost/Benefits of 3 Implementation Strategies § Implications and Summary Pfizer, Inc. Slide: 2 6/8/2021

CDISC SDTM Version 3. 1 A brief model overview 3

CDISC SDTM Material (www. cdisc. org) Pfizer, Inc. Slide: 4 6/8/2021 Source of model information: www. cdisc. org

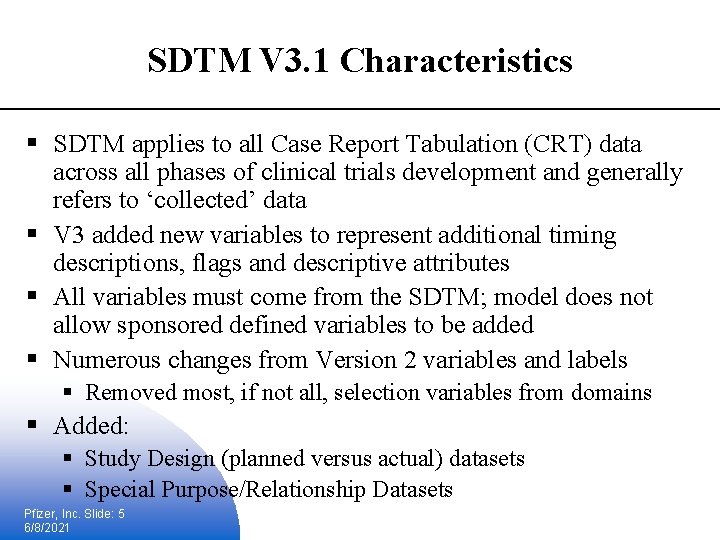
SDTM V 3. 1 Characteristics § SDTM applies to all Case Report Tabulation (CRT) data across all phases of clinical trials development and generally refers to ‘collected’ data § V 3 added new variables to represent additional timing descriptions, flags and descriptive attributes § All variables must come from the SDTM; model does not allow sponsored defined variables to be added § Numerous changes from Version 2 variables and labels § Removed most, if not all, selection variables from domains § Added: § Study Design (planned versus actual) datasets § Special Purpose/Relationship Datasets Pfizer, Inc. Slide: 5 6/8/2021

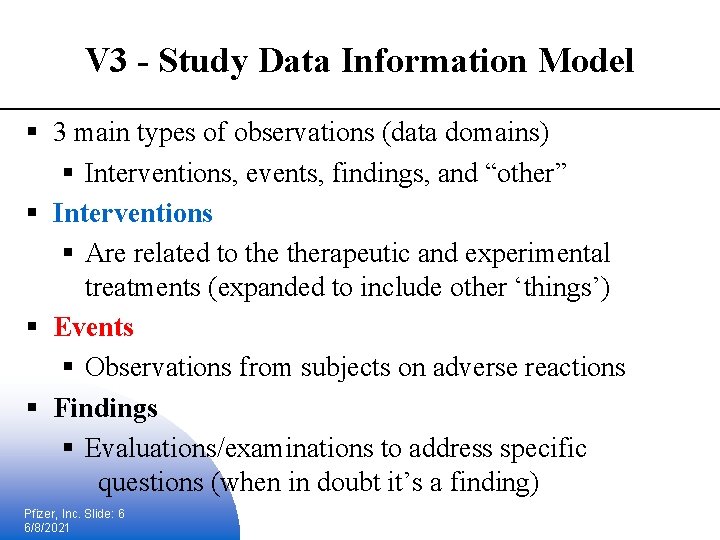
V 3 - Study Data Information Model § 3 main types of observations (data domains) § Interventions, events, findings, and “other” § Interventions § Are related to therapeutic and experimental treatments (expanded to include other ‘things’) § Events § Observations from subjects on adverse reactions § Findings § Evaluations/examinations to address specific questions (when in doubt it’s a finding) Pfizer, Inc. Slide: 6 6/8/2021

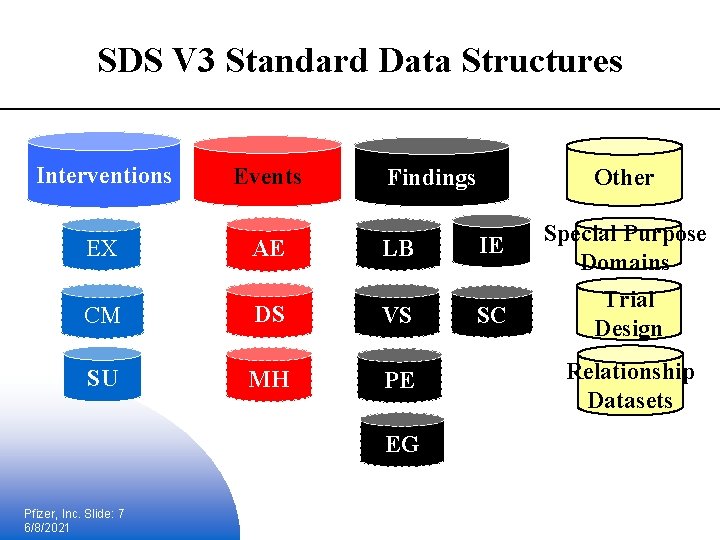
SDS V 3 Standard Data Structures Interventions EX Events AE LB CM DS VS SU MH PE EG Pfizer, Inc. Slide: 7 6/8/2021 Other Findings IE Special Purpose Domains SC Trial Design Relationship Datasets

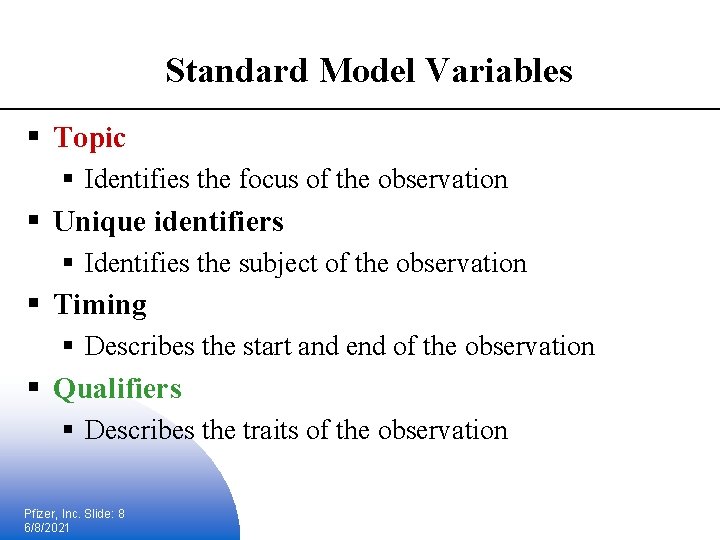
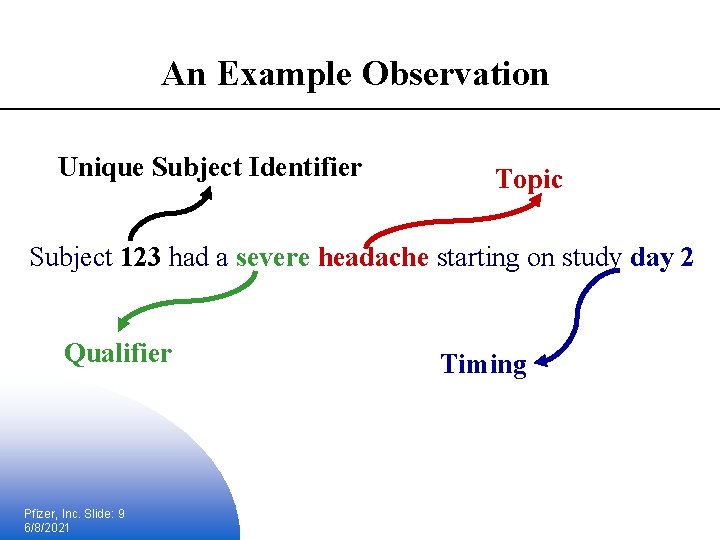
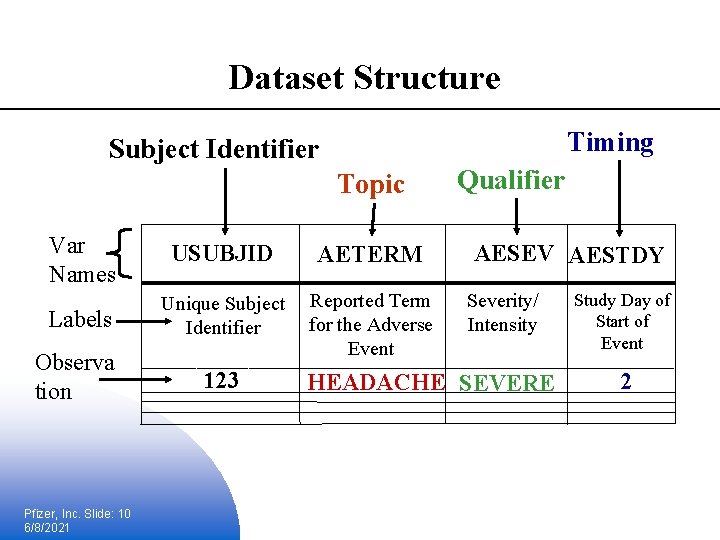
Standard Model Variables § Topic § Identifies the focus of the observation § Unique identifiers § Identifies the subject of the observation § Timing § Describes the start and end of the observation § Qualifiers § Describes the traits of the observation Pfizer, Inc. Slide: 8 6/8/2021

An Example Observation Unique Subject Identifier Topic Subject 123 had a severe headache starting on study day 2 Qualifier Pfizer, Inc. Slide: 9 6/8/2021 Timing

Dataset Structure Timing Subject Identifier Topic Var Names USUBJID AETERM Labels Unique Subject Identifier Observa tion Reported Term for the Adverse Event 123 Pfizer, Inc. Slide: 10 6/8/2021 Qualifier AESEV AESTDY Severity/ Intensity HEADACHE SEVERE Study Day of Start of Event 2

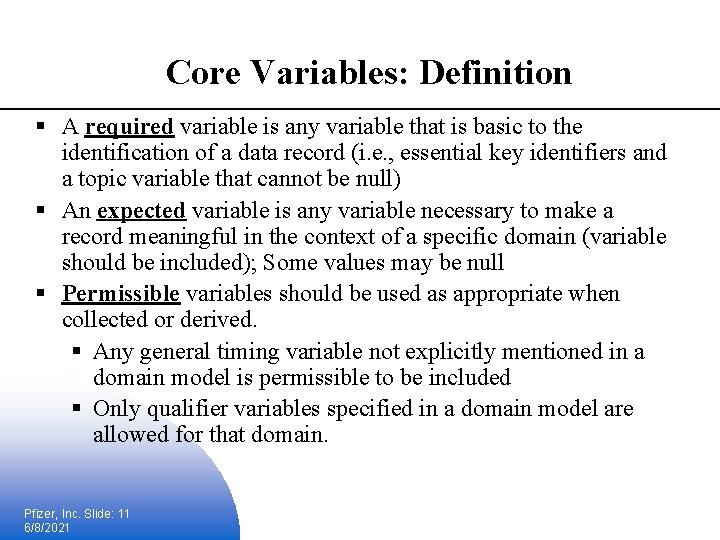
Core Variables: Definition § A required variable is any variable that is basic to the identification of a data record (i. e. , essential key identifiers and a topic variable that cannot be null) § An expected variable is any variable necessary to make a record meaningful in the context of a specific domain (variable should be included); Some values may be null § Permissible variables should be used as appropriate when collected or derived. § Any general timing variable not explicitly mentioned in a domain model is permissible to be included § Only qualifier variables specified in a domain model are allowed for that domain. Pfizer, Inc. Slide: 11 6/8/2021

A Brief Look at the Domain Classes 12

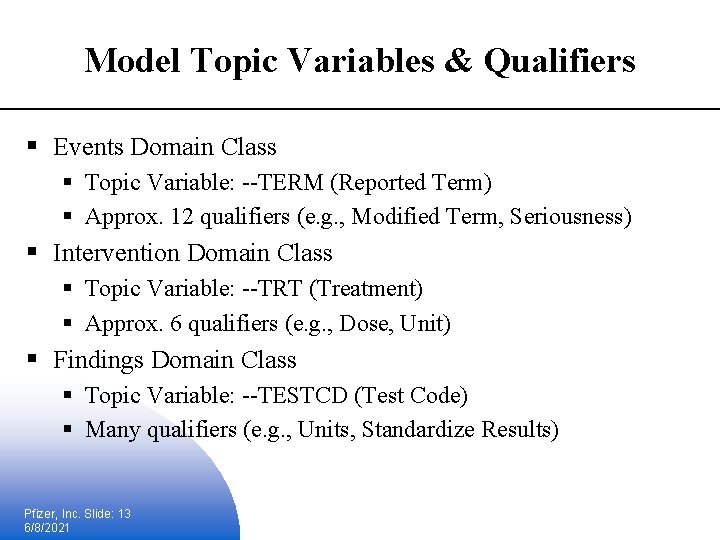
Model Topic Variables & Qualifiers § Events Domain Class § Topic Variable: --TERM (Reported Term) § Approx. 12 qualifiers (e. g. , Modified Term, Seriousness) § Intervention Domain Class § Topic Variable: --TRT (Treatment) § Approx. 6 qualifiers (e. g. , Dose, Unit) § Findings Domain Class § Topic Variable: --TESTCD (Test Code) § Many qualifiers (e. g. , Units, Standardize Results) Pfizer, Inc. Slide: 13 6/8/2021

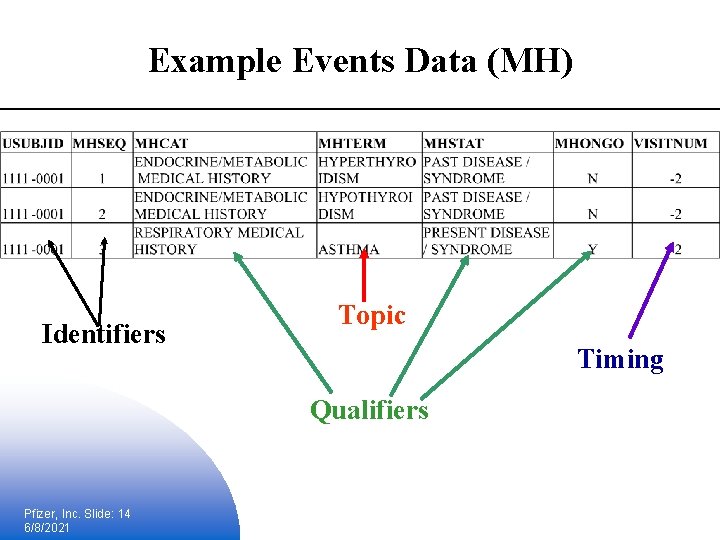
Example Events Data (MH) Identifiers Topic Timing Qualifiers Pfizer, Inc. Slide: 14 6/8/2021

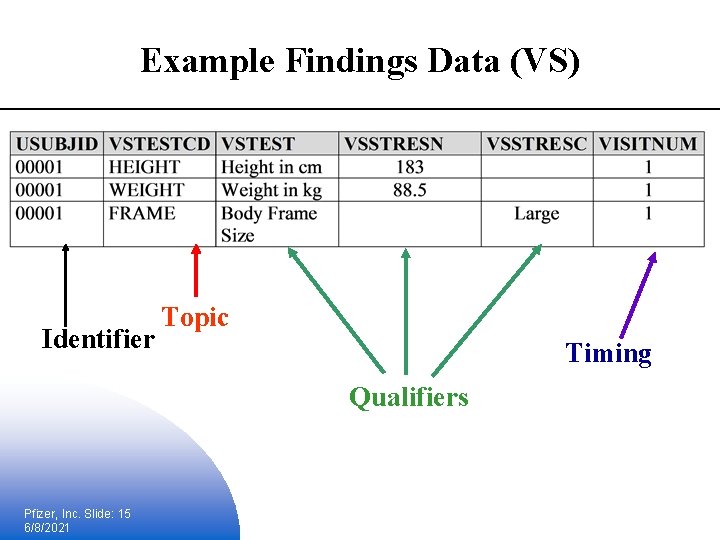
Example Findings Data (VS) Identifier Topic Timing Qualifiers Pfizer, Inc. Slide: 15 6/8/2021

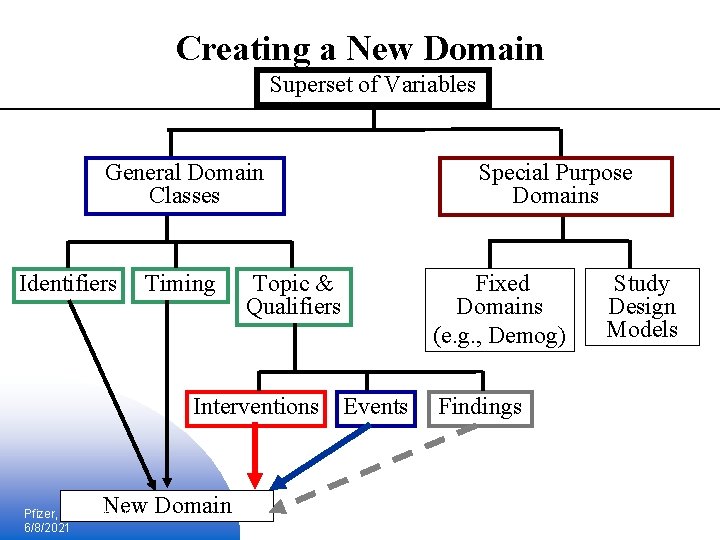
Creating a New Domain Superset of Variables General Domain Classes Identifiers Timing Topic & Qualifiers Interventions Events New Domain Pfizer, Inc. Slide: 16 6/8/2021 Special Purpose Domains Fixed Domains (e. g. , Demog) Findings Study Design Models

Programming, Statistical and Submission Considerations…. 17

PFE & CDISC SDTM § Pfizer has and continues to contribute to CDISC, participated in the FDA pilots and has implemented CDISC Version 2. 0 § We delivered our first CDISC SDTM compliant submission in August § Submitted 5 protocols of partial data § Over 11, 000 patients worth of data § Included all CDISC defined domains plus 5 additional as well as the define. xml § Converted several of the analysis datasets into SDTM compliant structures Pfizer, Inc. Slide: 18 6/8/2021

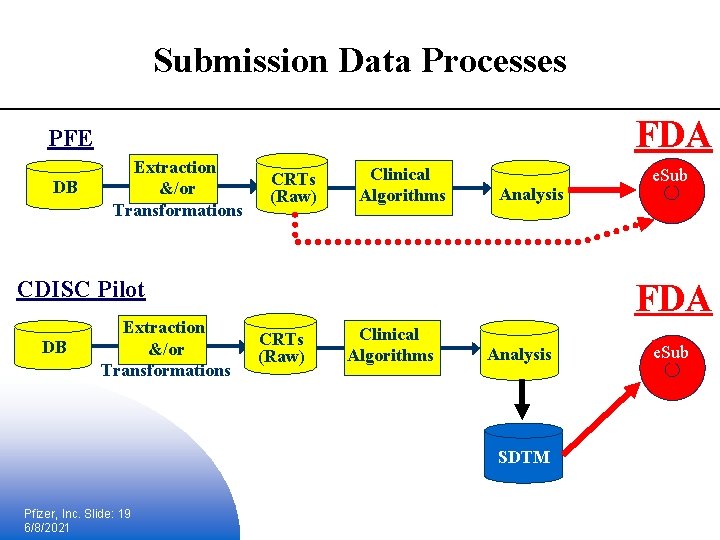
Submission Data Processes FDA PFE DB Extraction &/or Transformations CRTs (Raw) Clinical Algorithms Analysis CDISC Pilot DB Extraction &/or Transformations FDA CRTs (Raw) Clinical Algorithms Analysis SDTM Pfizer, Inc. Slide: 19 6/8/2021 e. Sub

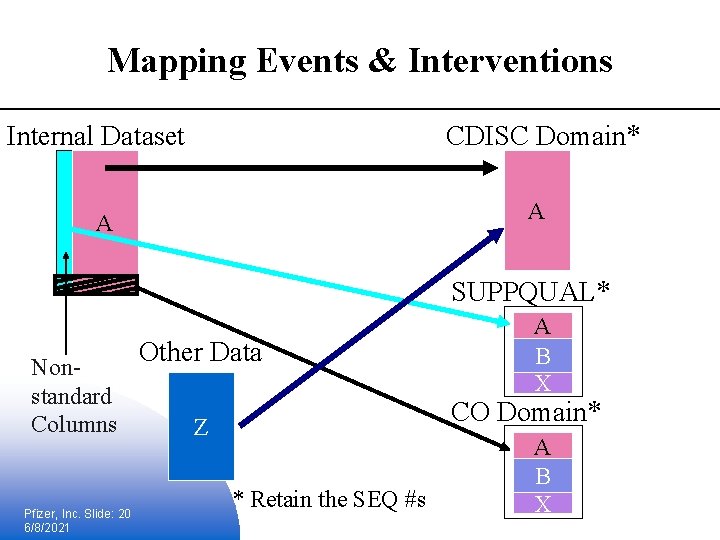
Mapping Events & Interventions Internal Dataset CDISC Domain* A A SUPPQUAL* Nonstandard Columns Pfizer, Inc. Slide: 20 6/8/2021 Other Data A B X CO Domain* Z * Retain the SEQ #s A B X

Lessons learned § Mapping was straight forward § e. Sub data documentation was not affected (e. g. , define. pdf) § Only a few variables were mapped to SUPPQUAL (the exception not the rule) § Technical challenges: § Increase dependencies; SUPPQUAL & CO become dependent on all contributing source datasets; 1 to many (source to target domain) § Several defined internal datasets may map to 1 domain target § May rethink how XPTs are generated – one at a time or in batches § No specific statistical considerations Pfizer, Inc. Slide: 21 6/8/2021

Lessons learned § May need to rethink how you organize your data into CDISC SDTM structures § For example, Pfizer, Inc. Slide: 22 6/8/2021

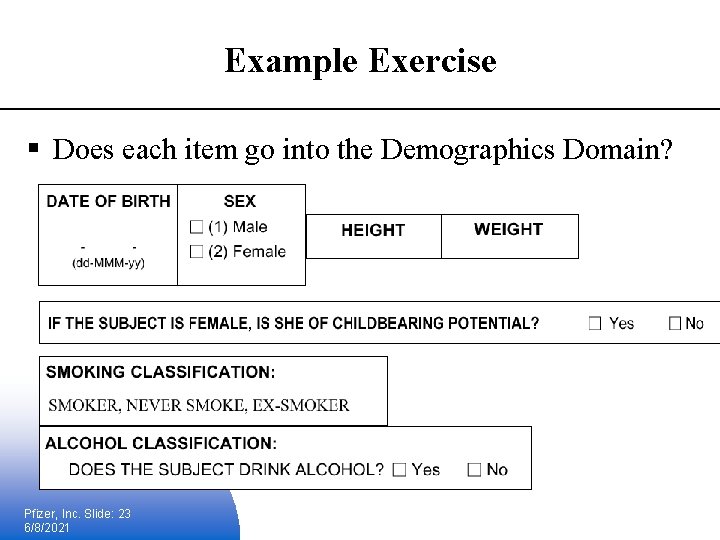
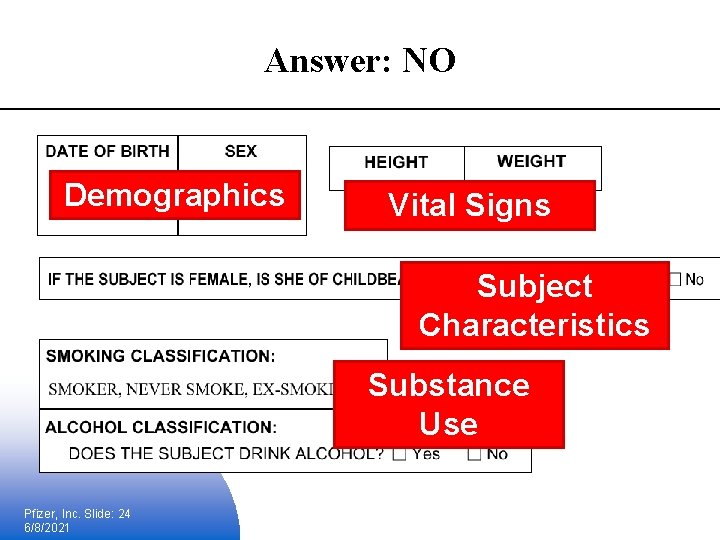
Example Exercise § Does each item go into the Demographics Domain? Pfizer, Inc. Slide: 23 6/8/2021

Answer: NO Demographics Vital Signs Subject Characteristics Substance Use Pfizer, Inc. Slide: 24 6/8/2021

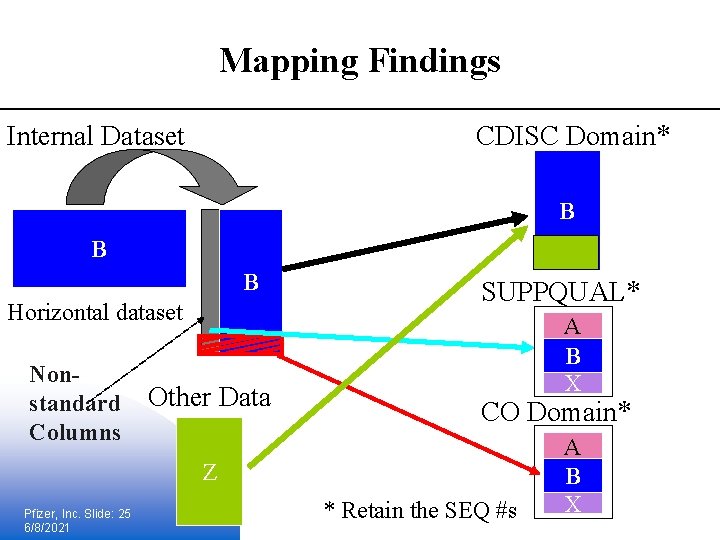
Mapping Findings Internal Dataset CDISC Domain* B B B Horizontal dataset Nonstandard Columns Other Data SUPPQUAL* A B X CO Domain* Z Pfizer, Inc. Slide: 25 6/8/2021 * Retain the SEQ #s A B X

Lessons learned § It describes the majority of the data in a submission § More complicated, b/c need to retain the transposed information and should be provided in define. xml § Statistical & programming considerations: § data stored in non-traditional structure § The structure is flexible enough to contain both collected analysis data; do you continue to keep them separate? § e. Sub data documentation is affected § Need to change CRF annotations and provide column (variable) and record-level Pfizer, Inc. Slide: 26 6/8/2021

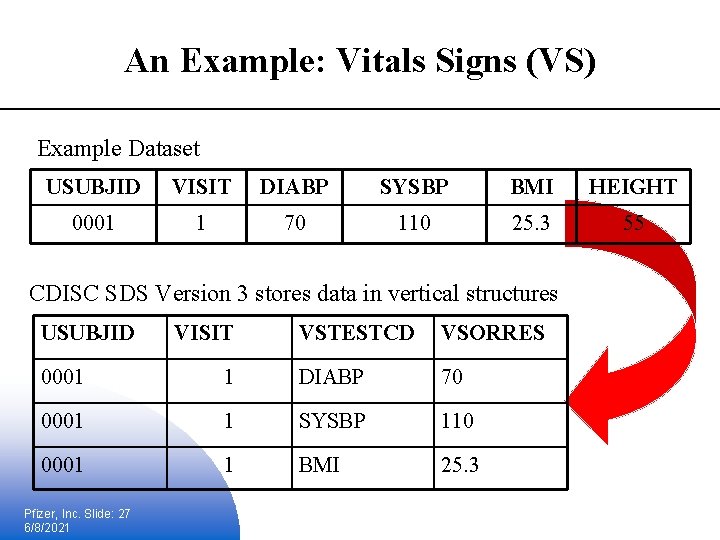
An Example: Vitals Signs (VS) Example Dataset USUBJID VISIT DIABP SYSBP BMI HEIGHT 0001 1 70 110 25. 3 55 CDISC SDS Version 3 stores data in vertical structures USUBJID VISIT VSTESTCD VSORRES 0001 1 DIABP 70 0001 1 SYSBP 110 0001 1 BMI 25. 3 Pfizer, Inc. Slide: 27 6/8/2021

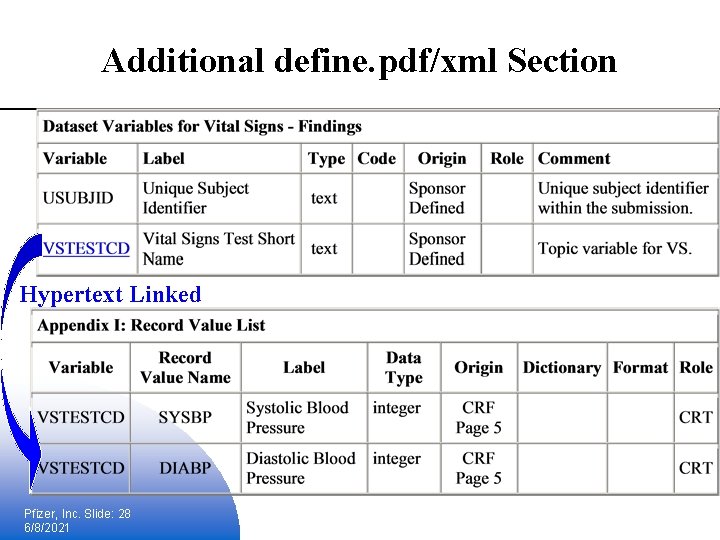
Additional define. pdf/xml Section Hypertext Linked Pfizer, Inc. Slide: 28 6/8/2021

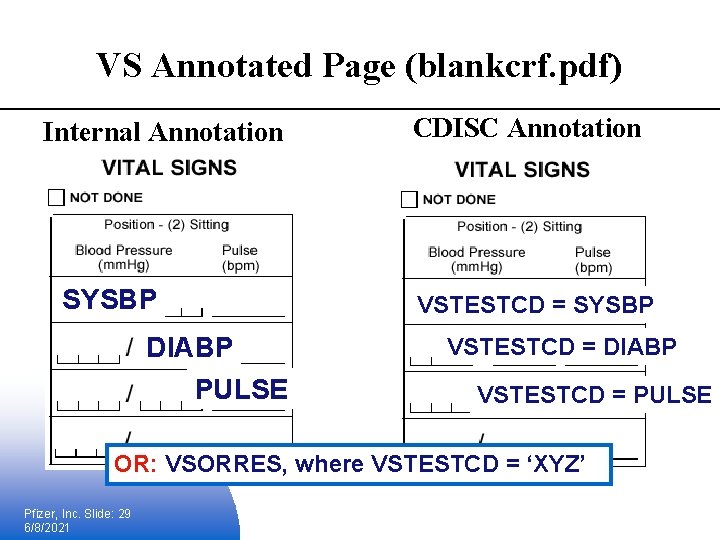
VS Annotated Page (blankcrf. pdf) Internal Annotation SYSBP DIABP PULSE CDISC Annotation VSTESTCD = SYSBP VSTESTCD = DIABP VSTESTCD = PULSE OR: VSORRES, where VSTESTCD = ‘XYZ’ Pfizer, Inc. Slide: 29 6/8/2021

Overall Statistical & Programming Considerations § Where to implement the data standards? § At the end (at XPT generation) § During the table production process § All the way back to the Database § Must prioritize what’s important: § Having minimal impact on your internal data storage &/or table creation process & algorithms § Implementing versions quickly (Time & Resource Issues) § End-game mapping costs § Software re-use? Pfizer, Inc. Slide: 30 6/8/2021

Implementation Strategies 31

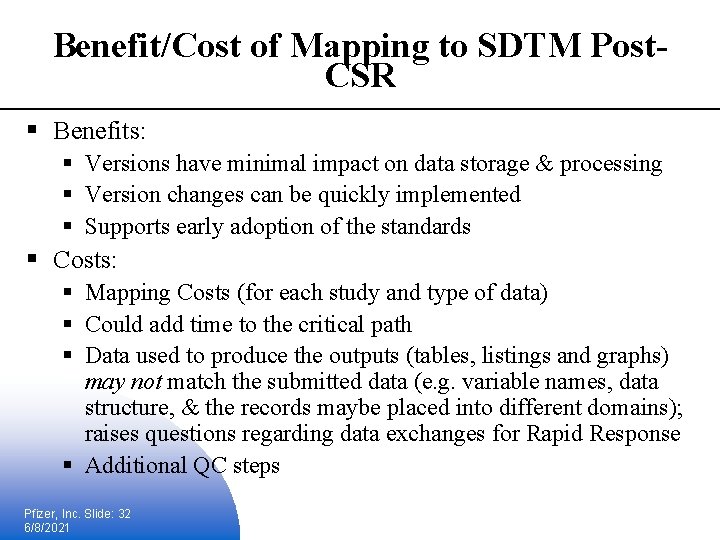
Benefit/Cost of Mapping to SDTM Post. CSR § Benefits: § Versions have minimal impact on data storage & processing § Version changes can be quickly implemented § Supports early adoption of the standards § Costs: § Mapping Costs (for each study and type of data) § Could add time to the critical path § Data used to produce the outputs (tables, listings and graphs) may not match the submitted data (e. g. variable names, data structure, & the records maybe placed into different domains); raises questions regarding data exchanges for Rapid Response § Additional QC steps Pfizer, Inc. Slide: 32 6/8/2021

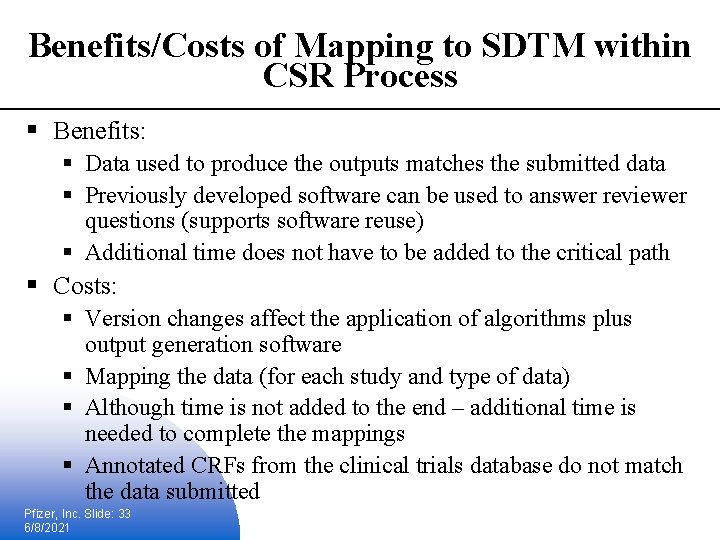
Benefits/Costs of Mapping to SDTM within CSR Process § Benefits: § Data used to produce the outputs matches the submitted data § Previously developed software can be used to answer reviewer questions (supports software reuse) § Additional time does not have to be added to the critical path § Costs: § Version changes affect the application of algorithms plus output generation software § Mapping the data (for each study and type of data) § Although time is not added to the end – additional time is needed to complete the mappings § Annotated CRFs from the clinical trials database do not match the data submitted Pfizer, Inc. Slide: 33 6/8/2021

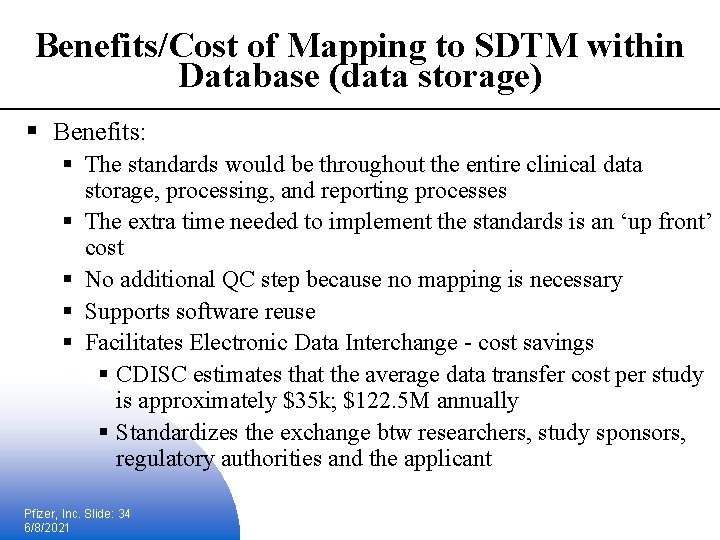
Benefits/Cost of Mapping to SDTM within Database (data storage) § Benefits: § The standards would be throughout the entire clinical data storage, processing, and reporting processes § The extra time needed to implement the standards is an ‘up front’ cost § No additional QC step because no mapping is necessary § Supports software reuse § Facilitates Electronic Data Interchange - cost savings § CDISC estimates that the average data transfer cost per study is approximately $35 k; $122. 5 M annually § Standardizes the exchange btw researchers, study sponsors, regulatory authorities and the applicant Pfizer, Inc. Slide: 34 6/8/2021

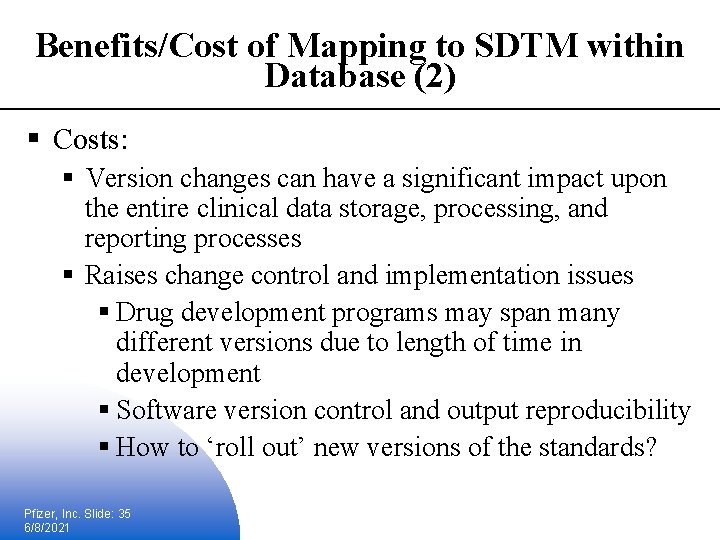
Benefits/Cost of Mapping to SDTM within Database (2) § Costs: § Version changes can have a significant impact upon the entire clinical data storage, processing, and reporting processes § Raises change control and implementation issues § Drug development programs may span many different versions due to length of time in development § Software version control and output reproducibility § How to ‘roll out’ new versions of the standards? Pfizer, Inc. Slide: 35 6/8/2021

Implications to the Industry § All sponsors are facing implementation strategy challenges § Analysis Datasets should also be provided in addition to the SDTM datasets § At this point they don’t need to conform to V 3 § Will be provided separately (e. g. , in a different submission directory) § Standardized datasets will enable the use of standardized review tools and could lead to more thorough and efficient reviews (e. g. , decreased learning curve) Pfizer, Inc. Slide: 36 6/8/2021

Summary § There are significant differences between CDISC SDS V 2 and V 3 in terms of scope, design and philosophy § For more information regarding SDTM Version 3. 1: www. cdisc. org Thank you! William_J_Qubeck@Groton. Pfizer. com Pfizer, Inc. Slide: 37 6/8/2021