ALUMINUM Isotopes 27 Al and 26 Al 1







- Slides: 11

ALUMINUM

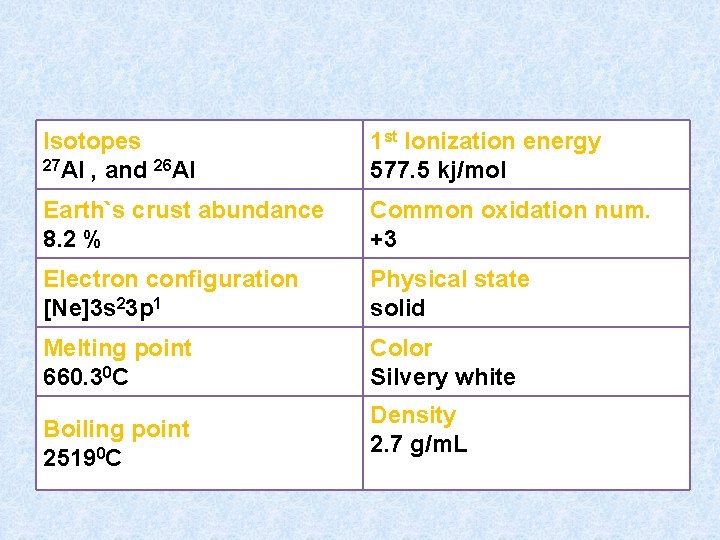
Isotopes 27 Al , and 26 Al 1 st Ionization energy 577. 5 kj/mol Earth`s crust abundance 8. 2 % Common oxidation num. +3 Electron configuration [Ne]3 s 23 p 1 Physical state solid Melting point 660. 30 C Color Silvery white Boiling point 25190 C Density 2. 7 g/m. L

• Isolated in 1827 by the German chemist F. Woehler. • Aluminum comes from the word alumen means bitter taste in Latin. • Silvery white, and soft metal, ductile and malleable, • Hammered easily into wire, sheet and plate, • Conductor of electricity.

Occurrence • Third most abundant element in the earth’s crust. • The most abundant metal in the earth’s crust. • The main aluminum ores are Feldspar, K 2 Al 2 Si 6 O 16, Kaolinite, Al 2 Si 2 O 7. 2 H 2 O, Corundum , A 12 O 3, Cryolite, Na 3 Al. F 6, Bauxite, Al 2 O 3. 2 H 2 O.

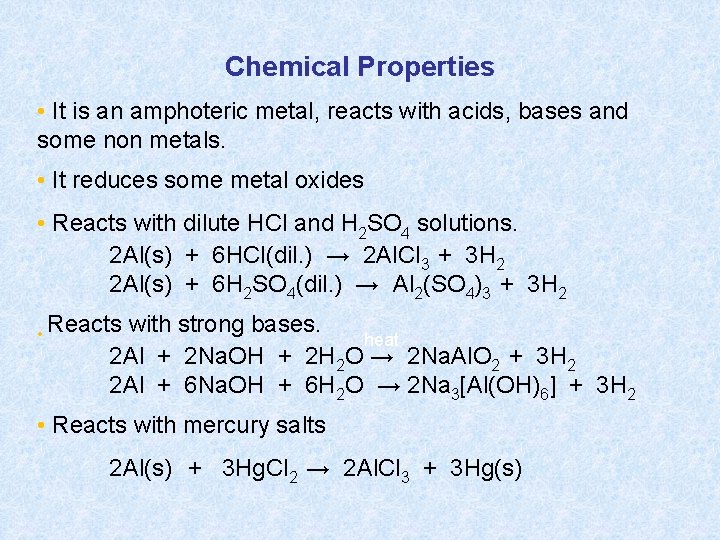
Chemical Properties • It is an amphoteric metal, reacts with acids, bases and some non metals. • It reduces some metal oxides • Reacts with dilute HCl and H 2 SO 4 solutions. 2 Al(s) + 6 HCl(dil. ) → 2 Al. Cl 3 + 3 H 2 2 Al(s) + 6 H 2 SO 4(dil. ) → Al 2(SO 4)3 + 3 H 2 • Reacts with strong bases. heat 2 Al + 2 Na. OH + 2 H 2 O → 2 Na. Al. O 2 + 3 H 2 2 Al + 6 Na. OH + 6 H 2 O → 2 Na 3[Al(OH)6] + 3 H 2 • Reacts with mercury salts 2 Al(s) + 3 Hg. Cl 2 → 2 Al. Cl 3 + 3 Hg(s)

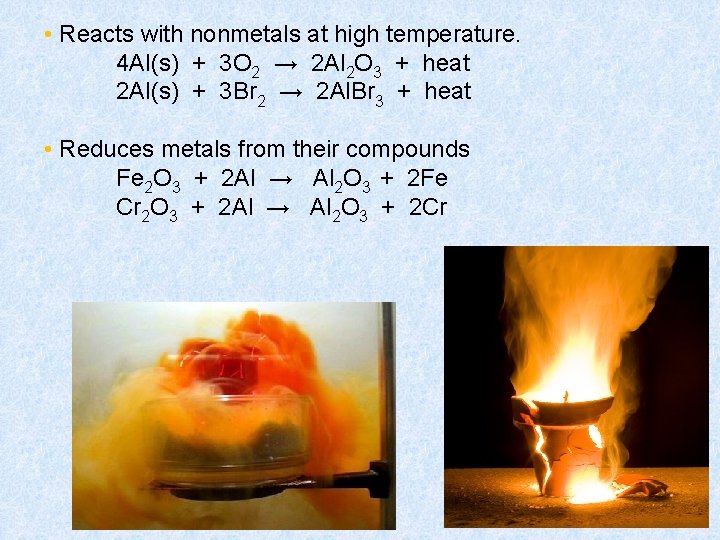
• Reacts with nonmetals at high temperature. 4 Al(s) + 3 O 2 → 2 Al 2 O 3 + heat 2 Al(s) + 3 Br 2 → 2 Al. Br 3 + heat • Reduces metals from their compounds Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe Cr 2 O 3 + 2 Al → Al 2 O 3 + 2 Cr

Compounds Aluminum Oxide, Al 2 O 3 • It is called as alumina, white substance. • Hard substance • Melting point of 2045°C. • Almost insoluble in water. • Shows amphoteric properties.

Aluminum Hydroxide, Al(OH)3 • It is gray precipitate produced from the reaction of aluminum salts with bases. Al. Cl 3 + 3 Na. OH(aq) → Al(OH)3 + 3 Na. Cl(aq) Aluminum Sulfate, Al 2(SO 4)3 • Aluminum sulfate forms in nature as an important series of alums. • Alums have MAl(SO 4)2. 12 H 2 O, Where M may be Na+, K, NH 4+, and Ag+

Uses • Aluminum alloys are light, durable, and resistant to corrosion and have high electrical conductivity. • Some aluminum alloys are Duralumin (Al, Mg, Cu, Mn), Magnalium (Al, Mg), and Aluminum bronze (Al, Cu).

• Aluminum has a wide variety of uses because of its low cost, nice appearance, lightness, good conductivity of heat and electricity as well as a good material for packaging foods.

• It is used in buildings, ships, submarines, planes and space technology, and high voltage electrical lines. • Aluminum powder is used in camera flashes, dyes, alumina thermo processes, and napalm bombs.