Alzheimers Disease v AD is a fatal neurodegenerative














- Slides: 28


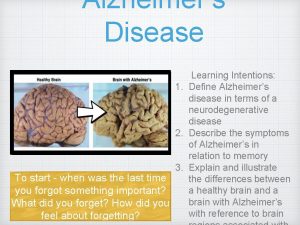
Alzheimer's Disease v AD is a fatal neurodegenerative disease associated with elderly age group & a major health problem in the geriatric subject's worldwide. v It was first described by German psychiatrist & neuropathologist Alois Alzheimer in 1906 & was named after him. v The most important risk factors for AD are old age & a positive family history.

v AD could be either early or late onset. Early onset AD is genetically determined; whereas late onset, which includes the majority of pts. , is believed to be a result of interaction between genetic & environmental factors. v Age is a major risk factor for AD. Other risk factors for late onset AD include: family history, & susceptibility genes Apolipoprotein e allele (APO-e 4 allele). v While the specific factors involved in the etiology & pathogenesis of AD are not well characterized, inflammation is thought to play important role in pathogenesis of AD.

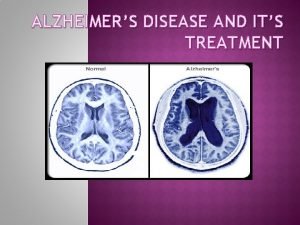
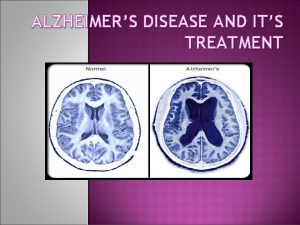
v AD has 3 consistent neuropathologic hallmarks: I. Extracellular Plaques of Amyloid beta Aβ protein (amyloid plaques) II. Intraneuronal Neurofibrillary Tangles (NFTs) III. Neuronal Degeneration.

v A distinguishing feature of AD is that the plaques & Tangles are localized to areas in the brain leading to gradual loss of neuronal synapses & ultimately neuronal degeneration with diminution of essential neurotransmitters. Causing gradual deterioration of memory & other cognitive functions, which eventually leads to a complete incapacity & death.

Disease Biochemistry Ø In Alzheimer's disease, an unknown process causes Senile plaques. Ø Senile plaques are mainly composed of Aβ peptide that is produced from proteolytic cleavage into smaller fragments of the amyloid precursor protein APP (transmembrane protein that penetrates through the neuron's membrane) that deposit outside neurons in dense formations.

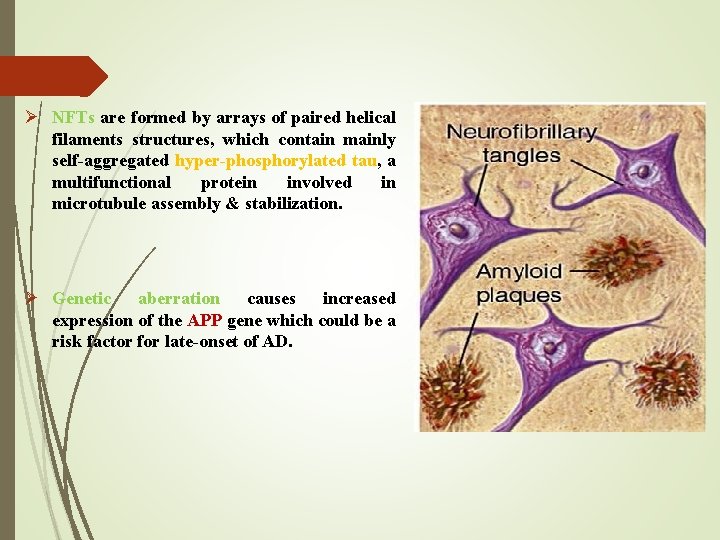
Ø NFTs are formed by arrays of paired helical filaments structures, which contain mainly self-aggregated hyper-phosphorylated tau, a multifunctional protein involved in microtubule assembly & stabilization. Ø Genetic aberration causes increased expression of the APP gene which could be a risk factor for late-onset of AD.

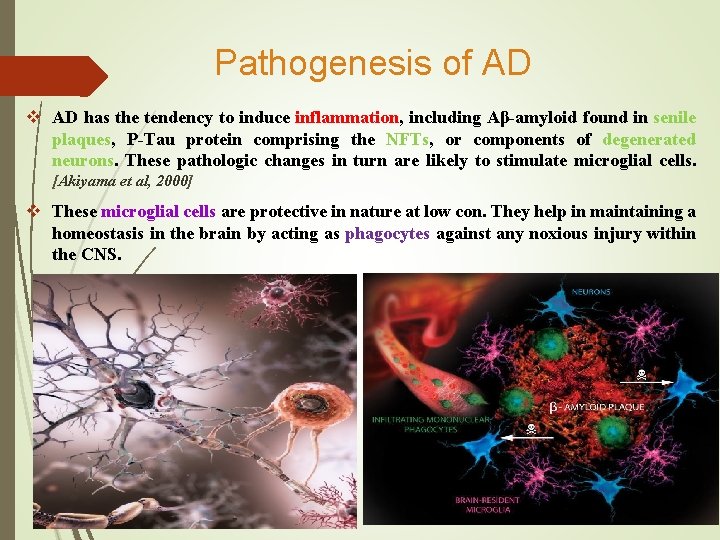
Pathogenesis of AD v AD has the tendency to induce inflammation, including Aβ-amyloid found in senile plaques, P-Tau protein comprising the NFTs, or components of degenerated neurons. These pathologic changes in turn are likely to stimulate microglial cells. [Akiyama et al, 2000] v These microglial cells are protective in nature at low con. They help in maintaining a homeostasis in the brain by acting as phagocytes against any noxious injury within the CNS.

v In healthy individuals, microglial cells play a neuroprotective function by clearing the plaques. [Fetler & Amigorena, 2005] v With advancing age & genetic predisposition, the normal function is compromised, resulting in persistence of chronic inflammatory response within the CNS. [Schram et al, 2007] v This results to produce neurotoxic substances in the brain, when they are exposed to the systemic inflammatory signals. Such response of the microglial cells contributes to the pathogenesis of AD instead of providing with a protective response to the systemic inflammatory signals. v The induced microglial cells now referred to as “activated microglial cells” alters its morphology & secrete cell antigens, which in turn results in uncontrolled expression of pro-inflammatory factors. v This can induce neurodegeneration, suggesting that the expression of inflammatory molecules will contribute to the progression of the AD. [Bernhardi & Eugenin, 2004] by accumulation of aggregated amyloid fibrils, which are believed to be the toxic responsible for disrupting the cell's homeostasis, induces programmed cell death (apoptosis).

Periodontal Disease Ø PDD is a group of conditions that cause inflammation & destruction of the periodontal tissues. Ø The etiology is complex involving the presence of pathogenic bacteria found in dental plaque, genetic & individual variation in host immune response. Ø PDD is a common source of chronic systemic infection in humans. [Taylor et al, 2006]. Ø More than 400 species of microorganisms are found in subgingival plaque. The bacterial pathogens most strongly implicated in chronic periodontal disease are: - o Aggregatibacter actinomycetemcomitans o Porphyromonas gingivalis, o Tannerella forsythensis, o Treponema denticola.

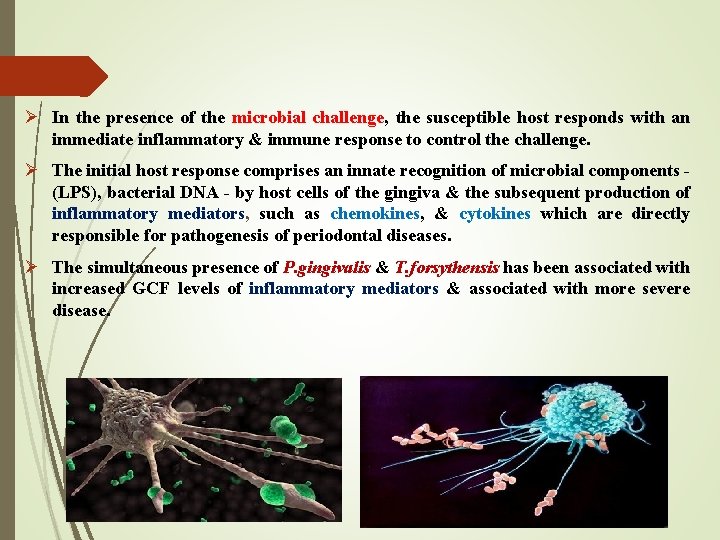
Ø In the presence of the microbial challenge, the susceptible host responds with an immediate inflammatory & immune response to control the challenge. Ø The initial host response comprises an innate recognition of microbial components - (LPS), bacterial DNA - by host cells of the gingiva & the subsequent production of inflammatory mediators, such as chemokines, & cytokines which are directly responsible for pathogenesis of periodontal diseases. Ø The simultaneous presence of P. gingivalis & T. forsythensis has been associated with increased GCF levels of inflammatory mediators & associated with more severe disease.

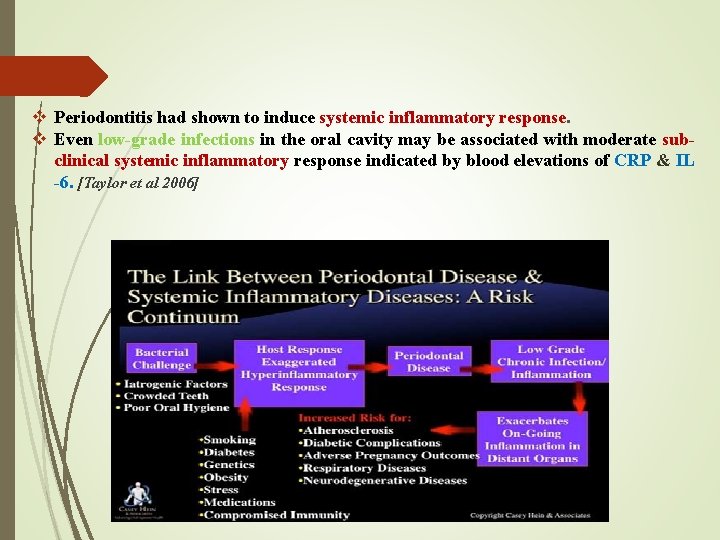
v Periodontitis had shown to induce systemic inflammatory response. v Even low-grade infections in the oral cavity may be associated with moderate subclinical systemic inflammatory response indicated by blood elevations of CRP & IL -6. [Taylor et al 2006]

v PDD may also be associated with increased risk for AD. v In Swedish study, [Gatz et al, 2006] found that those who had lost half or more of their teeth before age 35 had a 1. 7 fold greater risk for AD. v A case control study in Japan found that loss of more than half of teeth by age 50– 60 increased AD risk by 2. 6 fold. [Kondo et al, 1994] v In USA [Stein et al, 2007] 144 nuns, having few or no teeth, predicted a 4. 3 -fold higher risk of dementia. v These studies included dental records that indicated teeth were lost, which are resulted in exposure to bacteria.

Systemic Infection From Mouth to Bloodstream v The mouth is a primary channel by which external organisms enter the body. Gram +ve & -ve oral bacteria can induce proinflammatory cytokines (IL-1, IL-6 & TNF-α). v The oral cavity has several barriers (physical, electrical, & chemical) that inhibit penetration by pathogens. If oral barriers are compromised by (PD, trauma, or immune suppression) microbes can extend to cause acute or chronic infection. v In individuals with good oral & immune health, the transient bacteremia has few consequences. Transient bacteremia occurs after tooth brushing & flossing as well as after normal dental procedures. v In individuals with PD infection have higher levels of pathogenic accumulation & may experience transient bacteremia multiple times per day. [Forner et al, 2006] v Once in the bloodstream, these bacteria can induce acute phase response characterized by increased WBC counts & the release of inflammatory cytokines.

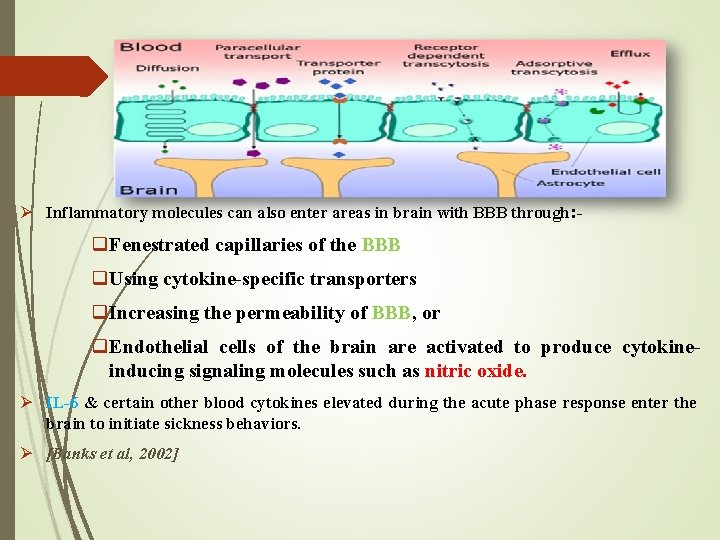
Mechanisms Involved in Spread of Inflammation to Brain Ø Although BBB generally prevents entry of substances into the brain, there is evidence that inflammatory cytokines can enter the brain under certain circumstances. Ø There are 2 mechanisms involved in the brain, that is, via: systemic circulation &/or neural pathways. Ø In the systemic circulation: proinflammatory molecules enter brain through areas which lack BBB. Ø Other mechanism include the presence of receptors for CD 14 present in the brain: which can get activated by LPS or AβP, which in turn will activate CD 14 cells. These CD 14 cells are exposed to systemic circulation; thus increasing further brain cytokines & hypothetically contributing to the inflammatory burden of AD.

Ø Inflammatory molecules can also enter areas in brain with BBB through: - q. Fenestrated capillaries of the BBB q. Using cytokine-specific transporters q. Increasing the permeability of BBB, or q. Endothelial cells of the brain are activated to produce cytokineinducing signaling molecules such as nitric oxide. Ø IL-6 & certain other blood cytokines elevated during the acute phase response enter the brain to initiate sickness behaviors. Ø [Banks et al, 2002]

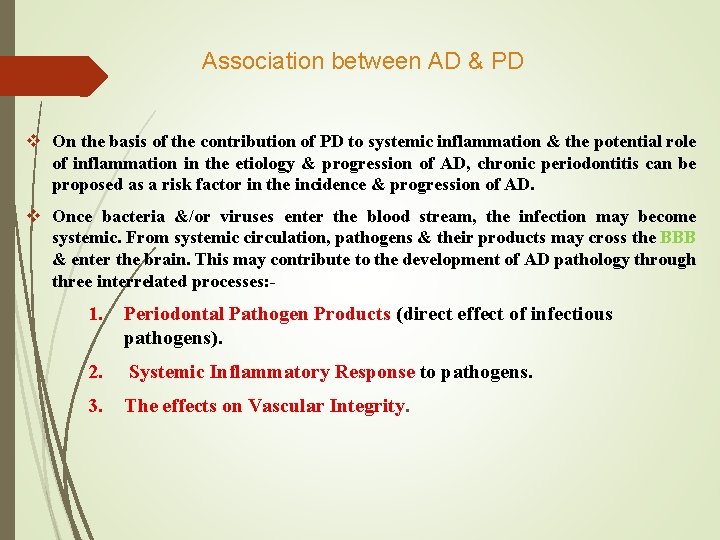
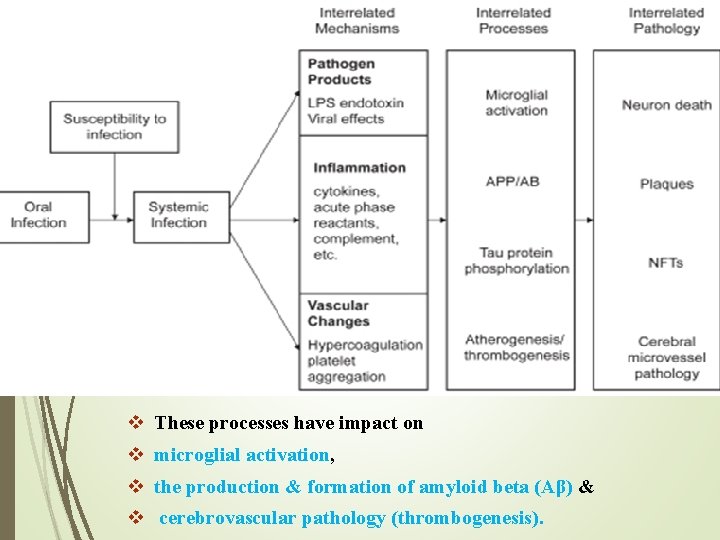
Association between AD & PD v On the basis of the contribution of PD to systemic inflammation & the potential role of inflammation in the etiology & progression of AD, chronic periodontitis can be proposed as a risk factor in the incidence & progression of AD. v Once bacteria &/or viruses enter the blood stream, the infection may become systemic. From systemic circulation, pathogens & their products may cross the BBB & enter the brain. This may contribute to the development of AD pathology through three interrelated processes: - 1. Periodontal Pathogen Products (direct effect of infectious pathogens). 2. Systemic Inflammatory Response to pathogens. 3. The effects on Vascular Integrity.

v These processes have impact on v microglial activation, v the production & formation of amyloid beta (Aβ) & v cerebrovascular pathology (thrombogenesis).

1 -Pathogen Products v The cell walls of Gram-ve bacteria contain LPS that induces a number of host defenses. LPS inflammatory cytokines microglial activation & altered processing of (APP). [Mattson, 2004]. v periodontal pathogens are linked with AD as: - o o Aggregatibacter actinomycetemcomitans Porphyromonas gingivalis Tannerella forsythensis Treponema denticola

1 -Pathogen Products v The mechanism by which periodontal bacteria have access to the brain is not known. However, the mechanisms described for other bacteria such as access via systemic circulation are possible. Bacteremia of oral origin occurs quite frequently during dental & non-dental manipulations. [Kamer et al, 2008] v Other way is via peripheral nerves. [Riviere et al, 2002] v Treponema species have been detected more frequently in the trigeminal ganglia, brainstem, & cortex of brain, & AD, suggesting that oral bacteria are capable of invading brain tissue perhaps via peripheral nerve fibers. v Other spirochetes have been found in the brain tissue, blood & cerebrospinal fluid of AD patients.

1 -Pathogen Products v The role of periodontal pathogens in pathogenesis of AD. v A study showed the presence of LPS of P. gingivalis in brains of an individual with AD. [Poole et al, 2013] v Elevated plasma AB Ig. G to periodontal bacteria (A. a, P. gingivalis, or/& T. forsythia) found in individual with AD compared to normal controls. [Sparks et al, 2012] v Furthermore, elevated serum AB Ig. G to F. nucleatum & P. intermedia in subjects with AD had found before development of AD as compared to controls. [Kamer et al, 2009] v Animal studies show that chronic infusion of LPS into rat brains may result in long lasting inflammatory reaction with pathological changes. These changes include an increase in IL-1β, TNF-α & βAPP & the degeneration of neurons, impairment in working memory, & decreased size of hippocampus & temporal lobe. [Hauss. Wegrzyniak et al, 2000]

2 - Systemic Inflammation & AD v It is clear that inflammation is involved in AD. However, it is not clear if inflammatory processes initiate pathological processes or merely contribute to disease progression. v The interaction between PD bacteria & host response results in locally increased production of proinflammatory cytokines including IL-1 β, IL-6, IL-8, TNF-α & CRP. [Loos, 2005] v Pro-inflammatory molecules derived locally from PD tissue may also stimulate trigeminal nerve fibers, leading to increased brain cytokines. [Romeo et al, 2001] v There are several mechanisms through which systemic inflammation might contribute to pathogenesis in AD. These include: activation of microglia dysregulation of APP & Aβ processing & metabolism, activation of microglia in response to Aβ neurotoxic loop [Watts et al, 2008]

Inflammation & AD v Proinflammatory cytokines including IL-6 & TNF-α can be directly toxic in high concentrations or can stimulate Aβ production. v Nonspecific responses of phagocytic cells can remove Aβ deposits, as well as recruit more microglia which produce more immune mediators. v Cell attempts to dissolve plaques & tangles may cause toxicity via the release of neurotoxic substances including nitric oxide & other reactive oxygen species. v Over sustained periods of time, these products may contribute to neurodegeneration via injury of surrounding non-infected cells resulting in neuron loss. [Rogers et al, 2002]

Inflammation & AD v Systemic Inflammatory markers appear to be higher in persons with AD than normal age-matched controls. [Heneka & O’Banion, 2007] v Griffin et al, 1998 proposed that IL-1 is critical to the processing of βAPP, IL-1 stimulates APP synthesis & factors that lead to its amyloidogenic properties, responsible for the breakdown of acetylcholine which is important in learning & memory function. v TNF-α & interferon gamma alter the metabolism of βAPP , trigger Aβp production & inhibit soluble APP secretion. v In acute systemic inflammations IL-1, IL-6, & CRP are elevated in the temporal cortex of AD patients compared to controls. [Mc. Geer et al, 1994] v Both acute & chronic systemic inflammations, associated with increases in serum TNFα, in AD subjects compared to healthy controls. [Holmes et al, 2009] [Kamer et al, 2009]

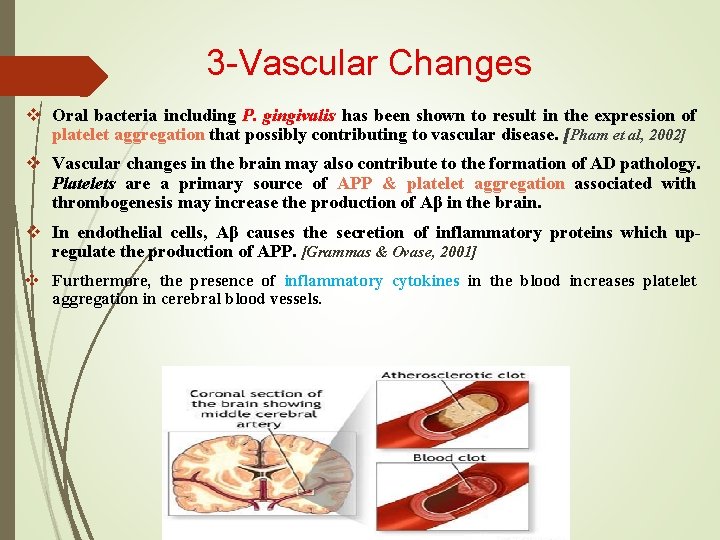
3 -Vascular Changes v Oral bacteria including P. gingivalis has been shown to result in the expression of platelet aggregation that possibly contributing to vascular disease. [Pham et al, 2002] v Vascular changes in the brain may also contribute to the formation of AD pathology. Platelets are a primary source of APP & platelet aggregation associated with thrombogenesis may increase the production of Aβ in the brain. v In endothelial cells, Aβ causes the secretion of inflammatory proteins which upregulate the production of APP. [Grammas & Ovase, 2001] v Furthermore, the presence of inflammatory cytokines in the blood increases platelet aggregation in cerebral blood vessels.

Conclusion v Bacterial & viral infections commonly found in PD may impact the brain, either directly by harmful pathogens products that contribute to the development of AD. v or via systemic inflammatory responses that contribute to the exacerbation of existing brain diseases. v Periodontal infections may also contribute to vascular pathology with the potential to impact brain function. v PDD & AD may also share common risk factors such as: genetic polymorphisms inflammatory mediators Age Gender Lifestyle


Thank You
 Alzheimers nz conference 2020
Alzheimers nz conference 2020 Georgia alzheimers planning
Georgia alzheimers planning Site:slidetodoc.com
Site:slidetodoc.com Alzheimers fast score
Alzheimers fast score Alzheimers society contented dementia
Alzheimers society contented dementia Vaskulär demens
Vaskulär demens Alzheimers eye test joke
Alzheimers eye test joke Bharathi viswanathan
Bharathi viswanathan What is percy's fatal mistake while battling the chimera
What is percy's fatal mistake while battling the chimera Oki error 980
Oki error 980 My attitude is fatal
My attitude is fatal Ms title
Ms title What is ambitious
What is ambitious Asma casi fatal
Asma casi fatal Romance de una fatal ocasion
Romance de una fatal ocasion Jcc v eisenhower
Jcc v eisenhower Evaluation of non fatal offences
Evaluation of non fatal offences Fatal error 823
Fatal error 823 13 fatal errors managers make cliff notes
13 fatal errors managers make cliff notes Which process discovers a fatal error?
Which process discovers a fatal error? Center fed room
Center fed room Indicadores verificables objetivamente
Indicadores verificables objetivamente The fatal equilibrium
The fatal equilibrium Fatality risk management
Fatality risk management Non-fatal injury evaluation
Non-fatal injury evaluation Balfour beatty 6 fatal risks
Balfour beatty 6 fatal risks When to use polling or interrupts
When to use polling or interrupts Fatal error in client side script
Fatal error in client side script I'm not yet born poem
I'm not yet born poem